Abstract
Background
The metabolic implications of intermuscular adipose tissue (IMAT) are poorly understood compared to those of visceral adipose tissue (VAT) even though the absolute quantities of both depots are similar in many individuals.
Objective
The aim was to determine the independent relationship between whole-body IMAT and cardiovascular risk factor parameters.
Design
Whole body magnetic resonance imaging (MRI) was used to quantify total skeletal muscle (SM), total adipose tissue (TAT) of which IMAT, defined as the AT visible by MRI within the boundary of the muscle fascia, is a sub-component. Fasting serum measures (n = 262) of glucose, total cholesterol (T-Chol), high-density lipoprotein cholesterol (HDL-Chol), triglycerides (TG), protein bound glucose (PBG, n = 206) and insulin (n = 119) were acquired in healthy African-American (AA, n = 78) and Caucasian (Ca, n = 109) women (body mass index (BMI) 26.5±5.7 kg/m2; 44.4±16.4 years) and men (39 AA, 62 Ca; BMI 25.6±3.5 kg/m2; 45.6±17.4 years). General linear models identified the independent effects of IMAT after covarying for SM, VAT, TAT, race, sex and two-way interactions.
Results
Significant independent associations were observed for IMAT with glucose (P < 0.001), PBG (P < 0.001) and T-Chol (P < 0.05). The association of IMAT with cholesterol differed by race in such a manner that for a unit increase in IMAT, T-Chol increased more rapidly in Ca compared to AA (P < 0.05). TG, HDL-Chol and insulin had no independent association with IMAT.
Conclusion
The strong independent associations of IMAT with fasting glucose and PBG suggest that IMAT may be related to glucose metabolism; however, IMAT is also associated with T-Chol in Ca.
Keywords: muscle fat, adipose tissue, imaging, body composition, health risk
Introduction
Adipose tissue (AT) has emerged as an important endocrine organ. Considerable evidence implicates altered fat topography and defects in adipocyte metabolism in the pathogenesis of adult disease including type 2 diabetes.1–3 AT and its distribution are risk factors for metabolic abnormalities.4–6 It is well established that excess visceral adipose tissue (VAT) mass is an important risk factor in the development of coronary artery disease7,8 and non-insulin-dependent diabetes mellitus.9,10 It is recognized that not all AT depots carry equivalent metabolic risk and lower-body AT has been shown to exert protective effects against cardiovascular disease. 11–13 Emerging evidence suggests that subcutaneous adipose tissue (SAT) or the lack of it may play a role in insulin resistance,14 and therefore SAT may not be the passive storage depot previously assumed. In HIV-positive obese women, 15 lower levels of leg SAT were found to be an independent determinant of insulin resistance.16
We have previously described a new fat depot called intermuscular adipose tissue (IMAT) mass located between muscle bundles using whole body magnetic resonance imaging (MRI).17,18 IMAT should not be confused with intramyocellular lipid which is the lipid within muscle fibers and quantifiable only using magnetic resonance spectroscopy.
With increasing adiposity, the African-Americans were found to have a significantly greater increment in the proportion of total adipose tissue (TAT) mass as IMAT compared to the Caucasians and the Asians, and VAT accumulation was greater than IMAT accumulation in the Asians and the Caucasians.18 Subsequently, Albu et al.19 found in premenopausal African-American women who had significantly higher insulin resistance and acute insulin response to glucose than did their Caucasian counterparts, that whole-body IMAT was an important independent correlate of insulin resistance. Others20,21 quantified IMAT in a single mid-thigh slice using computed tomography and found that insulin resistance was associated with increased subfascial AT in obese adults20 and thinner older persons.21 Goodpaster et al.20 found that AT located beneath the fascia lata (i.e., IMAT) and therefore adjacent to skeletal muscle was significantly negatively correlated with insulin resistance, whereas AT located above the fascia (i.e., SAT) and removed from skeletal muscle was not.20 A question of interest therefore is whether IMAT relates to other cardiovascular risk factors.
The primary aim of this study was to determine the relationship between whole-body IMAT and cardiovascular risk factor parameters namely, fasting glucose, protein-bound glucose, insulin, total cholesterol, and HDL cholesterol in healthy African-American and Caucasian adults.
Methods
Protocol and subjects
The study involved a retrospective analysis of archived data and was carried out by evaluating the relationship between IMAT and specific cardiovascular risk factors in healthy subjects. Subjects were established as healthy on the basis of a medical history and physical examination. Subjects with elevated fasting glucose (> 126 mg/dl) were retained in this study. Subjects were African-American and Caucasian men and women (aged ≥ 18 years) who had participated in studies at St Luke’s-Roosevelt Hospital’s Body Composition Unit between 1996 and 2002. Two hundred and eighty-eight healthy subjects were selected from among those on whom whole-body MRI and CVD risk factor information was available. A separate glucose tolerance test was not performed. The total selected sample included African- American (AA, n = 78) and Caucasian (Ca, n = 109) men and women (39 AA, 62 Ca). Race was self-reported by the subject and both parents and all four grandparents were required to be of the same racial group. All studies were approved by the Institutional Review Board, and all subjects gave written consent to participate.
Anthropometric measurements
Subjects reported in the morning in a fasted state to the Body Composition Laboratory. While each subject was wearing a hospital gown and foam slipper, body weight was measured to the nearest 0.1 kg and height to the nearest 0.1cm with the use of appropriately calibrated scale (Weight Tronix, New York, NY, USA) and stadiometer (Holtain Stadiometer, Crosswell, Wales, UK).
Magnetic resonance imaging
Whole-body MRI was carried out as reported previously.17,18 Subjects were placed in a 1.5 T scanner ( × 6 Horizon; General Electric, Milwaukee, WI, USA) with their arms extended above their heads. T1-weighted MRI images were acquired using a whole-body coil with a matrix of 256 × 256, a field of view of 48 cm, and a spin echo sequence with repetition time/echo time (TR/TE) of 300/15 ms. The protocol involved the acquisition of 40 axial images of 10mm thickness at 40mm intervals across the whole body. SLICEOMATIC image analysis software (version 4.2; Tomovision, Montreal, QC, Canada) was used to analyze images on a personal computer workstation (Gateway, Madison, WI, USA). Total-body skeletal muscle (SM) and TAT, including total SAT, VAT and IMAT were measured from whole-body multi-slice MRI images. IMAT in our laboratory (Image Reading Center, New York, NY, USA) is defined as the AT visible between muscle groups and beneath the muscle fascia.17,18 MRI volume estimates were converted into mass by using assumed density of 1.04 kg/l for SM and 0.92 kg/l for AT.22 All scans were read by the same analyst. The technical errors for four repeated readings of the same four whole-body scans by the same observer of MRI-derived SM, SAT, VAT and IMAT volumes in our laboratory are 1.4, 1.7, 2.3 and 5.9%, respectively.
Biochemical assays
Blood samples were drawn in the morning after an overnight fast and were sent to a commercial laboratory (Corning Clinical Laboratories and Quest Diagnostics, Teterboro, NJ, USA) for analysis. Glucose, protein bound glucose (PBG), triglycerides (TG), total cholesterol (T-Chol) and HDL cholesterol (HDL-Chol) were measured by enzymatic method, and insulin by radioimmunoassay. PBG refers to glycated serum proteins (fructosamines) and reflects the degree of glycemia or glucose control for the preceding 2–3 weeks. PBG was used as a short-term index of glucose control before the currently used glycated hemoglobin.23 The range for normal PBG from this commercial laboratory at the time of data collection was 0–1.19 mg/g.
Statistical analysis
Group data are presented as means±s.d. Comparisons between groups were made using t-test and analysis of variance (ANOVA). Pearson partial correlation coefficients were used to assess the linear relationships of IMAT with weight, height, body mass index (BMI), SM, SAT, VAT and TAT. General linear models (GLM) were used in analyses to identify independent effects of IMAT after covarying for SM, VAT, TAT, race, sex and two-way interactions, on cardiovascular risk factors (glucose, PBG, insulin, T-Chol, TG and HDL cholesterol). Model coefficients were tested for significance. Residuals from the GLM were checked for normality and natural log-transformed values were used when a significant deviation from normality was detected (e.g., triglycerides). In all analyses, a two-tailed level of 0.05 was used, except when adjustments for multiple comparisons were made. A P-value < 0.05 was considered statistically significant. All analyses were performed with SAS 9.1 for Windows (SAS Institute Inc, Cary, NC, USA).
Results
Subject characteristics
The subject characteristics are presented in Table 1. There were no differences between the Caucasian and the African-American men for age, weight, height and BMI. Among women, the Caucasians weighed less (P < 0.05) and had a lower BMI (P < 0.05) compared with the African- Americans.
Table 1.
Anthropometric and body composition characteristics of subjects
| Men |
Women |
|||
|---|---|---|---|---|
| Ca | AA | Ca | AA | |
| (n = 62) | (n = 39) | (n = 109) | (n = 78) | |
| Age (year) | 45.6±16.7 | 45.5±18.8 | 43.6±16.1 | 45.6±16.8 |
| Weight (kg) | 81.4±12.4 | 80.1±11.6 | 66.0±14.1 | 76.9±16.3b |
| Height (cm) | 178.3±6.9 | 177.0±7.5 | 163.2±7.0 | 163.0±7.3 |
| BMI (kg/m2) | 25.6±3.8 | 25.6±3.2 | 24.8±5.2 | 28.9±5.5b |
| SM (kg) | 32.4±5.2 | 33.3±5.2 | 20.1±3.4 | 22.6±3.8b |
| SAT (kg) | 16.9±6.6 | 16.0±5.3 | 21.9±9.7 | 28.4±10.5b |
| IMAT (kg) | 0.7±0.5 | 0.9±0.6 | 0.9±0.6 | 1.4±0.8b |
| VAT (kg) | 2.5±1.8 | 1.9±1.5a | 1.4±1.2 | 1.7±1.1 |
| TAT (kg) | 20.2±8.5 | 18.7±6.3 | 24.2±10.9 | 31.5±11.8b |
All values are means±s.d. AA, African American; BMI, body mass index; Ca, Caucasian; IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; SM, skeletal muscle; TAT, total adipose tissue; VAT, visceral adipose tissue.
Significantly different at P < 0.05 for men.
Significantly different at P < 0.001 for women.
Cardiovascular risk factors
The mean values for age and TAT-adjusted cardiovascular risk factors by race and sex are presented in Table 2. There were no differences in insulin and cholesterol levels by race or sex. In women, fasting glucose levels were lower in the African- Americans than the Caucasians. In men, HDL-Chol levels were higher in the African-Americans than the Caucasians. In both men and women, PBG levels were higher (P < 0.05) and TG (P < 0.05) lower in the African-Americans than in the Caucasians.
Table 2.
Cardiovascular risk factors values of subjects
| Men |
Women |
|||
|---|---|---|---|---|
| Ca | AA | Ca | AA | |
| Glucose (mg/dl) | 87.3±8.4ab | 88.1±8.9ab | 88.9±7.5a | 86.0±10.3b |
| PBG (mg/g) | 0.9±0.1a | 1.0±0.1b | 0.9±0.1a | 1.0±0.1b |
| Insulin (μU/ml) | 9.3±4.0 | 9.3±3.1 | 8.9± 3.3 | 9.8±3.7 |
| T-Chol (mg/dl) | 194.8±34.8 | 198.5±30.6 | 199.0±38.9 | 192.7±39.0 |
| TG (mg/dl) | 95.6±59.9a | 69.1±36.4b | 90.9±41.0a | 77.8±36.4b |
| HDL Chol (mg/dl) | 51.4±10.8a | 57.2±13.7b | 54.2±13.4ab | 53.0±14.8ab |
All values are means±s.d. AA, African American; Ca, Caucasian; HDL Chol, high-density lipoprotein cholesterol; PBG, protein-bound glucose; T-Chol, total cholesterol; TG, triglycerides. Cardiovascular risk factors were adjusted for TAT, age and sex. Values with different superscript lettersa,b,ab within each measure are significantly different at P < 0.05.
Independent association of IMAT with cardiovascular risk factors
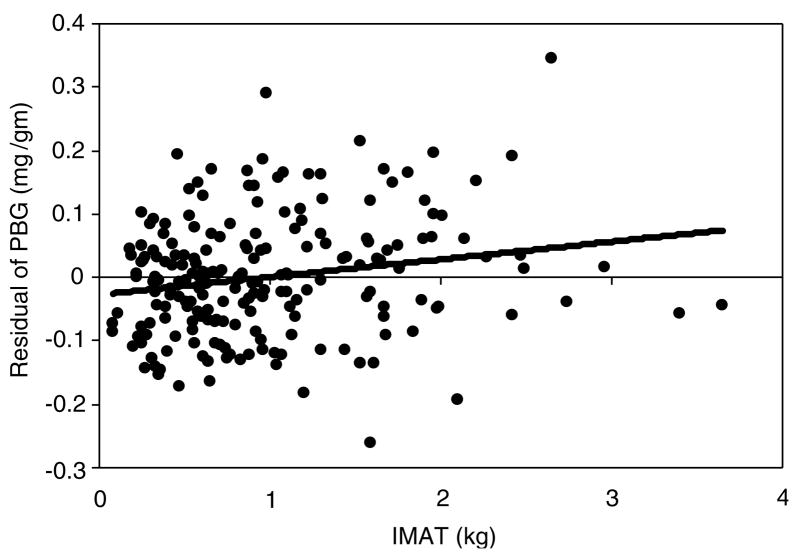
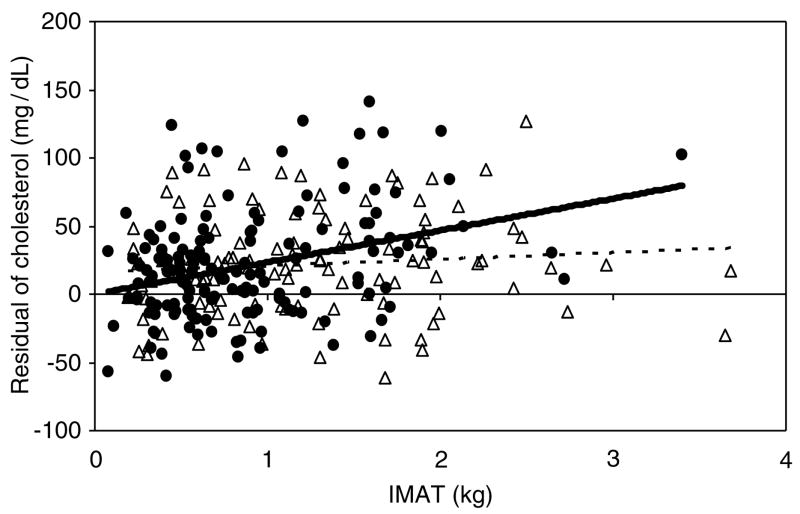
Using a general linear model, the relationship between IMAT and cardiovascular risk factors was assessed (Table 3) and the significance of covariates (skeletal muscle, VAT, TAT, sex, race, IMAT-by-race and IMAT-by-sex interactions) was tested. This allows us to evaluate the association of IMAT with these cardiovascular risk factors after taking into account the association of VAT and TAT with the same risk factors. Independent associations were observed for IMAT with glucose and PBG (P < 0.001; Figure 1). No independent associations were found for IMAT with insulin, TG and HDL-Chol. An analysis involving the combined variable of glucose and insulin representing Homeostatic model assessment (HOMA) index was conducted and an association was found between HOMA index and VAT (P < 0.001) but not with IMAT. T-Chol was independently associated with IMAT in the full sample (P < 0.05) but the relationship differed by race. T-Chol levels were comparable between the two race groups for small amounts of IMAT but increased more rapidly with accumulation of IMAT in the Caucasians compared to the African-American (23.4 mg/dl per kg IMAT in Ca; 4.9 mg/dl per kg IMAT in AA; interaction of slopes P < 0.05; Figure 2).
Table 3.
Independent association of IMAT with CVD risk factors
| Variables | Glucose | PBG | Insulin | T-Chol | Log TG | HDL-Chol |
|---|---|---|---|---|---|---|
| IMAT | 5.44±1.39*** | 0.07±0.02*** | −0.03±1.07 | 23.39±7.59* | 0.15±0.09 | 3.36±2.69 |
| SM | −0.23±0.14 | −0.001±0.002 | 0.12±0.09 | −1.04±0.61 | 0.01±0.01 | −0.59±0.22** |
| VAT | 1.02±0.63 | 0.01±0.01 | 1.23±0.37** | 2.98±2.72 | 0.13±0.03*** | −1.29±0.97 |
| TAT | −0.02±0.09 | −0.002±0.001 | 0.06±0.07 | 0.49±0.40 | 0.004±0.004 | −0.32±0.14* |
| Sexa | 5.57±2.50* | −0.002±0.03 | −3.01±1.54 | 13.52±10.68 | 0.01± 0.12 | −0.79±3.78 |
| Raceb | −2.16±1.23 | 0.04±0.01** | 1.17±0.73 | 15.90±9.11 | 0.03±0.10 | 2.17±3.24 |
| IMAT* Racec | – | – | – | −18.49±7.46* | −0.15±0.08 | −1.35±2.66 |
| Intercept | 84.59±2.91*** | 0.93±0.03*** | 2.28±1.84 | 177.75±13.11*** | 3.67±0.15*** | 75.29±4.63*** |
| R2 | 0.21 | 0.24 | 0.37 | 0.17 | 0.28 | 0.17 |
| s.e.e. | 8.94 | 0.09 | 3.44 | 38.20 | 0.43 | 13.55 |
Values are regression coefficients±s.e.e. HDL-Chol, high-density lipoprotein cholesterol; IMAT, intermuscular adipose tissue; IMAT*Race, interaction variable between IMAT and race; log TG, log-transformed triglycerides; PBG, protein bound glucose; s.e.e., standard error of estimation; SM, skeletal muscle; TAT, total adipose tissue; T-Chol, total cholesterol; VAT, visceral adipose tissue. IMAT was adjusted for SM, VAT, TAT, race, sex and two-way interactions.
Significantly different from zero at:
P < 0.05;
P < 0.01;
P < 0.001.
Dummy codes are 0 = women, 1 = men.
Dummy codes are 0 = Caucasians, 1 = African America.
Figure 1.
Residual of PBG after adjustment for SM, VAT, TAT, race and sex according to the regression equation: PBG = 0.93 − 0.001(SM) + 0.01(VAT) − 0.002(TAT) + 0.04(AA) − 0.002(male), (s.e.e. = 0.09mg/g) as a function of IMAT (kg).
Figure 2.
Residual of total cholesterol after adjustment for SM, VAT, TAT and sex according to the regression equation: T-Chol = 177.75 − 1.04(SM) + 2.98(VAT) − 0.49(TAT) + 13.52(male), (s.e.e. = 38.20 mg/dl) as a function of IMAT (kg) in Ca (●) and AA (△). Solid and dashed lines represent Ca and AA, respectively.
Discussion
It is recognized that not all AT depots carry equivalent metabolic risk. In the current study of healthy African-American and Caucasian adults, we found an association between IMAT and levels of glucose and PBG, independent of other AT depots (VAT and TAT). We also found that at low levels of IMAT, the African Americans and the Caucasians have similar levels of T-Chol, and only at higher levels of IMAT, the Caucasians have higher T-Chol than the African Americans. Given that IMAT is in close proximity to skeletal muscle, the primary organ critical in glucose uptake and metabolism, we speculate that IMAT could be a factor along the pathway to metabolic impairment/disease, although the mechanism at this time remains unknown. Although the association of VAT with cardiovascular risk factors is well documented and is replicated in this dataset, nevertheless, an even stronger association is found between the IMAT compartment and glucose, PBG and T-Chol, and the latter within the Caucasians only. Thus, a unique feature of these data is that these findings are generating hypotheses.
IMAT and glucose metabolism
A higher prevalence of obesity, diabetes mellitus and a higher degree of insulin resistance exists among the African- Americans compared to the Caucasians; however, reasons for these differences remains unclear. Although the mechanism of the effect of IMAT on glucose utilization or vice versa is unknown, it is possible that IMAT affects peripheral insulin dynamics by impairing muscle blood flow,15 enhancing rates of lipolysis within skeletal muscle24 and increasing concentrations of glucose and PBG. Given that skeletal muscle is the primary site for glucose uptake and metabolism, and even though IMAT is outside of the muscle fibers, the close proximity of IMAT to the muscle bundles could influence the muscle environment.
We have previously shown that the African-Americans have the greatest increment in IMAT with increasing total adiposity compared to Asian and Caucasian adults. In the current study, we show a strong independent association of IMAT with fasting glucose and PBG. PBG is a marker of short-term glucose control/homeostasis and has similar clinical indications to the currently more common measure of hemoglobin A1c. These combined findings point to higher rates of abnormal glucose metabolism with increasing adiposity in the African Americans. These results add to the recent findings of Albu et al.,19 where higher IMAT was found to be an independent predictor of lower insulin sensitivity. Goodpaster et al.15 originally described the IMAT compartment from a single slice in the thigh, showing a negative association with insulin sensitivity in lean and obese glucose-tolerant subjects and obese subjects with diabetes mellitus. Also, higher IMAT was associated with metabolic syndrome in normal-weight and over-weight, but not in obese men and in women.14
The range in insulin values and HOMA index in the current study was narrow and primarily fell within the normal range. Accordingly, the lack of a wide spread in these values minimized the chances of finding a stronger relationship between insulin or HOMA index and IMAT.
IMAT and lipid metabolism
We found T-chol had an independent association with IMAT, and the association of IMAT to T-Chol differed by race. T-Chol was more strongly related to IMAT in the Caucasians than the African Americans. TG and HDL cholesterol had no independent associations with IMAT. In a study of women aged 18–69 years, low-density lean tissue of the thigh as measured using CT was found to be correlated with age (r = 0.52), insulin (r = 0.34), triglycerides (r = 0.41), T-Chol (r = 0.50) and LDL cholesterol (r = 0.53).25 Unfavorable changes in plasma lipids (triglycerides, T-Chol, and LDL cholesterol) are strongly associated with increases in VAT,26–28 but there have been no previous reports on the relationship between plasma lipids and IMAT.
Regional fat distribution effects on lipids
With regard to VAT and lipid metabolism, it has been proposed that insulin resistance in the presence of VAT accumulation may be due to an overload of liver free fatty acids produced by the high lipolytic activity of VAT.29 Alternatively, VAT may be a marker of increased free fatty acids released from SAT.30 Overexposure of hepatic and extrahepatic tissues to free fatty acids leads to the promotion of aberrations in insulin action and dynamics, and may cause insulin resistance.31,32 Another possible mechanism responsible for insulin resistance related to increased VAT is the contribution of bioactive substances (adipocytokines). 2,33,34
Snijder et al.35 found that abdominal SAT was related to unfavorable glucose and lipid levels that contributed to higher non-esterified fatty acid (NEFA) levels, whereas thigh SAT was independently related to more favorable glucose and lipid levels. The latter supported their hypothesis that thigh SAT acts as a metabolic sink for circulating NEFA.35 It is possible that regional difference in the secretion of adipokines between abdominal SAT and thigh SAT and IMAT could contribute to the different associations of these fat depots with glucose and lipid levels.
Study limitations
All components of body composition including IMAT are potentially influenced by dietary intake, levels of physical activity and/or inactivity and exercise, for which no independent measures were acquired. Diet composition in particular has a direct influence on serum lipids, insulin and glucose levels; however, cardiovascular risk factor measures were all acquired after a 12-hour overnight fast. This study used a convenience sample of urban dwelling healthy adults and cannot be considered representative of the general adult population. The presence of unreported and undiagnosed medical conditions that could affect body composition cannot be ruled out. Race group was determined by self-report which is reported to be a suitable proxy for genetic ancestry, especially when assessing disease risk36 but does not take into account degrees of admixture. It is possible, given the significant differences in BMI among the ethnic subgroups in our sample, that the variables in our models do not adequately take into account the differences in BMI.
Study strengths
We had for this study a relatively large sample of African- American and Caucasian adults, whose total body IMAT had been measured by MRI using a semi-automatic method with a reasonably low technical error. All scans were acquired in one Center under high levels of quality control by welltrained examiners skilled in performing a protocol for body composition measurements.
Conclusions
The strong independent associations of IMAT with fasting glucose and PBG suggest that IMAT may be related to glucose metabolism. IMAT also shows an association with T-Chol in the Caucasian, however, the mechanism responsible for this relationship is unknown.
Acknowledgments
Supported by NIH AG14715, DK42618; RR00645, DK40414; and a contract from the NIA.
We acknowledge the contributions of Mark Punyanitya, the Director of Image Reading Center where MRI analyses were performed and Dr Blandine Laferrere and Dr F Xavier Pi-Sunyer for their critical input.
References
- 1.Boden G. Obesity, free fatty acids, and insulin resistance. Cur Opin Endo Diabetes. 2001;8:235–239. [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 4.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Cupples LA, Ramaswami R, Stokes J, III, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 6.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 7.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference as a screening tool for cardiovascular risk factors: evaluation of receiver operating characteristics (ROC) Obes Res. 1996;6:533–547. doi: 10.1002/j.1550-8528.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 10.Seidell JC, Han TS, Feskens EJ, Lean ME. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med. 1997;242:401–406. doi: 10.1046/j.1365-2796.1997.00235.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in post-menopausal women. J Clin Endo Meta. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn HS, Austin H, Williamson DF, Arensberg D. Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol. 1996;49:1017–1024. doi: 10.1016/0895-4356(96)00113-8. [DOI] [PubMed] [Google Scholar]
- 13.Tan GD, Goossens GH, Humphreys SM, Vidal H, Karpe F. Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obesity Res. 2004;12:114–118. doi: 10.1038/oby.2004.15. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 16.Albu JB, Engelson ES, He Q, Berk E, Kotler DP. Upper body subcutaneous adipose tissue distribution and increased intermuscular adipose (IMAT) tissue independently predict insulin resistance (IR) in HIV-infected obese women. Obesity Res. 2005;13(Suppl):A48. (Abstract) [Google Scholar]
- 17.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased muscle adipose tissue infiltration in elderly African-American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1010–1017. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 22.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. International Commission on Radiological Protection no. 23. Pergamon Press; Oxford, United Kingdom: 1975. Report of the Task Group on Reference Men. [Google Scholar]
- 23.O’Brien JE, Brookes M. Determination of reference values for a novel ketoamine-specific fructosamine assay for assessment of diabetic glycemic control. Diabetes Technol Ther. 1999;1:457–459. doi: 10.1089/152091599316982. [DOI] [PubMed] [Google Scholar]
- 24.Maggs DG, Jacob R, Rife F, Lange R, Leone P, During MJ, et al. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadricep muscle and adipose tissue. Evidence for significant lipolysis in skeletal muscle. J Clin Invest. 1995;96:370–377. doi: 10.1172/JCI118043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in midthigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 26.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring study. Arterioscler Thromb Vasc Biol. 1996;16:1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 27.Lemieux S, Prud’homme D, Moorjani S, Tremblay A, Bouchard C, Lupien PJ, et al. Do elevated levels of abdominal visceral adipose tissue contribute to age-related difference in serum lipoprotein concentrations in men? Atherosclerosis. 1995;118:155–164. doi: 10.1016/0021-9150(95)05603-t. [DOI] [PubMed] [Google Scholar]
- 28.Miyawaki T, Abe M, Yahata K, Kajiyama N, Katsuma H, Saito N. Contribution of visceral fat accumulation to the risk factors for atherosclerosis in non-obese Japanese. Inter Med. 2004;43:1138–1144. doi: 10.2169/internalmedicine.43.1138. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model Obesity. 2006;14:20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 30.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E552–E560. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: The Japanese experience. Diabetes Metab Rev. 1997;13:3–13. doi: 10.1002/(sici)1099-0895(199703)13:1<3::aid-dmr178>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Bjorntorp P. “Portal”. adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 33.Bertin E, Nguyen P, Guenounou M, Durlach V, Potron G, Leutenegger M. Plasma levels of tumor necrosis factor-alpha (TNF-α) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab. 2000;26:178–182. [PubMed] [Google Scholar]
- 34.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 35.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 36.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]