Abstract
Despite the knowledge that GABAA modulators can affect learning and memory, their capacity for disrupting each of these complex processes is rarely compared, and often mistakenly assumed to occur with identical potency. For these reasons, the effects of flunitrazepam (0.056–3.2 mg/kg), ethanol (0.25–1.5 g/kg), and β-CCE (ethyl-β-carboline-3-carboxylate; 1–17.8 mg/kg) were compared in groups of rats responding under baselines that assessed learning and memory separately. The first baseline was a multiple schedule of repeated acquisition and performance of tandem response sequences, whereas the second baseline was a retention or memory procedure where a tandem response sequence was acquired and then retested after a 30-min delay. Under both procedures, responding was maintained under a second-order fixed-ratio (FR) 2 schedule of food reinforcement, and incorrect responding (errors) produced a 5-sec timeout. With regard to the effects of the three drugs on sequence acquisition (learning), all three drugs dose-dependently decreased the overall response rate and increased the percentage of errors. Both flunitrazepam and β-CCE affected accuracy more potently than response rate, whereas ethanol was equipotent in affecting these two dependent measures. With regard to the effects of these drugs on sequence retention (memory), both flunitrazepam and ethanol dose-dependently decreased retention at doses that had little or no effect on sequence acquisition under the multiple schedule, whereas β-CCE decreased retention and sequence acquisition similarly at the doses tested. Together, these data demonstrate that drugs with differing capacities for altering the function of GABAA receptors differ in their capacity for disrupting the acquisition and retention of response sequences and that positive modulation of this receptor complex may be more predictive of disruptions in memory than disruptions in learning.
Keywords: flunitrazepam, ethanol, β-CCE, repeated acquisition, learning and memory, rat
Introduction
Benzodiazepines are often prescribed clinically to relieve anxiety, promote sleep, induce anesthesia, and treat signs and symptoms associated with ethanol withdrawal. However, depending upon their indication, some of the effects of the benzodiazepines are considered to be adverse and limit their clinical utility (Chouinard, 2004). For example, the anterograde amnestic effects of the benzodiazepines are viewed as beneficial in the context of a variety of minor invasive procedures that require conscious sedation such as third molar extraction (Loeffler, 1992; Sarasin et al., 1996) or routine endoscopic procedures (Reves et al., 1985), but they are viewed as adverse effects in the context of most outpatient indications such as insomnia (Roehrs et al., 1983) or anxiety (Barbee, 1993). Therefore, the capacity of the benzodiazepines to produce specific disruptions in complex processes such as learning and memory must be balanced with their wide ranging clinical utility, and this has also been true for other positive GABAA modulators (e.g., the barbiturates; Moerschbaecher and Thompson, 1980). Recent experimental comparisons between the classes of drugs that positively modulate the GABAA receptor have provided evidence that there may be important differences among them in terms of their capacity for disrupting complex behavioral processes, and that modulation of GABAA receptors through the various allosteric sites is not identical (cf. Gerak et al., 2004).
The purpose of the present study was to determine whether learning and memory in rats was equally sensitive to the effects of three drugs with varying capacities for modulating the GABAA receptor complex. The three drugs were flunitrazepam, ethanol and β-CCE. Both flunitrazepam and ethanol are considered to be positive GABAA modulators that act at distinct sites (Suzdak et al., 1986; Klein et al., 1995; Grobin et al., 1998). These two drugs, which are representative of the benzodiazepines and alcohols, respectively, were also chosen because of the interesting differences and similarities that occur between these classes of drug. For example, benzodiazepines can produce ethanol-lever responding in rats trained to discriminate ethanol, but ethanol does not necessarily produce benzodiazepine-lever responding (De Vry and Slangen, 1986). Benzodiazepines are also the current drugs of choice for treating ethanol withdrawal and for prevention of alcohol withdrawal seizures, largely because cross dependence can occur between these two classes of CNS depressant (for review see McKeon et al., 2008). In contrast, the beta carboline β-CCE binds to the benzodiazepine site on the GABAA receptor complex, but it is considered to be an inverse agonist at this site or a negative modulator of the GABAA receptor complex (Auta et al., 1997).
In order to assess the capacity of each of these drugs to disrupt learning and memory, responding under two procedures involving a repeated-acquisition technique (Boren, 1963) was used with different groups of rats. The first procedure was a multiple schedule of repeated acquisition and performance of tandem response sequences, which assayed learning in one component by reinforcing subjects to acquire a different sequence of responses each session and assayed performance in a second component by reinforcing responding on a sequence of responses that remained the same across sessions (e.g., Daniel et al., 2002). The second procedure assayed memory processes by requiring subjects to acquire a sequence of responses and then within that same session repeat that sequence of responses after a 30-min delay. A delay of this duration has been shown to produce high levels of retention for response sequences and to be sensitive to drug-induced disruptions in monkeys (Thompson et al., 1986) and rats (unpublished observations). The sequence to be retained (remembered) was changed each session (Thompson et al., 1986; Savage et al., 1996). Under both procedures, responding was maintained under a second-order fixed-ratio (FR) 2 schedule of food reinforcement, and incorrect responding (errors) produced a 5-sec timeout. One advantage of these procedures, in particular, is that both have been used in animals and humans to characterize the effects of a variety of drugs on learning and memory (e.g., Desjardins et al., 1982; Auta et al., 1995; Pakarinen and Moerschbaecher, 1995; Savage et al., 1996).
Methods
Subjects
Thirty-five adult male Long–Evans rats served as subjects. Eighteen were used to establish dose-effect curves with flunitrazepam on each of the two procedures (i.e., n=9 for the multiple schedule and n=9 for the retention procedure), whereas seventeen were used to establish the dose-effect curves for ethanol and β-CCE on each procedure (i.e., n=10 for ethanol and n=8 for β-CCE under the multiple schedule, and n=7 for each drug under the retention procedure). Subjects were housed singly in a colony room in polypropylene rodent cages containing hardwood chip bedding. The colony room was maintained at 21 ± 2°C with 50 ± 10% relative humidity on a 14L:10D light/dark cycle (lights on 0600 h; lights off 2000 h). Rats earned 45-mg food pellets (Research Diets, Inc., New Brunswick, NJ) during the experimental sessions, and, when necessary, were provided with standard rodent chow (Rodent Diet 5001, PMI Inc., Brentwood, MO) in their home cage after the test sessions in order to maintain them at 85–90% of their free-feeding weight. Water was freely available to all animals in their home cage. These studies were carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institutes of Health.
Apparatus
Nine identical modular test chambers (Coulbourn Instruments, Allentown, PA, Model E10-10TC) configured specifically for rodents were used. Located on the front wall of each chamber were a houselight, auditory feedback relay, pellet trough (5.5 cm above the floor and centered), and three response keys aligned horizontally (8 cm apart, center to center, and 14.5 cm above the floor). Each response key could be illuminated by a Sylvania 28ESB indicator lamp with a yellow plastic cap. Response keys required a minimum force of 0.15 N for activation and correct responses produced an audible click of the feedback relay. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and for masking extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT), and to cumulative recorders (Gerbrands, Arlington, MA) located within the same room.
Procedures
Multiple schedule of repeated acquisition and performance of tandem response sequences
Preliminary training for the repeated-acquisition tasks included magazine training, shaping of the response (nose press), and reinforcing responses on individually illuminated keys after shaping. To train repeated acquisition, all three response keys were illuminated with yellow light, but only one of the three response keys was correct for a particular session and each response emitted on that key resulted in the delivery of a food pellet. Responding on either of the other two illuminated keys was considered an error and resulted in a 5-s timeout during which the key lights were extinguished and responding had no programmed consequence. For each additional session during this stage of training, the position for the correct response was varied in a mixed order. After rats reliably acquired this task, regardless of the key position, a second response was added to create a sequence of responding such that two correct responses were necessary to obtain a food pellet. This type of sequential responding is procedurally defined as a “tandem” sequence because the same stimuli (yellow) were correlated with each response in the sequence (Thompson, 1970); however, the position for the correct responses varied both within the two-response sequence and across sessions. A third and fourth response were added to the sequence when stable responding was obtained under the two-response tandem sequence. When response rates and the percentage of errors did not vary by more than ±20% or ±10% of the mean, respectively, for 10 consecutive sessions, acquisition of the 4-response tandem sequences was considered stable, and a second component was added to the schedule so that subjects responded under a multiple schedule of repeated acquisition and performance of response sequences.
During the acquisition components under the multiple schedule, the three response keys were illuminated at the same time with yellow light. As described above, responding on the correct key in the presence of that stimulus changed the position for the next correct response. When the response sequence was completed by emitting four correct responses, the key lights were extinguished and the stimulus light in the pellet trough was illuminated for 0.4 s. Subsequently, the response keys were illuminated again with yellow stimuli and the sequence was reset. Within a given session, the correct responses that were associated with the yellow stimuli did not change, and the same sequence (e.g., center–left–right-center or CLRC) was repeated during all acquisition components of a given session. Responding on this sequence was maintained by food presentation under a second-order fixed-ratio (FR) 2 schedule such that every second completion of the sequence resulted in the presentation of a 45-mg food pellet. When rats responded on an incorrect key (in the example, the left or right key when the center was correct), the error was followed by a 5-s timeout. An incorrect response did not reset the four response sequence (i.e., the position of the correct response was the same before and after a timeout).
To establish a steady state of repeated acquisition, the sequence was changed from session to session. An example of a typical set of sequences for five consecutive sessions was: RLCL, LCRC, CRLR, RCRL, and CLCR. The sequences were carefully selected to be equivalent in several ways and there were restrictions on their ordering across sessions (Thompson, 1973). Briefly, 17, four-response sequences were arranged in a specific order and presented to each subject one after another until the list was completed. Once completed, the same list of sequences was presented again. In this list, each of the 17 sequences occurred three to five times and adhered to the other restrictions on their ordering (i.e., adjacent positions within a sequence were different from day to day and no position within a sequence was duplicated across days). This eliminated sequence presentations such as right-right-center-left (RRCL) and sequence ordering such as RLCR followed by CLRC where the second response in the sequence would be “left”, across days. Thus, each subject was exposed to a given sequence three to five times every 61 test sessions.
During performance components, the response keys and the houselight were illuminated. The houselight served as a discriminative stimulus for responding during this component, and unlike the acquisition component, the sequence in this component remained the same from session to session (i.e., LCLR) and its use was restricted to this component. In all other aspects (yellow stimuli for each response in the sequence, second-order FR-2 schedule of food presentation, 5-s timeout, etc.), the performance components were identical to the acquisition components. Experimental sessions always began with an acquisition component, which then alternated with a performance component after 34 reinforcers or 20 min, whichever occurred first. Each session terminated after 200 reinforcers or 100 min, whichever occurred first. Throughout testing, sessions were generally conducted 6 days per week, Monday through Saturday.
Retention of tandem response sequences
In this procedure, each session was composed of three phases: acquisition, delay, and retention test. Training for this procedure was similar to the training for responding under the multiple schedule in that subjects were first exposed to magazine training, shaping of the response (nose press), and reinforcing responses on individually illuminated keys after shaping. Then, all of the subjects were trained to acquire a four-response tandem sequence in which all three response keys were illuminated with yellow light. As in the above procedure, acquisition of each four-response tandem sequence was maintained by food presentation under a second-order FR-2 schedule of reinforcement, and errors produced a 5-sec timeout, but did not reset the sequence. During the acquisition phase of this procedure, however, subjects were required to meet an acquisition criterion in which they had to complete 7 sequences errorlessly (i.e., emit 28 consecutive correct responses) within 45 min. Otherwise, the session ended. This 45 min time limit for acquisition in the retention task was included to prevent opportunities for “overlearning”, which can increase retention above control levels and modulate drug effects (cf. Thompson et al., 1986). If the criterion was met before 45 min elapsed, the key lights were turned off and a 30-min delay phase (“retention interval”) began. Following the delay, the response keys and the houselight were illuminated for a 30-min retention test. In this procedure, the houselight served as a discriminative stimulus for the test of retention. Similar to the acquisition phase, responding during the retention test was maintained by food presentation under a second-order FR-2 schedule of reinforcement, and errors produced a 5-sec timeout, but did not reset the sequence. In summary, the subject acquired a sequence in the acquisition phase, and after a delay of 30 min, the subject responded on the same tandem sequence during retention test.
Drug Administration
Flunitrazepam (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in a vehicle consisting of ethanol (20%), saline (40%), and propylene glycol (40%), whereas β-CCE (ethyl β-carboline-3-carboxylate, Sigma-Aldrich Corp.) was dissolved in a vehicle of saline (33%), emulphor (33%), and ethanol (33%). Except for the largest dose of β-CCE, which had to be administered in a larger volume due to poor solubility, the injection volume for flunitrazepam, β-CCE, and their respective vehicles was 0.1 ml/100 g body weight. To determine the dose-effects for ethanol, it was always diluted to a 20% (v/v) solution with sterile saline, and the injection volume varied accordingly for each dose; control (saline) injections matched these various volumes. Injections for all three drugs were administered i.p. 15 min before the start of the session or 15 min before the retention test. All the dosages for a given drug were administered in a mixed order on Tuesdays and Fridays throughout behavioral testing. However, to avoid the development of acute tolerance or any “carry over” effects, the highest doses of each drug were administered only once per week. A drug-free period of approximately 10 days also occurred between drugs. Saline or vehicle (control) injections were administered on Thursdays. Baseline sessions (no injections) occurred on the remaining days.
Data Analysis
The data collected for both behavioral procedures were analyzed in terms of: (1) the overall response rate (total responses/min, excluding timeouts), and (2) the overall accuracy, expressed as the percentage of errors [(incorrect responses/total responses) × 100]. However, when the response rate was less than 5 responses per min, data were excluded from the analyses for percent errors because of the small number of responses emitted. Retention was quantified under the retention procedure using a percent savings measure. Percent savings was calculated by subtracting the errors-to-criterion (ETC) during the retention test from the ETC during the acquisition phase, and then expressing this difference as a percentage of the ETC in the acquisition phase. For example, if a subject made 200 errors before the acquisition criterion was met, but made only 50 errors before the same criterion was met in the retention test, the percent savings would be 75 (i.e., (200-50/200) × 100). If there was no retention, then ETC in the retention test would be equal to, or greater than, the ETC in the acquisition phase; thus, the percent savings would be 0 (any negative values were considered to be zero retention as well).
The mean data for each subject were grouped and analyzed statistically for an effect of each drug treatment using a one-way ANOVA with repeated measures (SigmaStat Statistical Software, SYSTAT Software, Inc. Point Richmond, CA, USA). Holm-Sidak post-hoc tests were used to compare drug sessions with control (saline or vehicle) sessions. Significance was accepted at α level ≤ 0.05 for all statistical tests. In addition to these measures based on session totals, within-session changes in responding were monitored by a cumulative recorder and the computer. For example, acquisition of a response sequence under the multiple schedule procedure was indicated by within-session error reduction; that is, a decrease in the number of errors between food presentations as the session progressed.
Results
Multiple Schedule
Stable responding in both components of the multiple schedule was evident for all subjects during the baseline sessions that preceded drug administration. In addition, the daily pattern of responding of each subject in the acquisition components was characterized by a steady state in terms of within-session error reduction, which was indicated by a distinct decrease in the number of errors and concomitant increase in consecutive correct completions of the response sequence.
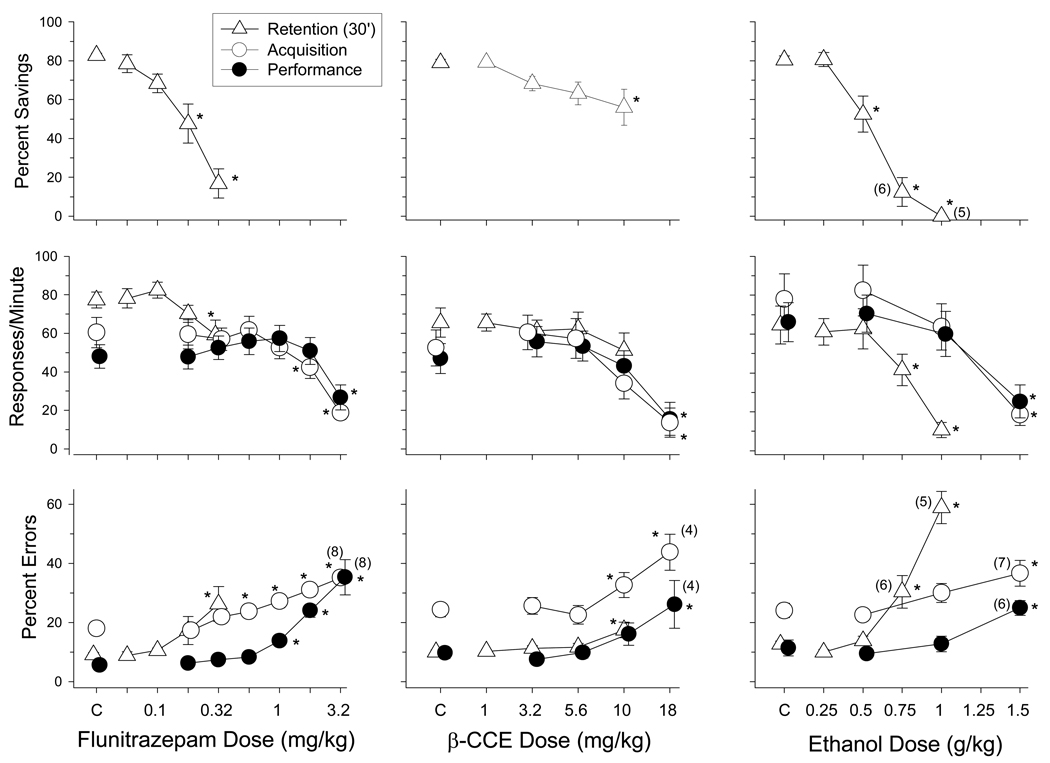
The overall response rates were also comparable in both components of the multiple schedule following control (saline or vehicle) injections for each drug, although some variation was evident (see Figure 1). More specifically, the mean rates of responding in the acquisition components following control injections for flunitrazepam, β-CCE, and ethanol were 61, 57.6, and 78 responses per minute, respectively, whereas the mean rates of responding in the performance components following control injections for each drug were 48, 50.7, and 66.2 responses per minute, respectively. With regard to the accuracy of responding, the percentage of errors was higher in the acquisition components than in the performance components following the administration of either saline or vehicle (filled and unfilled circles above C). For example, the mean percentages of errors in the acquisition components under control conditions for flunitrazepam, β-CCE, and ethanol were 17.9, 24.5, and 24.1, respectively, whereas the mean percentages of errors in the performance components under the control conditions for each drug were 5.7, 8.9, and 11.4, respectively.
Figure 1.
Effects of ethanol, β-CCE, and flunitrazepam on overall response rates and percentage of errors in rats responding under a multiple schedule of repeated acquisition and performance, and a delayed retention of acquired sequences procedures. The mean data from the acquisition components are represented by the unfilled circles, whereas the mean data from the performance components are represented by the filled circles. Unfilled triangles represent mean data from the delayed-retention phase of that procedure. Error bars represent ± SEM. Any points without error bars indicate instances in which the SEM is encompassed by the data point. Data points and error bars above “C” indicate the mean and SEM for control sessions in which either saline or vehicle was administered. Asterisks reflect doses that were significantly (α ≤ 0.05) different from control as determined by Holm-Sidak post-hoc tests. The numbers in parentheses adjacent to some data points indicate cases where the data represented by that point is less than the total number of subjects for that group (e.g., when the rate was below 5 responses per minute for an individual subject, the percentage of errors could not be calculated for that subject).
Compared to control conditions where either saline or vehicle was administered, flunitrazepam, β-CCE, and ethanol produced significant dose-dependent decreases in overall rates of responding in the acquisition [F(9,39) = 21.61, P < 0.001; F(7,39) = 13.66, P < 0.001; F(8,62) = 14.18, P < 0.001, respectively] and performance [F(9,39) = 18.19, P < 0.001; F(7,39) = 12.223, P < 0.001; F(8,62) = 5.70, P < 0.001] components. After the largest doses of each drug were administered, responding in both components was decreased by more than 50% of the control rate to approximately 20 responses per minute. All three drugs also produced significant increases in errors in both the acquisition [F(9,36) = 9.63, P < 0.001; F(7,35) = 14.61, P < 0.001; F(8,61) = 17.53, P < 0.001] and performance [F(9,35) = 25.47, P < 0.001; F(7,35) = 7.14, P < 0.001; F(8,61) = 23.85, P < 0.001] components, with the largest dose for each drug increasing the percentage of errors nearly two-fold in both components. Thus, all three drugs produced dose-dependent rate-decreasing and error-increasing effects. However, there were some differences among the drugs in their capacity for disrupting responding. For example, ethanol only disrupted response rate and the percentage of errors at the largest dose tested (1.5 g/kg), and this dose had comparable effects in the two components. Flunitrazepam increased the percentage of errors in the acquisition component at a dose that was two-fold smaller than the dose required to increase errors in the performance component. In the acquisition component, for example, error-increasing effects of flunitrazepam were evident at doses of 0.56 mg/kg and higher, whereas doses of 1 mg/kg or higher increased errors in the performance component. Furthermore, flunitrazepam disrupted accuracy in both components before decreasing the overall response rate, indicating some differential sensitivity of these measures to the effects of this drug. β-CCE also disrupted the accuracy of responding prior to decreasing the overall response rate, but not nearly to the same extent as flunitrazepam.
Retention Procedure
The data plotted in Figure 1 for this procedure represent the data obtained during the retention test. As shown, when the respective vehicles for each drug were administered 15 minutes prior to the 30-minute retention test, the mean values for percent savings, response rate, and the percentage of errors were comparable (unfilled triangles above C in each plot). For example, the mean percent savings obtained when vehicle was administered during the 30-minute delay was near 80%, the mean response rate was between 60 and 80 responses per minute, and the mean percentage of errors was approximately 10%. In contrast, when flunitrazepam was administered during the 30-minute delay, retention of the acquired sequence was disrupted and this was indicated by a significant decrease in percent savings and response rate [F(8,44) = 3.57, p < 0.02], and an increase in percent errors [F(8,44) = 4.83, p < 0.005]. More specifically, 0.18 to 0.32 mg/kg of flunitrazepam dose-dependently disrupted retention, with the 0.32-mg/kg dose decreasing the percent savings for the group from 82.8% under control conditions to less than 20%. With regard to the other dependent measures, 0.32 mg/kg of flunitrazepam decreased response rate from 77.4 to 59.2 responses per minute and increased percent errors from 9.1% to 26.3%.
When β-CCE was administered to subjects during the delay phase, the effects on retention were minimal. In fact, only the highest dose tested on this procedure (10 mg/kg) decreased percent savings [F(6,34)=4.19, p<0.01] and increased percent errors [F(6,34) = 5.83, p < 0.002], but it did not significantly decrease response rate [F(6,34) = 1.77, NS]. regard to retention specifically, percent savings was reduced from 79% to 56%.
In a manner similar to flunitrazepam, ethanol disrupted retention of the acquired sequence, as indicated by the dose-dependent decrease in percent savings [F(6,31) = 45.48, P< 0.001] and response rate [F(6,34) = 17.05, P< 0.001], and the concomitant increase in percent errors [F(6,30) = 30.67, P< 0.001]. Although the disruption of retention after ethanol administration was significant at doses larger than 0.5 g/kg, the magnitude of its disruptive effects are probably best exemplified by the effects obtained after 1 g/kg, where percent savings for the group was decreased from 80% under control conditions to 0%, response rate was decreased from 64.7 to 10.6 responses per minute, and the percentage of errors was increased from 12.7% to 58.9%. Thus, in terms of their effects on retention, and in comparison to their effects on responding under the multiple schedule, flunitrazepam was the most disruptive and β-CCE was the least disruptive. Ethanol was far more disruptive of retention than β-CCE, but was not as selectively disruptive to sequence acquisition as flunitrazepam, in that flunitrazepam disrupted sequence acquisition under the multiple schedule more potently than it disrupted response rate in the acquisition components or responding in the performance components. In contrast, only the highest dose of ethanol (1.5 g/kg) disrupted sequence acquisition under the multiple schedule and this dose was equally potent in disrupting response rate and accuracy in both the acquisition and performance component.
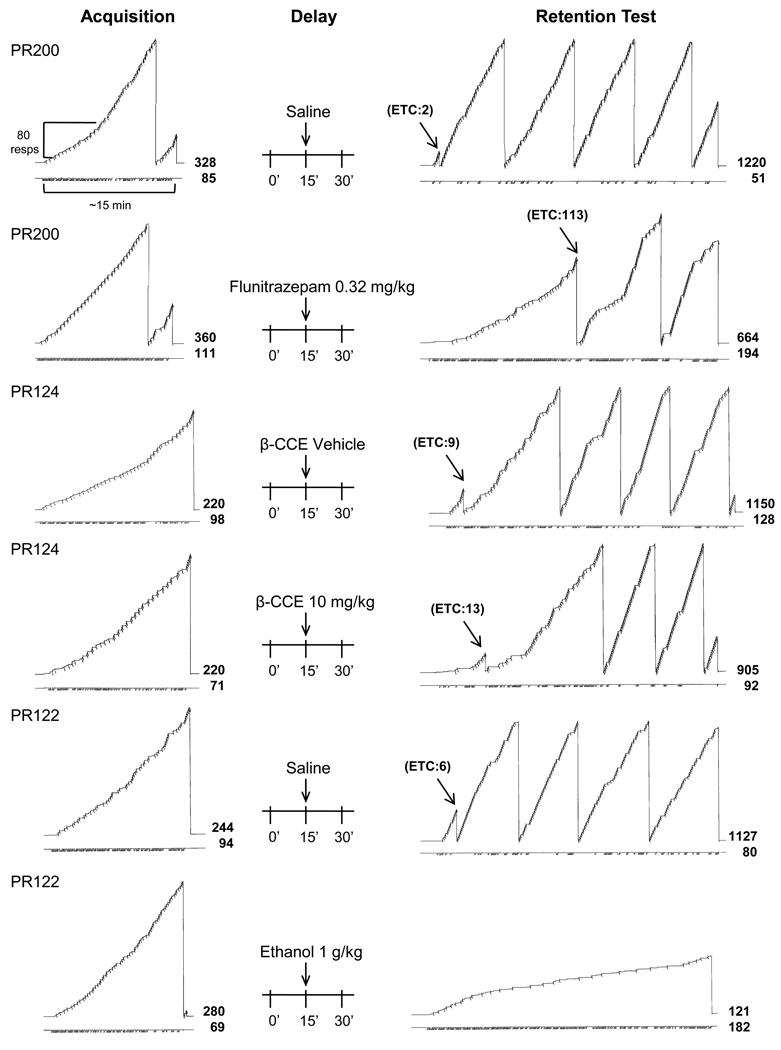
The within-session effects of flunitrazepam, β-CCE, and ethanol on responding under the retention procedure in three different subjects are shown in Figure 2. Each of the doses of drug depicted under this procedure is the dose that produced the maximum effect on retention (see Figure 1) for the group. As shown in the top record, when saline was administered 15 minutes before the delay phase terminated, retention of the acquired sequence was unimpaired (i.e., the subject met the criterion of 7 errorless sequences shortly after the start of the retention test and after emitting only 2 errors). In contrast, when 0.32 mg/kg of flunitrazepam was administered 15 minutes before the delay phase terminated, retention was substantially impaired, as the time and numbers of errors to criterion were almost the same for the initial acquisition and the retention test (i.e., 111 and 113, respectively). In other words, this subject essentially had to re-acquire the sequence after flunitrazepam administration. Unlike the effects of flunitrazepam, the 10 mg/kg dose of β-CCE produced only a small disruption of retention compared to vehicle administration. As shown by the record in the fourth row, the time and number of errors to criterion were larger than for vehicle, but these increases were negligible for this subject. The effects of 1 g/kg of ethanol were substantially larger, in that this dose decreased retention and overall response rate, and increased errors. Compared with saline administration, ethanol produced a marked reduction in the number of correct sequence completions and a high error rate throughout the retention phase.
Figure 2.
The within-session pattern of responding when flunitrazepam, β-CCE, or ethanol was administered 15 minutes prior to the retention test of a delayed retention procedure in PR200, PR124, and PR122, respectively. Each record shown represents a session that consisted of three phases that occurred in the following order: acquisition, delay, and retention. In both the acquisition (A) and retention (R) phases, the response pen stepped upward with each correct response and deflected downward after the four-response tandem sequence was completed. The response pen reset when the criterion was met (acquisition and retention test), at the top of the page, or at the end of a phase. The black arrows are another indication of when criterion was met during the retention phase. Errors in the acquisition and retention phases were indicated by the downward deflection of the event pen (below each record). The two numbers after the acquisition and retention phases and in line with the stepping pen and event pen indicate the total number of correct and incorrect responses that occurred during that phase, respectively. The maximum duration for the acquisition and retention phases was 45 and 30 minutes, respectively.
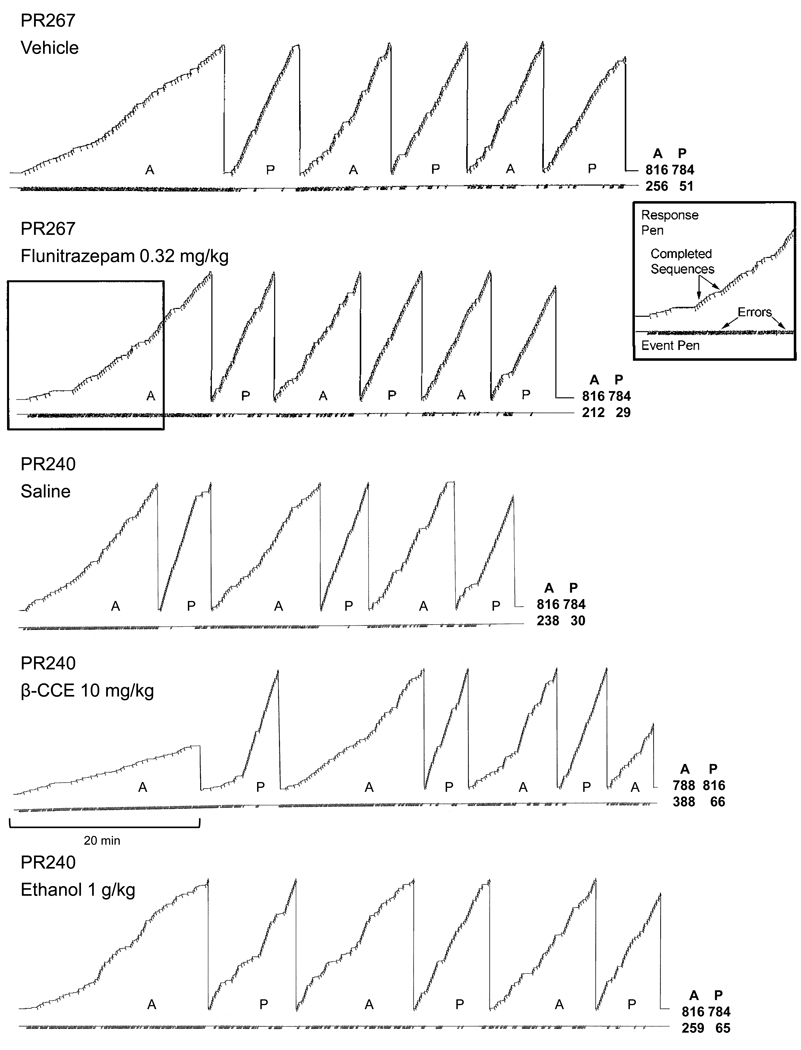
Some of the within-session effects on responding under the multiple schedule of repeated acquisition and performance are shown for each drug in the cumulative records of responding in Figure 3. Each of the doses of drug to be compared under this procedure is the dose that produced the maximum effect on retention and closely represented the overall effects shown in Figure 1. The top two records compare the effects of a vehicle injection with a 0.32-mg/kg dose of flunitrazepam in subject PR267, whereas the bottom three records compare a vehicle injection with 10 mg/kg of β-CCE and 1 g/kg of ethanol in subject PR240. When vehicle or saline was administered, a similar within-session pattern of responding was obtained, and in each case, this pattern was comparable to that obtained during sessions when these subjects did not receive injections (not shown). More specifically, these subjects typically acquired the four-response tandem sequence for each session over the course of the two initial acquisition components (i.e., errors occurred at a fairly high rate until they transitioned to a pattern where fewer errors were emitted and there was a large number of consecutive errorless sequences). In subsequent acquisition components, therefore, responding was characterized by a lower error rate and a high rate of correct responding compared to the initial acquisition component. Conversely, responding in the performance components was characterized by relatively few errors and a high rate of correct responding throughout the sessions.
Figure 3.
Cumulative records for subject PR267 and PR240 depicting the within-session pattern of responding under the multiple schedule, 15 minutes after flunitrazepam (0.32 mg/kg), ethanol (1 g/kg) or β-CCE (10 mg/kg) administration. The response pen stepped upward with each correct response and deflected downward after the four-response tandem sequence was completed. Errors are indicated by the downward deflection of the event pen (below each record). The response pen reset after each component or at the end of the session. Each session began with an acquisition (A) component, which then alternated with a performance (P) component. The four numbers at the end of each record below “A” and “P”, and in line with the stepping pen and event pen indicate the total number of correct and incorrect responses that occurred during each component. Components alternated after 40 reinforcers or 20 minutes, whichever occurred first. Sessions ended after 200 reinforcers or 120 minutes, whichever occurred first.
When 0.32 mg/kg of flunitrazepam was administered to PR267, this dose produced little or no disruption in the rate or accuracy of responding in either component of the multiple schedule. Compared to vehicle administration, there was no observable difference in the within-session pattern of responding. In contrast, both 10 mg/kg of β-CCE and 1 g/kg of ethanol decreased response rates and increased errors in each component in PR240. As evident by examination of the cumulative records, the transition to more errorless responding was slowed in the acquisition components and there was an increase in the error rate in both the acquisition and performance components. The within-session disruptions in responding were also somewhat different from each other, in that the effects on responding tended to be more uniform across components after ethanol administration than after β-CCE administration. Compared to the effects of ethanol in this subject, β-CCE administration produced larger error-increasing effects in the acquisition components and larger effects at the beginning of the session, as indicated by the decrease in the number of correct responses in the initial acquisition component.
Discussion
In the present study, flunitrazepam, β-CCE, and ethanol were administered to groups of rats to assess their effects on the acquisition of a tandem response sequence and on the retention of a tandem response sequence. The value of administering these drugs to rats responding under these two procedures, which both utilized repeated-acquisition techniques, was that the capacity of each GABAA modulator for disrupting learning or memory processes could be compared easily. For example, flunitrazepam was more potent at disrupting the accuracy of responding than the rate of responding under the multiple schedule, and more potently disrupted the retention of an acquired sequence than the acquisition of a response sequence. In contrast, the potency of ethanol for disrupting the rate and accuracy of responding under the multiple schedule was similar in that only the highest dose disrupted both dependent measures, yet its relative potency for disrupting retention of a response sequence was greater, and more graded, than its potency for disrupting the acquisition of a response sequence. β-CCE disrupted responding under the multiple schedule similarly to flunitrazepam, but produced only a small, significant disruption of retention compared to flunitrazepam and ethanol.
The fact that different modulators of the GABAA receptor complex can produce different effects on responding under the multiple-schedule procedure, and across procedures, is fairly well established. Flunitrazepam and other benzodiazepines have been shown to decrease overall response rate and increase the percentage of errors in both the acquisition and performance components of a multiple schedule (e.g., Winsauer et al., 1996; Gerak et al., 2004). Many benzodiazepines, particularly the triazolo-substituted benzodiazepines, have also been shown to disrupt accuracy more potently than response rate, and to produce these types of selective effects more potently in the acquisition components than in the performance components in monkeys (Auta et al., 1995) and humans (Bickel et al., 1990). This was true for flunitrazepam in the present study. as 0.56 and 1 mg/kg produced small increases in percent errors, but had no significant effect on response rate in the acquisition components until 1.8 mg/kg. Likewise, accuracy of responding in the acquisition components was disrupted at doses lower than those that disrupted responding in the performance components, as the first dose to significantly disrupt accuracy in this component was 1 mg/kg.
As mentioned above, ethanol did not show the same differential potency for disrupting rate and accuracy of responding in each component of the multiple schedule, nor did it show the same differential potency for disrupting responding in the acquisition and performance components. More specifically, a dose of 1.5 g/kg produced a substantial decrease in overall response rate and a small, significant increase in the percentage of errors in both components of the multiple schedule. Thus, even though ethanol shares many effects with other positive GABAA modulators (Grobin et al., 1998), and has been shown to effect chloride flux (Suzdak et al., 1986), there are important differences among the different classes of positive modulators. For example, both the benzodiazepines and ethanol enhance GABA-induced chloride conductance; however, ethanol has effects at a variety of other ion channels (Crew et al., 1996) and this could translate directly to differences in their effects on rate and accuracy of responding. To what extent the differences observed between ethanol and flunitrazepam can be attributed to their different pharmacodynamic interactions with the GABAA receptor complex is unclear, especially because ethanol, similar to some of the neuroactive steroids, has been shown to have effects at receptors other than the GABAA receptor complex (for review see Crews et al., 1996). For example, both ethanol and the neuroactive steroids have been shown to have behavioral effects that are also mediated by NMDA receptors (e.g., Grant, 1994; Engel et al., 2001). With the neuroactive steroids, however, there is at least some evidence that their interaction with the GABAA receptor complex is more potent than their interaction with NMDA receptors as it relates to the rate-decreasing and error-increasing effects of these drugs on responding under a multiple schedule involving both repeated acquisition and performance components (Gerak et al., 2004). A similar interaction study where ethanol is administered with both positive GABAA modulators and NMDA receptor antagonists is needed to show that the interaction of ethanol with the GABAA receptor complex is more potent than its interaction with NMDA receptors.
Although β-CCE produced both rate-decreasing and error-increasing effects in rats responding under the multiple schedule, and affected accuracy of responding prior to affecting the rate of responding in the acquisition component (effects shared with flunitrazepam), β-CCE is a negative modulator of the GABAA receptor complex because of its ability to decrease chloride influx via the benzodiazepine site (Obata and Yamamura, 1986; Malatynska et al., 1989). The fact that β-CCE negatively modulates the GABAA receptor complex also suggests that it should have behavioral effects that are opposite to those of the positive modulators (Stephens and Sarter, 1988). For example, positive GABAA modulators produce sedation and are anticonvulsant, whereas negative GABAA modulators produce insomnia and are convulsant (Venault et al., 1986). Consistent with this relationship, positive GABAA modulators have been shown to disrupt the acquisition of behavioral tasks in numerous species, including humans (Bickel et al., 1990), whereas the negative modulators have been shown to enhance acquisition and retention in certain behavioral tasks in several species (e.g., Venault et al., 1986; Mayo et al., 1992). With regard to their effects on repeated-acquisition procedures, however, the negative modulators have thus far only been shown to be disruptive to responding (Auta et al., 1997; Campbell et al., 2004). For example, in squirrel monkeys responding under two different repeated-acquisition procedures (one involving tandem stimuli similar to the present study, and another involving “chain” stimuli), three negative GABAA modulators (β-CCE, β-CCM and FG-7142) decreased overall response rate and increased percent errors in a manner similar to the positive modulator triazolam (Campbell et al., 2004). The findings in that study with squirrel monkeys and the findings in the present study with rats broadens the generality of the disruptive effects obtained with the negative modulators on repeated-acquisition behavior and suggests that species differences do not explain the contrasting behavioral results reported with negative modulators.
Another finding from the present study that was consistent with previous studies involving nonhuman primates was the finding that a benzodiazepine (flunitrazepam) could disrupt the retention of an acquired sequence and that the magnitude of the disruption depends on the efficacy for positively modulating the GABAA receptor complex. In a study by Auta et al. (1995), for example, 0.32 mg/kg of triazolam administered half-way through a 60-min delay virtually eliminated retention of a conditional discrimination in patas monkeys. In addition, two low efficacy positive modulators, imidazenil and bretazenil, decreased retention after a 60-min delay by 40 to 50% at doses that had little or no effects on response rate and the percentage of errors. Interestingly, in this study by Auta et al. (1995), both imidazenil and bretazenil were able to antagonize the memory-disrupting effects of triazolam, a full positive allosteric modulator, at doses that had no effect on response rate, percent savings or percent error. A study by Pakarinen et al. (1996) involving squirrel monkeys also indicated how efficacy as a positive modulator is required to disrupt memory processes. Using a similar baseline to the one used in the present study, these investigators administered convulsants such as strychnine and pentylenetetrazole that directly inhibit the GABAA receptor complex and found that they had no effect on retention compared to triazolam when they were administered immediately after acquisition of the sequence or 24 h prior to retention. Therefore, one of the conclusions of the study by Pakarinen et al. (1996) was that these convulsants drugs, which inhibit the chloride influx through the GABAA receptor complex, neither enhanced nor disrupted memory in squirrel monkeys responding under this memory task involving repeated acquisition at subconvulsant doses.
Regarding the present study involving rats, the positive allosteric modulator flunitrazepam dose-dependently disrupted retention at doses that had no effects on either response rate or percent errors, whereas the negative allosteric modulator β-CCE only decreased retention by approximately 20% at the highest dose administered (10 mg/kg) and this dose also increased the percentage of errors in rats responding under the multiple schedule procedure. Higher doses of β-CCE were not administered to rats responding under the delayed-retention procedure because only 4 of 7 subjects responding under the multiple schedule had a response rate large enough after 18 mg/kg to allow for the calculation of the percentage of errors (i.e., a response rate greater than 5 responses per minute). Thus, consistent with the results obtained with nonhuman primates, retention of an acquired sequence or discrimination in rats decreased as the efficacy as a positive modulator increased.
Similar to a finding by Hoffman and Mathews (2001) showing that disruptions in learning were not necessary to produce disruptions in retention, the present data indicated that ethanol more potently disrupted the retention of an acquired sequence than the acquisition of a sequence (an effect similar to flunitrazepam); however, additional data would be required to directly address the question of whether or not the disruptive effects of ethanol on retention are also mediated by positive modulation of GABAA receptors. This is because the behavioral effects of ethanol have been shown to be mediated by multiple receptor systems and both positive GABAA modulators and NMDA receptor antagonists have been shown to disrupt sequence-retention procedures. For example, in one of the first studies utilizing a sequence-retention procedure, Thompson et al. (1986) demonstrated that the noncompetitive NMDA receptor antagonist phencyclidine produced both dose-dependent and delay-dependent disruptions of retention in patas monkeys. Given that ethanol has been shown to produce behavioral effects that are mediated by both the GABAA receptors and NMDA receptors, there is no way to determine which receptor (or combination of receptors) might have mediated its effects on retention in the present study. Currently, some of the most convincing evidence for the involvement of GABAA receptors in the behavioral effects of ethanol at the doses tested in the present study comes from drug-discrimination studies such as the one by Grant and Columbo (1993), which found that the discriminative stimulus effects of NMDA receptor antagonists in rats were more similar to ethanol when doses of 1.5 g/kg or higher were used as the training dose. In the present study, the effects of ethanol on retention occurred at doses as low as 0.5 g/kg.
In summary, the data from the present study demonstrate that drugs with differing capacities for altering the function of GABAA receptors differ substantially in their capacity and potency to disrupt the acquisition and retention of response sequences, and that positive allosteric modulation of this receptor complex may be more predictive of disruptions in memory than disruptions in learning. These results also provide additional evidence of the capacity of positive GABAA modulators to produce some degree of retrograde amnesia as well as anterograde amnesia, because drug administration occurred after sequence acquisition in the retention procedure. Given the benzodiazepines’ capacity for producing frank anterograde amnesia (cf. Lister, 1985; Weingartner, 1985), only a few studies have focused on their retrograde amnestic effects in animals and humans. In addition, the results from these studies have not always been in agreement. For example, similar to the present study, Auta et al. (1995) demonstrated retrograde amnestic effects after triazolam administration in patas monkeys responding under a similar retention procedure. However, Ott et al. (1988) reported that the benzodiazepines lormetazepam and flunitrazepam produced “promnesic” effects rather than retrograde amnestic effects in a between-group design, because the information presented before drug administration was recalled to a greater extent than information presented before placebo administration. The suggestion by Ott et al. (1988) and others (cf. Lister, 1985) since that time has been that improved retention of any information obtained prior to drug information might occur because there is no interference from the information obtained after drug administration due to the anterograde effects of the benzodiazepines. To what extent the differing results between these studies can be attributed to the specific variables that can influence retention, (e.g., the drug, the type of memory, the type of information and its encoding) needs to be examined further. Certainly, there is also the possibility that positive GABAA modulators can affect other memory processes such as consolidation and retrieval, but the data to support these possibilities is also quite limited.
Acknowledgements
This study was supported in part by Grant AA009803 from the National Institute on Alcohol Abuse and Alcoholism and Grant DA019625 from the National Institute on Drug Abuse.
References
- 1.Auta J, Faust WB, Lambert P, Guidotti A, Costa E, Moerschbaecher JM. Comparison of the effects of full and partial allosteric modulators of GABA(A) receptors on complex behavioral processes in monkeys. Behav Pharmacol. 1995;6:323–332. [PubMed] [Google Scholar]
- 2.Auta J, Winsauer PJ, Faust WB, Lambert P, Moerschbaecher JM. Effects of negative allosteric modulators of gamma-aminobutyric acidA receptors on complex behavioral processes in monkeys. J Pharmacol ExpTher. 1997;280:316–325. [PubMed] [Google Scholar]
- 3.Barbee JG. Memory, benzodiazepines, and anxiety: integration of theoretical and clinical perspectives. J Clin Psychiatry. 1993;54 Suppl::86–97. [PubMed] [Google Scholar]
- 4.Bickel WK, Hughes JR, Higgins ST. Human behavioral pharmacology of benzodiazepines: Effects on repeated acquisition and performance of response chains. Drug Development Research. 1990;20:53–65. [Google Scholar]
- 5.Boren JJ. Repeated acquisition of new behavioral chains. American Psychologist. 1963;18:421. [Google Scholar]
- 6.Campbell UC, Winsauer PJ, Stevenson MW, Moerschbaecher JM. Effects of GABA modulators on the repeated acquisition of response sequences in squirrel monkeys. J Exp Anal Behav. 2004;82:37–56. doi: 10.1901/jeab.2004.82-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry. 2004;65 Suppl 5:7–12. [PubMed] [Google Scholar]
- 8.Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- 9.Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav Neurosci. 2002;116:989–998. [PubMed] [Google Scholar]
- 10.De Vry J, Slangen JL. Effects of training dose on discrimination and cross-generalization of chlordiazepoxide, pentobarbital and ethanol in the rat. Psychopharmacology (Berl) 1986;88:341–345. doi: 10.1007/BF00180836. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins PJ, Moerschbaecher JM, Thompson DM, Thomas JR. Intravenous diazepam in humans: effects on acquisition and performance of response chains. Pharmacol Biochem Behav. 1982;17:1055–1059. doi: 10.1016/0091-3057(82)90493-2. [DOI] [PubMed] [Google Scholar]
- 12.Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- 13.Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 2004;173:195–202. doi: 10.1007/s00213-003-1717-2. [DOI] [PubMed] [Google Scholar]
- 14.Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol. 1994;5:383–404. doi: 10.1097/00008877-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- 16.Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann SE, Matthews DB. Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol Clin Exp Res. 2001;25:856–861. [PubMed] [Google Scholar]
- 18.Klein RL, Mascia MP, Harkness PC, Hadingham KL, Whiting PJ, Harris RA. Regulation of allosteric coupling and function of stably expressed gamma-aminobutyric acid (GABA)A receptors by chronic treatment with GABAA and benzodiazepine agonists. J Pharmacol Exp Ther. 1995;274:1484–1492. [PubMed] [Google Scholar]
- 19.Lister RG. The amnesic action of benzodiazepines in man. Neurosci Biobehav Rev. 1985;9:87–94. doi: 10.1016/0149-7634(85)90034-x. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler PM. Oral benzodiazepines and conscious sedation: a review. J Oral Maxillofac Surg. 1992;50:989–997. doi: 10.1016/0278-2391(92)90061-4. [DOI] [PubMed] [Google Scholar]
- 21.Malatynska E, Knapp R, Ikeda M, Yamamura HI. Beta-carboline interactions at the BZ-GABA receptor chloride-ionophore complex in the rat cerebral cortex. Brain Res Bull. 1989;22:845–848. doi: 10.1016/0361-9230(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 22.Mayo W, Dellu F, Cherkaoui J, Chapouthier G, Dodd RH, Le Moal M, Simon H. Cognitive enhancing properties of beta-CCM infused into the nucleus basalis magnocellularis of the rat. Brain Res. 1992;589:109–114. doi: 10.1016/0006-8993(92)91168-e. [DOI] [PubMed] [Google Scholar]
- 23.McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79:854–862. doi: 10.1136/jnnp.2007.128322. [DOI] [PubMed] [Google Scholar]
- 24.Moerschbaecher JM, Thompson DM. Effects of phencyclidine, pentobarbital, and d-amphetamine on the acquisition and performance of conditional discriminations in monkeys. Pharmacol Biochem Behav. 1980;13:887–894. doi: 10.1016/0091-3057(80)90224-5. [DOI] [PubMed] [Google Scholar]
- 25.Obata T, Yamamura HI. The effect of benzodiazepines and beta-carbolines on GABA-stimulated chloride influx by membrane vesicles from the rat cerebral cortex. Biochem Biophys Res Commun. 1986;141:1–6. doi: 10.1016/s0006-291x(86)80325-4. [DOI] [PubMed] [Google Scholar]
- 26.Ott H, Rohloff A, Aufdembrinke B, Fichte K. Anterograde and retrograde amnesia after lormetazepam and flunitrazepam. Psychopharmacol Ser. 1988;6:180–193. doi: 10.1007/978-3-642-73288-1_13. [DOI] [PubMed] [Google Scholar]
- Pakarinen ED, Moerschbaecher JM. Effects of competitive and noncompetitive GABA(A) antagonists on the acquisition of a discrimination in squirrel monkeys. Behav Pharmacol. 1995;6:156–166. [PubMed] [Google Scholar]
- Pakarinen ED, Faust WB, Moerschbaecher JM. Effects of convulsant and anticonvulsant agents on memory in squirrel monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:883–898. doi: 10.1016/0278-5846(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 29.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- 30.Roehrs T, Zorick FJ, Sicklesteel JM, Wittig RM, Hartse KM, Roth T. Effects of hypnotics on memory. J Clin Psychopharmacol. 1983;3:310–313. [PubMed] [Google Scholar]
- 31.Sarasin DS, Ghoneim MM, Block RI. Effects of sedation with midazolam or propofol on cognition and psychomotor functions. J Oral Maxillofac Surg. 1996;54:1187–1193. doi: 10.1016/s0278-2391(96)90348-1. [DOI] [PubMed] [Google Scholar]
- 32.Savage UC, Faust WB, Lambert P, Moerschbaecher JM. Effects of scopolamine on learning and memory in monkeys. Psychopharmacology (Berl) 1996;123:9–14. doi: 10.1007/BF02246275. [DOI] [PubMed] [Google Scholar]
- 33.Stephens DN, Sarter M. Bidirectional nature of benzodiazepine receptor ligands extends to effects on vigilance. Psychopharmacol Ser. 1988;6:205–217. doi: 10.1007/978-3-642-73288-1_15. [DOI] [PubMed] [Google Scholar]
- 34.Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson DM. Repeated acquisition as a behavioral baseline. Psychonomic Science. 1970;21:156–157. [Google Scholar]
- 36.Thompson DM. Repeated acquisition as a behavioral base line for studying drug effects. J Pharmacol Exp Ther. 1973;184:506–514. [PubMed] [Google Scholar]
- 37.Thompson DM, Mastropaolo J, Winsauer PJ, Moerschbaecher JM. Repeated acquisition and delayed performance as a baseline to assess drug effects on retention in monkeys. Pharmacol Biochem Behav. 1986;25:201–207. doi: 10.1016/0091-3057(86)90253-4. [DOI] [PubMed] [Google Scholar]
- 38.Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, Rossier J. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature. 1986;321:864–866. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]
- 39.Weingartner H. Models of memory dysfunctions. Ann N Y Acad Sci. 1985;444:359–369. doi: 10.1111/j.1749-6632.1985.tb37601.x. [DOI] [PubMed] [Google Scholar]
- 40.Winsauer PJ, Bixler MA, Mele PC. Comparison of the effects of typical and atypical anxiolytics on learning in monkeys and rats. J Pharmacol Exp Ther. 1996;276:1111–1127. [PubMed] [Google Scholar]