Abstract
Saccadic oscillations threaten clear vision by causing image motion on the retina. They are either purely horizontal (ocular flutter) or multidimensional (opsoclonus). We propose that ion channel dysfunction in the burst cell membrane is the underlying abnormality. We have tested this hypothesis by simulating a neuromimetic computational model of the burst neurons. This biologically realistic model mimics the physiologic properties and anatomic connections in the brainstem saccade generator. A rebound firing after sustained inhibition, called post-inhibitory rebound (PIR), and reciprocal inhibition between premotor saccadic burst neurons are the key features of this conceptual scheme. PIR and reciprocal inhibition make the circuits that generate the saccadic burst inherently unstable and can lead to oscillations unless stabilized by external inhibition. Our simulations suggest that alterations in membrane properties that lead to an increase in PIR, a reduction in external glycinergic inhibition, or both can cause saccadic oscillations.
DISORDERS OF SACCADES AFFECTING STEADY FIXATION
A fundamental requirement for clear vision is stabilization of the image of an object on the retina. Steady fixation is threatened by spontaneous abnormal eye movements, which include nystagmus, saccadic dysmetria, and saccadic intrusions (1).
Nystagmus is characterized by drift of the eyes away from the desired target that is usually followed by a corrective saccade (quick phase) back to the target of interest. In saccadic dysmetria, the eyes overshoot or undershoot the target, such that a corrective gaze movement is required to bring the eyes to fixation of the new target. When the hypermetria is extreme, large saccades, separated by an intersaccadic interval, move the eyes back and forth around the fixation point. Such eye movements are called macrosaccadic oscillations.
Saccadic intrusions, or uncalled-for saccades occurring when fixation is desired, include square-wave jerks (SWJ), macro square wave jerks (macro SWJ), saccadic pulses, and saccadic oscillations. SWJ are pairs of small horizontal saccades (<2 degrees), one away and one back to the target of interest, separated by a 200-ms interval. SWJ often occur in series. Macro SWJ are not simply enlarged SWJ but are pairs of large saccades, the first away from and the second back to the target, separated by a short (75-150 ms) interval (1). Saccadic pulses are brief; small eye movements away from the target followed by a rapid drift back to an object of interest. Unlike SWJ, saccadic pulses lack a step change in innervation to hold the eyes in a new position, and the eyes come back to the object of interest by a relatively fast drift (1).
Saccadic Oscillations
Saccadic oscillations consist of continuous uncalled-for back-to-back saccades without an intersaccadic interval. They are called ocular flutter when purely horizontal and opsoclonus when multidimensional (2-4). Saccadic oscillations occur most commonly in paraneoplastic syndromes, postinfectious encephalitis, and demyelinating disorders, in which the underlying etiology is thought to be an autoimmune or cross-immune mechanism (1,5). Saccadic oscillations may also occur in healthy subjects, as a physiologic phenomenon during eye closure and with convergence (6,7). Saccadic oscillations can be familial (4), caused by drug or other intoxications (8,9), associated with migraine (2), or transiently present in the newborn (10).
We propose that alterations in the properties of the membranes of burst neurons underlie saccadic oscillations. We test this hypothesis by simulating a neuromimetic computational model of the burst neurons. A neuromimetic model is one that implements physiologically realistic parameters of the biophysical properties of the membranes of neurons within an anatomically realistic circuit.
The concept of a membrane-based etiology of saccadic oscillations stems from the characteristics of the neural connections in the brainstem saccade generator. Therefore, we will first outline the functional features of the central areas that generate saccades and that are the cause of saccadic oscillations in pathologic circumstances. Then we will demonstrate how a neuromimetic model with its membrane-based hypothesis explains saccadic oscillations (2,4,8,9).
Functional Organization of a Neural Circuit That Generates Saccades and Saccadic Oscillations
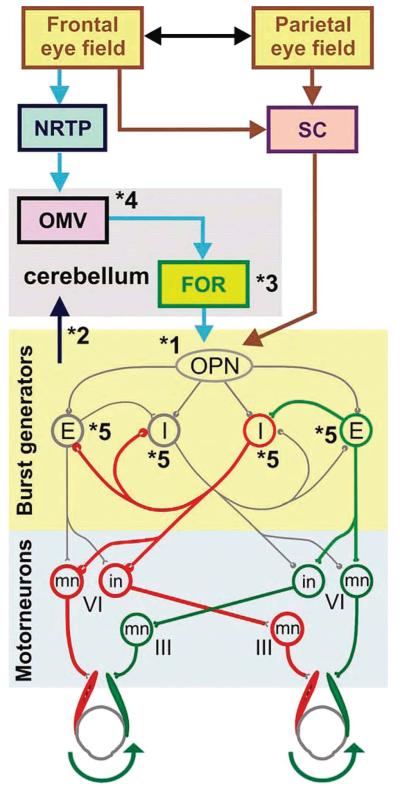
Voluntary saccades are initiated by circuits within the frontal eye field (FEF) and parietal eye field (PEF) in the cerebral cortex. Saccade-related signals from the cerebral cortex follow two main pathways as schematized with the blue and brown arrows in Fig. 1. One of these pathways (blue arrows) projects to the nucleus reticularis tegmenti pontis (NRTP) of the pontine reticular formation. The other pathway (brown arrows) projects to the superior colliculus (SC). Neurons of NRTP then project to the oculomotor vermis (OMV) (lobules 5-7) of the cerebellar cortex that sends GABAergic inhibitory signals to the underlying caudal fastigial nucleus (fastigial oculomotor region [FOR]. The FOR projects to the omnidirectional pause neurons (OPN) of the saccadic burst generator area in the nucleus raphe interpositus of the midline pons (11,12) as well as to the burst neurons themselves (not shown for clarity). The OPN also receive parallel projections directly from the SC (brown arrow in Fig. 1). Excitatory burst neurons (EBN), located in the caudal pontine reticular formation, and inhibitory burst neurons (IBN), located in the nucleus paragigantocellularis dorsalis in the medulla, receive inhibitory projections from OPN. A key role of the OPN is to maintain sustained inhibition of EBN and IBN when steady fixation is required. When a rapid shift of gaze (saccade) is warranted, inhibition of the OPN upon the EBN and IBN is removed suddenly, causing a rebound increase in the firing rate of these burst neurons. Postinhibitory rebound (PIR) is a rebound increase in neuronal membrane discharge after sustained membrane hyperpolarization. Although hypothetical, such a rebound burst in discharge would help saccades by contributing to their fast movement (13,14).
FIG. 1.
The neural circuit that generates saccades. Saccades are initiated by activity in neurons of the frontal and parietal eye fields of the cerebral cortex. These signals then follow two pathways projecting to the nucleus reticularis tegmenti pontis (NRTP) of the pontine reticular formation and the superior colliculus (SC). The NRTP sends projections to the oculomotor vermis (OMV) (lobules 5-7) of the cerebellar cortex which, in turn, sends GABAergic inhibitory signals to the underlying caudal fastigial nucleus (fastigial oculomotor region [FOR]; gray box is the cerebellum). The FOR projects to the omnidirectional pause neurons (OPN) of the saccadic burst generator area in the nucleus raphe interpositus of the midline pons. Excitatory burst neurons (EBN) and inhibitory burst neurons (IBN) receive inhibitory projections from OPN. The OPN have a key role in maintaining sustained inhibition of the EBN and IBN when steady fixation is required. When a rapid shift of gaze is needed, OPN-triggered inhibition on the EBN and IBN is suddenly removed, causing a rebound increase in the firing rate of these neurons. The EBN project directly to the ipsilateral abducens nucleus (VIth nucleus), where they synapse upon abducens motoneurons (mn) and internuclear neurons (in) (green excitatory projections). The internuclear neurons of the abducens nucleus project to the medial rectus subgroup of the contralateral oculomotor nucleus (IIIrd nucleus) (see green excitatory projections crossing the dashed midline). Hence, for making rightward saccades, the excitatory burst of activity reaches the right abducens and left IIIrd nerve nucleus motoneurons from EBN in the right paramedian pontine reticular formation (PPRF). The right side (ipsilateral) IBN, which receives inhibitory inputs from OPN and the left (contralateral) IBN, projects to the contralateral (left) abducens nucleus, inhibiting the abducens motoneurons that innervate the antagonistic muscles.
EBN and IBN are the critical neurons in the generation of saccades and are schematized in Figure 1 as “E” and “I,” respectively. The EBN project directly to the ipsilateral sixth cranial nerve nucleus, where they synapse upon motoneurons and internuclear neurons (green excitatory projections in the schematic diagram of Fig. 1). The internuclear neurons of the sixth cranial nerve nucleus project to the medial rectus subgroup of the contralateral third cranial nerve nucleus (schematized by green excitatory projections crossing the midline in Fig. 1). Hence, for rightward saccades, the excitatory burst of activity reaches the right sixth cranial nerve nucleus and left third cranial nerve nucleus motoneurons from EBN on the right side. The IBN, which project to the contralateral abducens nucleus, are normally inhibited by the OPN and the contralateral IBN. For a rightward saccade, the right side (ipsilateral) IBN are released from inhibition and then inhibit the contralateral sixth cranial nerve motoneurons (red inhibitory projection). The latter innervate the antagonistic extraocular muscles (schematized as red projections in Fig. 1). The ipsilateral IBN also project to the contralateral IBN, presumably to prevent them from becoming active (via rebound after OPN disinhibition) and inhibiting the agonist premotor and motor neurons (15). The reciprocal inhibition across the midline between two IBN populations (with PIR) forms a neural circuit with a positive feedback loop. This feedback loop is unstable if the strengths of the connection around the loop are too strong and could lead to high-frequency saccadic oscillations, such as ocular flutter (4,15). During steady fixation, oscillations that may emerge from the inherently unstable burst neuron network are prevented by inhibition from the OPN.
Hypothesis for Saccadic Oscillations
We propose a mechanism intrinsic to the neurons that generate saccadic bursts (4) (Fig. 1). In particular, we propose that saccadic oscillations arise from the instability in the saccadic burst neuron circuit because of an imbalance between burst neuron excitability and external inhibition. Such an imbalance might arise from an abnormal increase in burst neuron excitability, reduced external inhibition, or increased amplitude of PIR. The PIR is a rebound increase in neuronal membrane discharge after sustained membrane hyperpolarization. Maximal conductance through pacemaker ion channels, including hyperpolarization-activated, inward-mixed, cation currents (Ih) and low threshold calcium currents (IT), determines neural excitability and the amplitude of PIR (16-18) (Fig. 2). Although PIR has yet to be directly identified in excitatory and inhibitory burst neurons, the necessary ion channels for carrying currents generating PIR (Ih and IT) have been identified in human saccadic burst neurons (19).
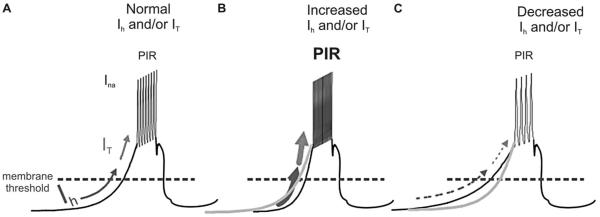
FIG. 2.
The influence of currents on the strength of postinhibitory rebound (PIR). A. Schematic diagram of the normal physiologic state. Ih is a pacemaker conductance activated by a hyperpolarizing membrane potential (in the figure, Ih activates below membrane threshold). This inward cationic conductance further depolarizes the membrane. Notice the direction of the arrow in the figure. At approximately 60 mV membrane potential, Ih gradually ceases and IT begins to activate. IT further depolarizes the membrane until a sodium conductance (INa) mediates a burst of action potential spikes. This burst of spikes characterizes normal PIR. B. Schematic diagram of the state when Ih or IT increases beyond physiologic limits. As Ih and/or IT increase, the membrane depolarizes faster. Notice thicker arrows in this panel and a steeper slope of the membrane potential compared with the gray line in A. In this situation, action potential spikes are also more frequent and thus PIR is stronger. C. Illustration of a state when Ih and/or IT are decreased below their physiologic limits When Ih and/or IT are weaker, the rate of membrane potential depolarization is relatively slower. Compare the shallower slope of membrane depolarization to the gray line in C. Hence the action potential spikes are less frequent, reflecting a weaker PIR. Black traces represent membrane potential. Lower arrows represent hyperpolarization activated inward cation currents (Ih). Upper arrows are low threshold calcium currents (IT). The dashed line represents the membrane threshold.
We hypothesize that increases in neural excitability and/or in PIR due to pathologically increased Ih and/or IT reduce the effects of external inhibition so that saccadic oscillations can emerge. Alternatively, a reduction in external inhibition because of malfunction of the strychnine-sensitive glycine receptors could also cause saccadic oscillations.
Testing the Hypothesis: A Neuromimetic Model of Saccadic Oscillations
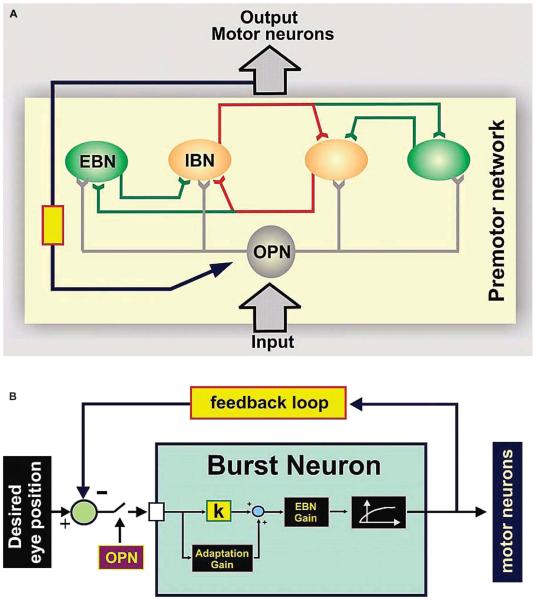
We have simulated a neuromimetic model of saccade generation with physiologically realistic membrane properties to test this hypothesis (4,15). This neuromimetic model is consistent with anatomic organization of saccadic burst neuron circuits and is based on the traditional local feedback burst neuron model (red is inhibitory and green is excitatory local feedback as schematized in Fig. 3A) (14,20-24). When membrane parameters are in the physiologic range, the model produces saccades with normal amplitudes and velocities. When the neural excitability in the model is increased by altering membrane parameters consistent with increased Ih and/or IT or by reducing the effects of the external inhibition, the simulation produces saccadic oscillations without any assumed structural alterations. The anatomic framework of the neuromimetic model is illustrated in Figure 3A. Excitatory and inhibitory burst neurons send local feedback projections (red inhibitory and green excitatory pathways as schematized in Fig. 3A) implementing the reciprocal inhibition necessary to generate accurate yet high-velocity saccades. The saccadic burst neuron and its membrane properties that play a key role are illustrated in Fig. 3B (see reference 15 for model methodology details).
FIG. 3.
The saccade generator. A. The premotor network. The excitatory burst neurons (EBN) and inhibitory burst neurons (IBN) send local feedback projections that implement reciprocal inhibition for generating accurate and high-velocity saccades. OPN, omnipause neurons; yellow box: feedback gain. B. The model burst neuron membrane. The short latency negative feedback loop is schematized as a blue arrow with a yellow box. This loop is around a high-gain (k) amplifier built into the burst neurons. Although the gain of the negative feedback loop around the burst neuron circuit determines the amplitude of the saccade, the delay time is not the main determinant of the frequency of saccadic oscillations. (Red lines indicate inhibitory projections. Green lines indicate excitatory projections. The blue line indicates feedback projection.)
Consistent with the anatomic and physiologic architecture of the neural network that generates saccades (25), this model has a short-latency negative feedback loop around a high-gain amplifier (built into the burst neurons) (yellow box and blue pathway in Fig. 3A-B). When a saccade is called for, this feedback loop generates a burst of neural discharge proportional to the eye velocity and produces a saccade of the correct amplitude and duration. However, because of the inherent high gain (neural discharge rate per unit amplitude of the desired eye displacement), the position error signal of the burst neurons, and the possible delay in the feedback loop, this system is prone to oscillate (4,15,20). Because of the high gain of the output nonlinearity of the burst neurons for small or null desired eye movement, this feedback system can oscillate even when there is a small input such as a small spontaneous saccade, unless it is suppressed by adequate inhibition from OPN. In other words, when burst neuron excitability and OPN inhibition are balanced, the saccadic system remains stable. Either an increase in neural excitability or a reduction of OPN inhibition can cause instability and oscillations. One way to increase the gain is to increase the amplitude of PIR due to increased membrane excitability.
This hypothesis raises an important question. Does PIR exist in saccadic burst neurons? PIR appears to be the property of many neurons that must develop a prompt increase in their discharge after inhibition (26-28). PIR is known to occur at the offset of hyperpolarization and is mediated by IT carried by Cav3.1, Cav3.2, and Cav3.3 calcium channels and Ih carried by HCN1, HCN2, HCN3, and HCN4 channels (16). Human saccadic burst neurons express these subtypes of ion channels carrying IT and Ih (19). A burst discharge due to PIR after marked hyperpolarization could occur in the paramedian pontine reticular formation (PPRF) burst neurons owing to their low membrane threshold voltage. Further, with this biophysical property, a single neuron is capable of firing at high rates automatically and without stimulation when released from inhibition (4,13,14). The strong PIR could be attributed to the increased activation (increase in maximal conductance) of specific subtypes of ion channels carrying IT and/or Ih.
Correlates of PIR in the Neuromimetic Model
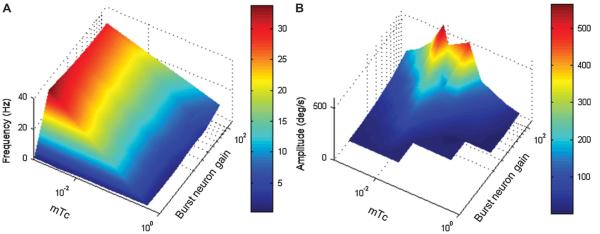
In vivo and in vitro electrophysiology experiments from neurons with PIR suggest that the membrane time constant (mTc) (Fig. 3B) and neural response gain (EBN gain) (Fig. 3B) are determined by the strength of ion currents, including Ih and IT (16-18,29-31). Ion channel activation kinetics (for Ih and IT currents) and maximal conductance through these ion channel subtypes (and therefore the amplitude of PIR) were simulated by adjusting the neuronal mTc and neuronal membrane excitability (EBN gain). Indeed, mTc and burst neuron gain are the key determinants of the amplitude and the frequency of resultant saccadic oscillations (Fig. 4). Figure 4 illustrates that an increase in the burst neuron gain (i.e., increasing Ih and IT conductance to increase neural excitability and PIR) causes an increase in the frequency (Fig. 4A) and amplitude (Fig. 4B) of oscillations. Furthermore, the frequency is also determined by the value of mTc. A shorter mTc (faster membrane kinetics) simulates a higher oscillation frequency.
FIG. 4.
Oscillation kinematics and simulated parameters determining membrane properties. Model parameters such as membrane time constant (mTc) and burst neuron gain influence the frequency (A) and amplitude (B) of simulated saccadic oscillations. Values of simulated frequency and amplitude are color-coded. Burst neuron gain and mTC determine oscillation frequency. Burst neuron gain predominantly determines oscillation amplitude.
HOW THE MEMBRANE HYPOTHESIS EXPLAINS SACCADIC OSCILLATIONS FROM VARIOUS UNDERLYING CAUSES
Saccadic oscillations have been observed in patients with cocaine abuse and with strychnine poisoning, yet there is no apparent structural abnormality in their central nervous systems (1). We use the neuromimetic model to explain the saccadic oscillations reported in these patients. Cocaine reduces norepinephrine reuptake, causing elevation in its synaptic levels (32). Norepinephrine increases Ih conductance (33). Thus cocaine intoxication may cause saccadic oscillations by increasing the PIR and burst neuron excitability because of the increase in Ih conductance through the burst neuron membrane. Saccadic oscillations are reportedly associated with hyperammonemic and uremic states, although quantitative recording confirming the exact pattern of oscillations is lacking (1). The pH of the brain changes in such toxic states. Extracellular pH regulates the maximal Ih conductance (29). Thus, increased neural excitability due to increased Ih may cause saccadic oscillations in hyperammonemic and uremic states. Opsoclonus also is reported in hyperosmolar states. Perhaps this phenomenon is related to the effects of the osmolarity of the extracellular fluid on ion channel function. For example, the concentration of extracellular sodium influences the maximal conductance through the Ih channel (29).
Opsoclonus resulting from organophosphate poisoning may be the consequence of cholinergic excess causing an increased activation in the FOR (34). The FOR is incorporated in a feedback loop of the brainstem saccade generator. Therefore, hyperexcitability of the FOR could lead to instability of the saccadic burst generators by influencing their membrane excitability, causing saccadic oscillations (35-37). Saccadic oscillations are also associated with migraine (2). An inherent hyperexcitability is thought to underlie some of the physiologic disturbances in migraine (38,39). Hence, it is possible that microsaccadic oscillations associated with migraine are due to increased burst neuron excitability. Strychnine poisoning is known to cause saccadic oscillations. Strychnine blocks glycine channels. This blockade may manifest as decreased OPN inhibition on the burst neuron membrane, which could cause saccadic oscillations.
We also speculate that in addition to direct autoimmune damage to cerebellar Purkinje neurons and neurons in the brainstem saccade generators, paraneoplastic, and postinfectious opsoclonus could also be due to the autoimmune or cross-immune reaction to the ion channels or their modulators in burst neuron. The membrane hypothesis for saccadic oscillations can also explain why some subjects can generate voluntary nystagmus, which is a transient saccadic oscillation usually associated with convergence. We speculate that an individual’s specific complement of ion channel subtypes in the brainstem circuits that generate saccades influences the ability to voluntarily generate these oscillations as well as their dynamic characteristics. This innate ability would be under genetic influence and may explain why a relatively wide range of frequencies of physiological saccadic oscillations are seen across normal individuals (15) but that within families, the frequency of voluntary nystagmus and saccadic oscillations is similar (4,40). Carbamazepine, a calcium channel antagonist (41), is reported to ameliorate saccadic oscillations (42). The membrane-based mechanism of saccadic oscillations can explain this efffect of carbamazepine.
CONCLUSION
We hypothesize that, regardless of the primary cause, ocular flutter and opsoclonus are related to alterations in the membrane properties of the neurons that generate saccadic bursts. We also propose that the level of activity in OPN may play a role in the genesis of saccadic oscillations. However, the properties of the oscillations and the ease with which the system can be made to oscillate depend critically on membrane properties of the burst neurons. Finally, our hypothesis suggests novel approaches to the treatment of ocular flutter and opsoclonus. Selective ion channel blockers may offer therapeutic benefits. Alternatively, counterintuitive therapy, interfering with the function of a normal ion channel to decrease membrane excitability in the face of increased excitability or impaired external inhibition, may reduce oscillatory behavior.
Acknowledgments
The work was supported by grants from the National Institutes of Health (EY01849 and EY06717), Department of Veterans Affairs, Intramural Research Program of the National Eye Institute (National Institutes of Health, Department of Health and Human Services), Gustavus and Louise Pfeiffer Foundation, and Ataxia Telangiectasia Children’s Project.
REFERENCES
- 1.Leigh RJ, Zee DS. The Neurology of Eye Movements. 4th ed. Oxford University Press; New York: 2006. Contemporary Neurology Series. [Google Scholar]
- 2.Ashe J, Hain TC, Zee DS, et al. Microsaccadic flutter. Brain. 1991;114:461–72. doi: 10.1093/brain/114.1.461. [DOI] [PubMed] [Google Scholar]
- 3.Foroozan R, Brodsky MC. Microsaccadic opsoclonus: an idiopathic cause of oscillopsia and episodic blurred vision. Am J Ophthalmol. 2004;138:1053–4. doi: 10.1016/j.ajo.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Shaikh AG, Miura K, Optican LM, et al. A new familial disease of saccadic oscillations and limb tremor provides clues to mechanisms of common tremor disorders. Brain. 2007;130:3020–31. doi: 10.1093/brain/awm240. [DOI] [PubMed] [Google Scholar]
- 5.Ko MW, Dalmau J, Galetta SL. Neuro-ophthalmologic manifestations of paraneoplastic syndromes. J Neuroophthalmol. 2008;28:58–6. doi: 10.1097/WNO.0b013e3181677fcc. [DOI] [PubMed] [Google Scholar]
- 6.Hain TC, Zee DS, Mordes M. Blink-induced saccadic oscillations. Ann Neurol. 1986;19:299–301. doi: 10.1002/ana.410190315. [DOI] [PubMed] [Google Scholar]
- 7.Ramat S, Somers JT, Das VE, et al. Conjugate ocular oscillations during shifts of the direction and depth of visual fixation. Invest Ophthalmol Vis Sci. 1999;40:1681–6. [PubMed] [Google Scholar]
- 8.Blain PG, Nightingale S, Stoddart JC. Strychnine poisoning: abnormal eye movements. J Toxicol Clin Toxicol. 1982;19:215–7. doi: 10.3109/15563658208990383. [DOI] [PubMed] [Google Scholar]
- 9.Scharf D. Opsoclonus-myoclonus following the intranasal usage of cocaine. J Neurol Neurosurg Psychiatry. 1989;59:1447–9. doi: 10.1136/jnnp.52.12.1447-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyt CS. Neonatal opsoclonus. J Pediatr Ophthalmol. 1977;14:274–7. [PubMed] [Google Scholar]
- 11.Noda H, Sugita S, Ideka Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–48. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- 12.May PJ, Hartwich-Young R, Nelson J, et al. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience. 1990;36:305–24. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- 13.Miura K, Optican LM. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. J Comput Neurosci. 2006;20:25–41. doi: 10.1007/s10827-006-4258-y. [DOI] [PubMed] [Google Scholar]
- 14.Enderle JD, Engelken EJ. Simulation of oculomotor post-inhibitory rebound burst firing using a Hodgkin-Huxley model of a neuron. Biomed Sci Instrum. 1995;31:53–8. [PubMed] [Google Scholar]
- 15.Ramat S, Leigh RJ, Zee DS, et al. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res. 2005;160:89–106. doi: 10.1007/s00221-004-1989-8. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh AG, Finlayson PG. Excitability of auditory brainstem neurons, in vivo, is increased by cyclic-AMP. Hear Res. 2005;201:70–8. doi: 10.1016/j.heares.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh AG, Finlayson PG. Hyperpolarization-activated (Ih) conductances affect brainstem auditory neuron excitability. Hear Res. 2003;183:126–36. doi: 10.1016/s0378-5955(03)00224-7. [DOI] [PubMed] [Google Scholar]
- 19.Zerratte MC, Shaikh AG, Zee DS, et al. Ion channel profile of human brainstem saccadic burst neurons; Proceedings of 36 Annual Meeting of the Society for Neuroscience; Washington, DC: Society for Neuroscience. 2006. [Google Scholar]
- 20.Zee DS, Robinson DA. A hypothetical explanation of saccadic oscillations. Ann Neurol. 1979;5:405–14. doi: 10.1002/ana.410050502. [DOI] [PubMed] [Google Scholar]
- 21.Ramat S, Leigh RJ, Zee DS, et al. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35. doi: 10.1093/brain/awl309. [DOI] [PubMed] [Google Scholar]
- 22.Robinson DA. Editorial: How the oculomotor system repairs itself. Invest Ophthalmol. 1975;14:413–5. [PubMed] [Google Scholar]
- 23.Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142:439–62. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- 24.Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–64. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- 25.Jurgens R, Becker W, Kornhuber HH. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern. 1981;39:87–96. doi: 10.1007/BF00336734. [DOI] [PubMed] [Google Scholar]
- 26.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 27.Nelson AB, Krispel CM, Sekrnjak C, et al. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–20. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- 28.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–6. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 29.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990b;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moosmang S, Stieber J, Zong X, et al. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–52. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- 31.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–8. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 32.Whitby LG, Herting G, Axelrod J. Effects of cocaine on the disposition of noradrenaline labeled with tritium. Nature. 1960;187:604–5. doi: 10.1038/187604a0. [DOI] [PubMed] [Google Scholar]
- 33.McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–42. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang TW, Balcer LJ, Solomon D, et al. Supranuclear gaze palsy and opsoclonus after Diazinon poisoning. J Neurol Neurosurg Psychiatry. 2003;74:677–9. doi: 10.1136/jnnp.74.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong AMF, Musallam S, Tomlison RD, et al. Opsoclonus in three dimensions: oculographic, neuropathologic and modeling correlates. J Neurol Sci. 2001;189:71–8. doi: 10.1016/s0022-510x(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 36.Dean P. Modelling the role of the cerebellar fastigial nuclei in producing accurate saccades: the importance of burst timing. Neuroscience. 1995;68:1059–77. doi: 10.1016/0306-4522(95)00239-f. [DOI] [PubMed] [Google Scholar]
- 37.Lefevre P, Quaia C, Optican LM. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Netw. 1998;7-8:1175–90. doi: 10.1016/s0893-6080(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 38.van der Kamp W, Maassen VanDenBrink A, Ferrari MD, et al. Interictal cortical hyperexcitability in migraine patients demonstrated with transcranial magnetic stimulation. J Neurol Sci. 1996;139:106–10. doi: 10.1016/s0022-510x(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 39.Aurora SK, Welch KM. Brain excitability in migraine: evidence from transcranial magnetic stimulation studies. Curr Opin Neurol. 1998;11:205–9. doi: 10.1097/00019052-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Neppert B, Rambold H. Familial voluntary nystagmus. Strabismus. 2006;14:115–9. doi: 10.1080/09273970600701226. [DOI] [PubMed] [Google Scholar]
- 41.Walden J, Grunze H, Bingmann D, et al. Calcium antagonistic effects of carbamazepine as a mechanism of action in neuropsychiatric disorders: studies in calcium dependent model epilepsies. Eur Neuropsychopharmacol. 1992;2:455–62. doi: 10.1016/0924-977x(92)90009-w. [DOI] [PubMed] [Google Scholar]
- 42.Sharpe JA, Fletcher WA. Disorders of visual fixation. In: Smith JL, editor. Neuro-Ophthalmology Now. Year Book Medical Publishers; Chicago: 1986. pp. 267–84. [Google Scholar]