Abstract
Objective
To investigate the influence of age, sex, ethnicity and total fatness on central obesity in four ethnic populations.
Design
Cross-sectional analysis of study subjects enrolled from 1993 to 2005.
Subjects
A multi-ethnic (Caucasian (CA), African-American (AA), Hispanic-American (HA) and Asian (As)) convenience sample of 604 men and 1192 women (aged 18–96 years, body mass index 15.93–45.80 kg/m2).
Measurements
Total body fat (TBF) and truncal fat were measured by dual-energy X-ray absorptiometry. General linear regression models were used to test for independent associations with log10-transformed truncal fat.
Results
For all ethnicities, men had a lower percent body fat and more truncal fat than women. Log10-transformed truncal fat increased with TBF approximately as a square root function. At older ages, there was a greater amount of truncal fat in CA, HA and As men (~0.20–0.25 kg/decade) with the effect more pronounced in AA men (~0.33 kg/decade). For women, the increment of truncal fat per decade was reduced in CA and AA women (~0.07 kg) compared with As and HA women (~0.33 kg). Adjusted for mean values of covariates in our sample, AA had less truncal fat than As.
Conclusion
The accumulation of truncal fat is strongly related to age, ethnicity and total fatness in both men and women.
Keywords: central obesity, percent body fat, fat distribution, race, dual-energy X-ray absorptiometry
Introduction
Obesity is a pervasive problem in the United States1 and worldwide.2 It is well documented that obesity, especially fat accumulation in the abdominal or visceral area is highly correlated with cardiovascular risk factors and metabolic perturbations, such as elevated blood pressure, insulin resistance, glucose intolerance and dyslipidemia.3–6 A higher central fat accumulation may also be related to sex6–8 and ethnic9,10 differences in cardiovascular risk factors.
With increasing age in adults, the percent body fat (PBF) and abdominal fat increase until 55–71 years,11 and whereas PBF begins to decline during and beyond the seventh decade,11,12 abdominal fat continues to increase.12,13 What is known about the exact pattern of change in PBF and abdominal fat depends on the measurement methods used13 and the populations studied.11
Sexual dimorphism in total body fat (TBF) and PBF exists with women having greater TBF and PBF whereas men have greater abdominal adiposity.8,11,12 Combining the complex interactions of aging, variations in body size, total adiposity, and sex differences in fat distribution make the interpretation of sexual influences on fat patterning difficult to understand and should be approached with caution.14
Compared with Caucasians (CA), Asians (As) have higher amounts of visceral adipose tissue (VAT),15,16 whereas African-Americans (AA) have less VAT.17–20 The term Hispanic can refer to persons originating from Central America, Cuba, the Dominican Republic, Mexico, Puerto Rico and South America, and has been used in many publications,10,21,22 some of which report on TBF and fat distribution differences compared with African-American and European-American populations.21,22 This admixed population carries different cultural, dietary and genetic characteristics. The Hispanic population is increasing rapidly in the United States as is the Asian population. Therefore, understanding how TBF and fat distribution compare among these ethnic groups has potentially important implications for understanding health risk. Body fatness, as indicated by body mass index (BMI, kg/m2) and PBF, has variable interactions across age, sex and ethnicity.23–25 BMI and PBF do not fully explain the well-documented ethnic differences in cardiovascular morbidities. It is possible that ethnic differences in fat distribution, specifically greater central fatness, may contribute to the comorbidities and mortality of cardiovascular disease and metabolic perturbations.9,10,26,27 A twin study has also suggested that TBF and regional fat mass, as determined by dual-energy X-ray absorptiometry (DXA), may be under extensive genetic control.28 However, the relations between TBF, BMI or PBF and central obesity reflected by truncal fat, VAT or waist circumferences in different sexes or ethnicities are less well understood.
Accordingly, the interrelationships between age, sex, ethnicity and level of fatness are complicated. Since there is heightened clinical and scientific interest in understanding the determinants of central adiposity, the primary aim of this study was to investigate how truncal fat is associated with sex, age, ethnicity and TBF in healthy adults spanning the adult age range across four ethnic groups.
Materials and methods
Subjects
Subjects were independent, community-dwelling individuals who had participated in one of the 18 studies at St Luke’s-Roosevelt Hospital’s Body Composition Unit between 1993 and 2005. Recruitment occurred through advertisements in newspapers and flyers posted in the local community. Inclusion criteria for all studies required that subjects be ambulatory, weight stable (less than 2 kg change over past 6 months), non-exercising based on self-report of no participation in vigorous routine or structured exercise, and non-smoking. Of the 1962 available subjects, 48 subjects had a body weight greater than 250 lb (DXA maximum limit), five subjects had a height greater than the DXA upper limit, 66 subjects had body weight difference (scale versus DXA weight) >2 kg, and 53 subjects had undetermined race/ethnicity. Some subjects overlapped in the above exclusion criteria. A total of 604 men and 1192 women, ages 18–96 years, with BMI ranging from 15.9 to 45.8 kg/m2 were included in this analysis. Ethnicity/race was determined by self-report according to the following criteria: all parents and grandparents were required to be of the same ethnicity/race: non-Hispanic African-American and non-Hispanic CA, for African-American (AA) and CA subjects, respectively. As and Hispanic-Americans (HA) were required to report all parents and grandparents as being of Eastern Asian origin or Hispanic, respectively. It is estimated that approximately 80% of the Hispanic subjects included in this analysis had origins in Puerto Rico and/or the Dominican Republic. The As subjects were predominantly of Japanese, Chinese and Korean origin.
Each subject completed a medical examination and the majority of subjects had screening blood tests after an overnight fast that included a standard hematology and blood chemistry panel. Subjects with untreated diabetes mellitus, malignant/catabolic conditions, missing limb, who had had joint replacement, those currently taking estrogen replacement therapy and those taking medications (diuretics, thyroid-, osteoporosis- and anti-obesity medications) that could potentially influence body composition, were excluded from the study. Specific to the DXA scans, subjects with incomplete scans (due to movement or improper positioning), body weights greater than the manufacturer recommended upper body weight limit (250 lb) or body height limit (6 ft and 4 inches); and a difference of >2.0 kg between the DXA weight and scale weight were excluded.
All studies were approved by the Institutional Review Board and all subjects gave written consent to participate.
Body composition
Subjects were examined in the morning in a fasted state at the Luke’s-Roosevelt Hospital’s Body Composition Unit. Wearing a hospital gown, body weight and height were measured to the nearest 0.1 kg (Weight Tronix, New York, NY, USA) and 0.1cm (Holtain Stadiometer, Crosswell, Wales, UK), respectively.
Whole-body and regional (truncal)-body composition were estimated by DXA (GE Lunar DPX or DPX-L; Madison, WI, USA). The system software provided the mass of the fat, lean soft tissue and bone mineral for both the whole body and specific regions. The truncal region was isolated from the head, arms and legs by using the computer-generated default lines, with manual adjustment, on the anterior-view planogram.29 The truncal region extends from a line drawn parallel to and through the base of the neck to a line drawn through, separated from the arms by a line drawn through the arm socket and perpendicular to the axis of the femoral neck and angled with the pelvic brim. Truncal fat was obtained from the fat mass in the modified truncal region.
Repeated daily measurements over 5 days in four adult subjects showed a coefficient of variation (CV) of 3.1% for PBF.30 Repeated daily measurements in three adult subjects showed a coefficient of variation (CV) of 5% for arm fat, 1% for leg fat, and 2% for truncal fat, respectively.29 An anthropomorphic spine phantom made up of calcium hydroxyapatite embedded in a 17.5 × 15 × 17.5-cm block was scanned for quality control each morning before subject evaluation. The phantom was also scanned immediately before and after all DXA system manufacturer maintenance visits. The measured phantom spine bone mineral density was stable throughout the study period at 1.182 (1.173–1.193) g/cm2 for DPX and 1.194 (1.165–1.228) g/cm2 for DPX-L. Methanol and water bottles (8 L volume), simulating fat and fat-free soft tissues, respectively, were scanned as soft-tissue quality-control markers monthly. The range in measured R-values over the study period was 1.253–1.293 (CV = 0.155–1.172%) and 1.359–1.373 (CV = 0.073–0.218%) by DPX-L, 1.255–1.367 (CV = 0.079–2.83%) and 1.342–1.378 (CV = 0.146–0.514%) by DPX, for methanol and water, respectively.
A DXA-derived body weight was calculated by adding the values for TBF, total lean mass, and total bone mineral content. PBF was calculated from DXA-derived TBF divided by DXA-derived total body weight × 100. DXA-derived body weight and scale weight (measured on laboratory scale) were highly correlated (R = 0.999). Body composition parameters in the same subjects (n = 78) measured on both the DPX and DPX-L systems in our laboratory were in close agreement for total bone mineral content (R = 0.997), TBF (R = 0.994), truncal fat (R = 0.996) and truncal fat in proportion to total fat (R = 0.962).31
Statistical analysis
Data were analysed using the SPSSWIN software (version 10.0, SPSS Inc., Chicago, IL, USA). T-tests for independent groups and one-way analysis of variance (ANOVA) were used to test differences in basic characteristics and anthropometric parameters between sexes and among ethnic groups, respectively. Analysis of covariance (ANCOVA), adjusted for age and BMI, was used to compare the differences in DXA-derived truncal fat, TBF and PBF between sexes and among ethnic groups. A general linear model was used to test the independent contribution of variables to truncal fat as the dependent variables with age, TBF, ethnicity (dummy-coded), height and weight as independent variables for each sex. As the contributions of weight and height were no longer significant when TBF was included, only TBF was retained in the final model. When the absolute value of the residuals was found to change with the amount of truncal fat mass, truncal fat was log10-transformed for constant error stability and all further analyses were carried out on the log10-transformed dependent variable. The square root of TBF (srTBF) was included as an independent variable in regression models to test for non-linear relationship of TBF with truncal fat. Two-way and three-way interaction effects between independent variables were tested for each model.
To illustrate the relationship found by our regression models, we back-converted log10 truncal fat to original units and plotted estimated truncal fat versus TBF and estimated truncal fat versus age, over a typical range of values of covariates set at the means of our samples, by sex and for four ethnic groups. A two-tailed P<0.05 was considered statistically significant.
Results
Basic characteristics for the study population are presented in Tables 1 and 2. There was close agreement between scale weight and DXA weight within sex (R = 0.9999). The age distribution was similar across the four ethnic groups. The BMI was the lowest in As for both sexes and the highest in HA men and AA women. After adjustment for age, height and weight, women had a higher TBF and PBF than men. Among men, As had a higher PBF compared with the other three ethnic groups (P<0.05) and AA had a lower PBF compared with CA or HA (P<0.05). Among women, As had a higher PBF compared with CA and AA, and HA had a higher PBF compared with AA and CA (all P<0.05).
Table 1.
Comparison of truncal fat and total body fat among four ethnic groups in 604 men
| Ethnic groups | Caucasian (CA) N = 271 | African-American (AA) N = 111 | Hispanic-American (HA) N = 155 | Asian (As) N = 67 | P value |
|---|---|---|---|---|---|
| Age (year) | 42.7 (18.7) | 46.7 (19.1) | 44.6 (15.9) | 44.1 (20.9) | 0.27† |
| Height (cm) | 176.12 (7.35) | 175.61 (7.08) | 169.93 (7.27)bd | 170.81 (7.38)ce | <0.001† |
| Weight-scale (kg) | 79.81 (12.34) | 80.32 (13.13) | 78.42 (14.17) | 69.03 (9.47)cef | <0.001† |
| BMI (kg/m2) | 25.72 (3.73) | 26.03 (3.96) | 27.11 (4.36)b | 23.62 (2.47)cef | <0.001† |
| Weight-DXA (kg) | 79.80 (12.38) | 80.30 (13.24) | 78.33 (14.26) | 68.89 (9.67)cef | <0.001† |
| Truncal fat (kg) | 8.98 (5.32) | 8.24 (4.79)a | 10.41 (4.90)d | 7.56 (3.19)e | <0.001† |
| Truncal fat (kg) | 9.25 (0.16) | 7.93 (0.25)a | 9.15 (0.21)d | 9.88 (0.32)e | <0.001‡ |
| Total body fat (kg) | 16.56 (9.34) | 16.16 (9.05)a | 18.43 (8.78) | 13.33 (4.88)ef | <0.05† |
| Total body fat (kg) | 17.01 (0.28) | 15.70 (0.43)a | 16.13 (0.37) | 17.61 (0.57)ef | <0.05‡ |
| Body fat (%) | 19.91 (8.91) | 19.20 (8.65)a | 22.76 (8.07)d | 19.09 (5.92)cef | <0.001† |
| Body fat (%) | 20.36 (0.32) | 18.64 (0.50)a | 20.89 (0.43)d | 22.51 (0.66)cef | <0.001‡ |
Data are expressed as mean (s.d.) for
ANOVA or age and BMI-adjusted mean (s.e.m.) for
ANCOVA; statistical significance (P<0.05) between ethnic groups:
= CA versus AA,
= CA versus HA,
= CA versus As,
= AA versus HA,
= AA versus As,
= HA versus As. BMI, body mass index; DXA, dual-energy X-ray absorptiometry.
Table 2.
Comparison of truncal fat and total body fat among four ethnic groups in 1192 women
| Ethnic groups | Caucasian (CA) N = 522 | African American (AA) N = 384 | Hispanic American (HA) N = 178 | Asian (As) N = 108 | P-value |
|---|---|---|---|---|---|
| Age (years) | 46.1 (17.5) | 48.3 (17.7) | 48.4 (15.7) | 46.6 (20.1) | 0.19† |
| Body height (cm) | 162.75 (6.75) | 162.81 (6.86) | 155.78 (6.32)bd | 157.47 (6.30)ce | <0.001† |
| Weight scale (kg) | 71.25 (16.41) | 79.39 (16.16)a | 68.80 (13.60)d | 55.07 (8.90)cef | <0.001† |
| Body mass index (kg/m2) | 26.97 (6.36) | 29.92 (5.70)a | 28.35 (5.38)bd | 22.17 (3.04)cef | <0.001† |
| DXA scale (kg) | 70.69 (16.38) | 78.86 (16.25)a | 68.14 (13.66)d | 54.45 (9.01)cef | <0.001† |
| Truncal fat (kg) | 12.00 (6.56) | 14.23 (5.67)a | 13.33 (4.86)d | 8.24 (3.39)cef | <0.001† |
| Truncal fat (kg) | 12.69 (0.10) | 12.16 (0.12)a | 12.70 (0.17)d | 13.32 (0.23)cef | <0.001‡ |
| Total body fat (kg) | 25.86 (13.04) | 31.35 (11.44) | 26.93 (9.86)bd | 16.31 (6.10)f | <0.001† |
| Total body fat (kg) | 27.23 (0.17) | 27.13 (0.21) | 25.67 (0.30)bd | 26.76 (0.40)f | <0.001‡ |
| Body fat (%) | 34.51 (10.96) | 38.43 (8.66) | 38.43 (7.86)bd | 29.27 (7.18)ce | <0.001† |
| Body fat (%) | 35.58 (0.22) | 35.31 (0.26) | 37.43 (0.37)bd | 36.80 (0.50)ce | <0.001‡ |
Data are expressed as mean (s.d.) for
ANOVA or age and BMI-adjusted mean (s.e.m.) for
ANCOVA; statistical significance (P<0.05) between ethnic groups:
= CA versus AA,
= CA versus HA,
= CA versus As,
= AA versus HA,
= AA versus As,
= HA versus As. BMI, body mass index; DXA, dual-energy X-ray absorptiometry.
Modeling
Truncal fat mass multiple regression models using sex, age, total body fatness and ethnicity as independent variables for men and women are presented in Table 3.
Table 3.
General linear models for log10-transformed truncal fat in 604 men and 1192 women
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Adjusted R2 | 0.971 β | s.e. | P | 0.964 β | s.e. | P |
| Constant | −0.414 | 0.061 | <0.001 | −0.815 | 0.097 | <0.001 |
| Age | −0.00439 | 0.001 | <0.05 | 0.00108 | 0.000 | <0.001 |
| TBF | −0.0267 | 0.004 | <0.001 | −0.0428 | 0.006 | <0.001 |
| SrTBF | 0.440 | 0.030 | <0.001 | 0.590 | 0.047 | <0.001 |
| Ethnicity | ||||||
| Caucasian | −0.0171 | 0.015 | 0.26 | 0.194 | 0.102 | 0.057 |
| African-American | −0.0852 | 0.018 | <0.001 | 0.209 | 0.106 | <0.05 |
| Hispanic-American | −0.00912 | 0.018 | 0.60 | 0.140 | 0.119 | 0.24 |
| Asian (reference) | 0 | 0 | ||||
| Interaction effects between variables | ||||||
| Age × TBF | −0.00024 | 0.000 | <0.01 | |||
| Age × srTBF | 0.00229 | 0.001 | <0.001 | |||
| Caucasian × TBF | 0.0156 | 0.006 | <0.01 | |||
| African American × TBF | 0.0168 | 0.006 | <0.01 | |||
| Hispanic American × TBF | 0.0110 | 0.006 | 0.081 | |||
| Asian × TBF (reference) | 0 | |||||
| Caucasian × srTBF | −0.118 | 0.049 | <0.05 | |||
| African American × srTBF | −0.131 | 0.050 | <0.01 | |||
| Hispanic American × srTBF | −0.0844 | 0.055 | 0.12 | |||
| Asian × srTBF (reference) | 0 | |||||
| Caucasian × Age | −0.0000572 | 0.000 | 0.85 | −0.000839 | 0.000 | <0.001 |
| African American × Age | 0.000723 | 0.000 | <0.05 | −0.000848 | 0.000 | <0.01 |
| Hispanic American × Age | 0.000192 | 0.000 | 0.60 | −0.000115 | 0.000 | 0.72 |
| Asian × Age (reference) | 0 | 0 | ||||
Abbreviations: TBF, total body fat; srTBF, square root of TBF; height, weight and dual-energy X-ray absorptiometry machine type did not differ by sex. The choice of reference group is arbitrary.
Sex
Since sexual dimorphism in TBF and fat distribution are well documented and in view of the finding of statistically significant interactions between sex and the other variables, all analyses were performed separately by sex. For men, age, ethnicity, TBF, srTBF, interaction effects between age and ethnicity, age and TBF, age and srTBF were all independent predictors of log10 truncal fat (adjusted R2 = 0.971). In women, age, ethnicity, TBF, srTBF, interaction effects between age and ethnicity, ethnicity and TBF, ethnicity and srTBF were all independent predictors of log10 truncal fat (adjusted R2 = 0.964). The addition of DXA machine type, BMI or replacing scale weight and height with BMI or reciprocal BMI as independent variables (data not shown) to these models did not change the outcome.
Total body fatness
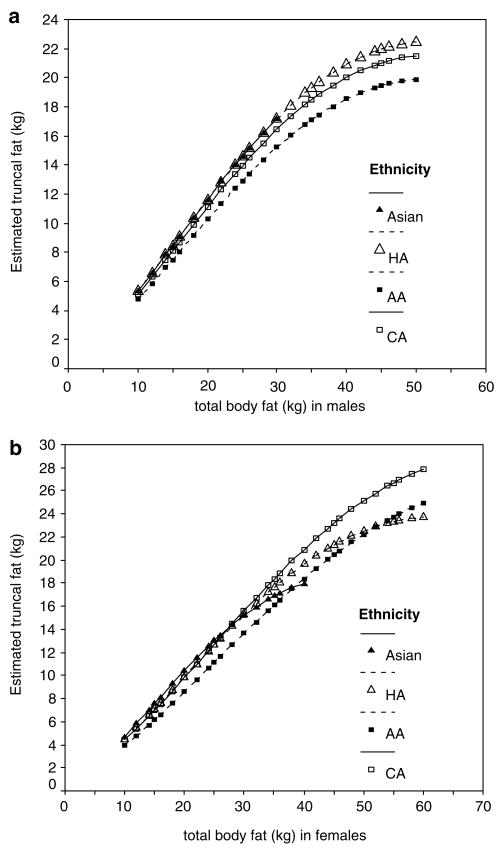
The dominant coefficient in the regression models for both men and women was that of the srTBF (β = 0.44 and 0.59, respectively) indicating that the major component of the association was a curvilinear (square root function) relationship between log10 truncal fat with greater TBF. Smaller but significant negative linear coefficients for TBF (β = −0.0267 and −0.0428 for men and women, respectively) reduced the amount of this change. Additionally, in men, a significant interaction of TBF and srTBF with age indicates that the associations of TBF with log10 truncal fat noted above was altered with age in such a manner that the coefficient for srTBF operates to increase log10 truncal fat with greater age more strongly (β = 0.00229/year) and the negative linear coefficient operates to reduce it (β = −0.00024/year) but the former is an order of magnitude larger than the latter. The interaction of TBF with age was not found in women. In general, Figures 1a and b illustrate the curvilinear and positive relationship of truncal fat with TBF taking into account the main effects of TBF and srTBF, ethnicity, and for women, interactions of TBF and srTBF with ethnicity.
Figure 1.
Truncal fat in CA (□), AA (■), HA (△) and As (▲) men (a) and women (b) estimated from regression model using TBF with mean age (44.1(18.4) years for men and 47.2 (17.6) years for women).
Age
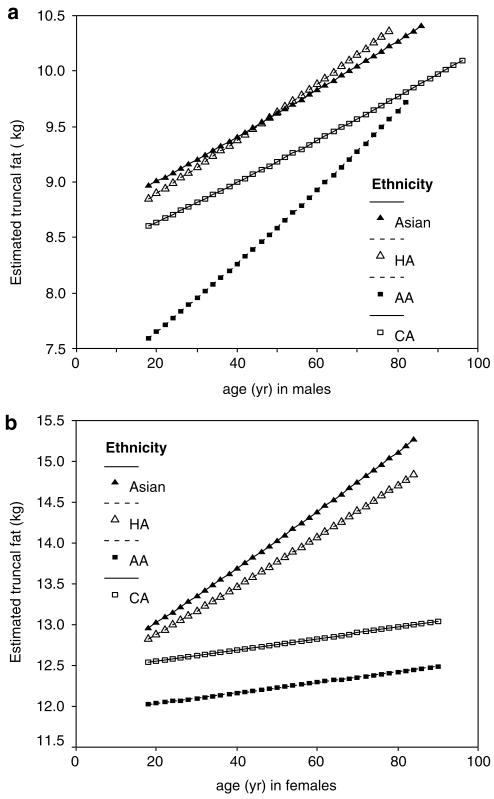
Although the regression coefficients of age and the age-by-TBF interaction were slightly negative for men, the effect of the positive coefficient of age with srTBF prevails, as noted above, resulting in a greater amount of truncal fat in CA, HA and As men (~0.20–0.25 kg/decade) with a more pronounced amount in AA men (~0.33 kg/decade). For women, there was no overall interaction of age with TBF or srTBF, however, the greater amount of truncal fat at older ages was moderated by ethnicity in a manner that reduced the overall amount with age in CA and AA women (~0.07 kg/decade) compared with As and HA women (~0.33 kg/decade). The resultant overall associations with age are illustrated in Figures 2a and b.
Figure 2.
Truncal fat in CA (□), AA (■), HA (△) and As (▲) men (a), and women (b) estimated from regression model using age with mean TBF (16.6 (8.9) kg for men and 26.9 (12.3) kg for women).
Ethnicity
Adjusted to the mean values of covariates seen in our sample, the model found main effects for ethnic group in men, specifically, less truncal fat overall in AA men compared with As (8.4 versus 9.5 kg, P<0.001, Table 3). For women, there were interactions of ethnicity with TBF and srTBF. The general pattern of this interaction was that, compared with As women, the curvilinear change in truncal fat with srTBF in CA and AA women was less (β = −0.118, P<0.05, and β = −0.131, P<0.01, respectively) but the negative linear coefficients for TBF were also less (i.e. became less negative: β = 0.0155, P<0.01, and β = −0.0168, P<0.01, respectively). The interaction of ethnicity with age was discussed above.
Hypothetical prediction models
Truncal fat from TBF
To illustrate truncal fat from hypothetical TBF values at mean (s.d.) ages of 44.1 (18. 4) years for men and 47.2 (17.6) years for women, ranges of TBF consistent with the TBF range of the original data were selected (10–30 kg for As men; 10–50 kg for AA, CA and HA men; 10–40 kg for As women; and 10–60 kg for AA, CA and HA women). These values were entered into the model equations to generate illustrative Figures 1a and b. There was more truncal fat in relation to TBF in each ethnic group in men (Figure 1a) and women (Figure 1b). In men, the slopes of the regression lines for the four ethnic groups were similar at lower TBF, but less steep in AA and CA at higher TBF. In women, the relationship between truncal fat and TBF differed by ethnic group according to the level of TBF. That is, the slopes were similar at lower TBF, crossed at middle levels of TBF and were steeper in CA and AA at higher TBF.
Truncal fat by age
To illustrate truncal fat at hypothetical ages, at mean (s.d.) TBF values of 16.6 (8.9) kg for men and 26.9 (12.3) kg for women, age ranges consistent with the age ranges of the original data were selected (18–86 year old for As men, 18–78 year-old for HA men, 18–96 year old for CA men, 18–82 year-old for AA men, 18–84 year old for As and HA women, 18–90 year old for CA and AA women). These values were entered into the model equations to generate illustrative Figures 2a and b. There was more truncal fat with greater age in each ethnic group in men (Figure 2a) and women (Figure 2b). The relationship between truncal fat and age differed by ethnic group according to age. In men, the slope was compatible in As, HA and CA, but steeper in AA. In women, the slope of the regression line was steeper in As and HA, especially at the higher ages.
Discussion
In this study, we demonstrate that the accumulation of truncal fat is strongly related to age, ethnicity and total fatness in both men and women. The interaction effects between variables also demonstrate the complex roles of age, sex, ethnicity and total fatness in contributing to the level of truncal fat accumulation. Consistent with pervious reports, PBF was higher in HA and As, and lowest in AA compared with CA.21,24,32 We further demonstrate (Figures 1 and 2) that the higher PBF in As and HA includes greater truncal fat. Greater truncal fat and PBF are both independently associated with a higher prevalence of cardiovascular comorbidities in HA10,26,27 and in As.33 The International Diabetes Federation places a greater emphasis on central obesity and proposes race-specific cutoffs for central obesity (reflected by waist circumference) but not general fatness (reflected by TBF or BMI) for the new criteria of the metabolic syndrome.34 Therefore, the factors associated with truncal fat deposits may provide information about the biological or physiological processes involved in partitioning fat deposits among regions.
There are three principal findings from the current study. In both men and women, with greater TBF, the amount of truncal fat was non-linear suggesting that at higher levels of TBF, the deposition of fat was proportionally less in the truncal region. Second, holding other variables constant, at older ages there was a greater amount of truncal fat in women which was less pronounced in CA and AA (~0.07 kg/decade) compared with As and HA (~0.33 kg/decade), and a greater amount of truncal fat in CA, HA and As men (~0.20–0.25 kg/decade) with a more pronounced amount in AA men (~0.33 kg/decade). Third, adjusted to the mean age and TBF values in our sample, AA men had less truncal fat (~1.1 kg) than As men. For women, there were interactions of ethnicity with TBF and srTBF. However, as noted above, among women, the greater amount of truncal fat at older ages was less pronounced in AA and CA compared with As and HA.
The truncal region as defined by DXA includes fat deposits other than those in the intra-abdominal or visceral cavity, including subcutaneous and intermuscular fat throughout the trunk region, in addition to epicardial and pelvic deposits. Since intra-abdominal or visceral fat has been shown to be one of the most detrimental fat deposit from a metabolic perspective, the question arises as to how representative of intra-abdominal fat is DXA-derived truncal fat? In a sample of 90 non-obese healthy men, we previously reported a high correlation (R = 0.825, P<0.01) between truncal fat and MRI-derived total VAT,35 which gives support to truncal fat as an appropriate reflection of intra-abdominal fat.
Strengths and limitations
The strengths of our study include a well-validated body composition measurement method; a relatively large sample where body composition measures were acquired in the same laboratory using daily calibration procedures that were consistent throughout the study period; a sampling of four ethnic groups; and models with high R2 values (0.964~0.971).
The inability of DXA to accommodate persons with body weights greater than 250 lb (113 kg) limits the study of persons with greater weight which includes severely or morbidly obese subjects. Whether the observed findings could be extrapolated to people weighing more than 250 lb is unknown. This study was also limited by the lack of information on smoking, exercise, menopausal status, dietary, socioeconomic status, factors that might influence the relationship between truncal and TBF. However, other studies have reported on ethnic differences in PBF across the adult age range even after controlling for socioeconomic status,22 physical activity,22 smoking21 and menopausal status.22 Since our regression models explained between 96.4 to 97.1% of the variation in truncal fat, the influence of these factors is probably small. Ethnic group was determined by self-report which is reported to be a suitable proxy for genetic ancestry, especially when assessing disease risk36 but does not take into account the degrees of admixture. The grouping of persons who self-identified as As and Hispanic into distinct ethnicities ignores within-group differences and does not allow for the examination of body composition differences. Whether the pattern of truncal fat is consistent across all As populations and persons self-identified as Hispanic needs further evaluation. On the other hand, the exact reasons for differences in truncal fat by ethnicity are unclear where genetics, body size, environmental or the interactions between these factors could be contributing factors and warrant further study.37–39 The present study employed a cross-sectional design that limits the interpretation of the data when examining the influence of age on truncal fat because age effects may include cohort differences as well as longitudinal changes. We also caution that the use of a regression model in an observational sample to estimate the effect of variation in a single variable while holding other variables constant ignores the known natural covariation of these variables. Thus, the relationships of truncal fat to TBF or age, while holding other variables constant are for illustrative rather than predictive purposes.
Main conclusion
The interrelationship between truncal fat, sex, age, ethnicity and total fatness is documented. Ethnic differences in truncal fat and its changing pattern with age and total fatness highlight the ethnicity-specific considerations in managing obesity.40
Acknowledgments
This work was supported by NIH DK PO1-42618; DK RO1-37352, RR00645, DK40414; P30 DK-26687; and R29-AG14715.
Footnotes
Contributors. Current study concept: Wang J and Gallagher. Provided data: Albu, Gallagher, Heymsfield, Heshka, Laferrère, Pierson, Pi-Sunyer, Wang J, Wang Z. Analysis and interpretation of data: Wu, Heshka, Gallagher. Critical review of manuscript for intellectual content: Gallagher, Wu, Heshka, Wang J, Wang Z, Albu, Laferrère, Pi-Sunyer. Statistical expertise: Heshka, Wu. Study supervision: Gallagher, Heshka.
Each author declared that she or he has no conflict of financial or personal interests in any company or organization sponsoring this study.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WPT. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 4.Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48:194–199. doi: 10.1016/s0026-0495(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14 (Suppl):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 6.Wu CH, Yao WJ, Lu FH, Wu JS, Chang CJ. Relationship between glycosylated hemoglobin, blood pressure, serum lipid profiles and body fat distribution in healthy Chinese. Atherosclerosis. 1998;137:157–165. doi: 10.1016/s0021-9150(97)00270-0. [DOI] [PubMed] [Google Scholar]
- 7.Lemieux S, Despres JP, Moorjani S, Nadeau A, Theriault G, Prud’homme D, et al. Are gender differences in cardiovascular disease risk factors explained by the level of visceral adipose tissue? Diabetologia. 1994;37:757–764. doi: 10.1007/BF00404332. [DOI] [PubMed] [Google Scholar]
- 8.Wu CH, Yao WJ, Lu FH, Yang YC, Wu JS, Chang CJ. Body fat distribution explains the sex differences in cardiovascular dysmetabolic factors in elderly non-diabetics. Age Aging. 2001;30:331–336. doi: 10.1093/ageing/30.4.331. [DOI] [PubMed] [Google Scholar]
- 9.Berman DM, Rodrigues LM, Nicklas BJ, Ryan AS, Dennis KE, Goldberg AP. Racial disparities in metabolism, central obesity, and sex hormone-binding globulin in postmenopausal women. J Clin Endocrinol Metab. 2001;86:97–103. doi: 10.1210/jcem.86.1.7147. [DOI] [PubMed] [Google Scholar]
- 10.Okosun IS, Liao Y, Rotimi CN, Prewitt TE, Cooper RS. Abdominal adiposity and clustering of multiple metabolic syndrome in White, Black and Hispanic Americans. Ann Epidemiol. 2000;10:263–270. doi: 10.1016/s1047-2797(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 11.Mott JW, Wang J, Thornton JC, Allison DB, Heymsfield SB, Pierson RN., Jr Relation between body fat and age in 4 ethnic groups. Am J Clin Nutr. 1999;69:1007–1013. doi: 10.1093/ajcn/69.5.1007. [DOI] [PubMed] [Google Scholar]
- 12.Chang CJ, Wu CH, Chang CS, Yao WJ, Yang YC, Wu JS, et al. Low body mass index but high percent body fat in Taiwanese – implications of obesity cut-offs. Int J Obes. 2003;27:253–259. doi: 10.1038/sj.ijo.802197. [DOI] [PubMed] [Google Scholar]
- 13.Silver AJ, Guillen CP, Kahl MJ, Morley JE. Effect of aging on body fat. J Am Geriatr Soc. 1993;41:211–213. doi: 10.1111/j.1532-5415.1993.tb06693.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee CC, Glickman SG, Dengel DR, Brown MD, Supiano MA. Abdominal adiposity assessed by dual energy X-ray absorptiometry provides a sex-independent predictor of insulin sensitivity in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:872–877. doi: 10.1093/gerona/60.7.872. [DOI] [PubMed] [Google Scholar]
- 15.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 (Suppl 1):S302–S304. doi: 10.1007/s00592-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74. doi: 10.1038/oby.2005.9. [DOI] [PubMed] [Google Scholar]
- 18.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 19.Tittelback TJ, Berman DM, Nicklas BJ, Ryan AS, Goldberg AP. Racial differences in adipocyte size and relationship to the metabolic syndrome in obese women. Obes Res. 2004;12:990–998. doi: 10.1038/oby.2004.121. [DOI] [PubMed] [Google Scholar]
- 20.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–771. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez JR, Heo M, Heymsfield SB, Pierson RN, Jr, Pi-Sunyer FX, Wang ZM, et al. Is percentage body fat differentially related to body mass index in Hispanic Americans, African Americans, and European Americans? Am J Clin Nutr. 2003;77:71–75. doi: 10.1093/ajcn/77.1.71. [DOI] [PubMed] [Google Scholar]
- 22.Casas YG, Schiller BC, Desouza CA, Seals DR. Total and regional body composition across age in healthy Hispanic and white women of similar socioeconomic status. Am J Clin Nutr. 2001;73:13–18. doi: 10.1093/ajcn/73.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Thornton JC, Burastero S, Shen J, Tanenbaum S, Heymsfield SB, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996;4:377–384. doi: 10.1002/j.1550-8528.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 25.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 26.Okosun IS. Ethnic differences in the risk of type 2 diabetes attributable to differences in abdominal adiposity in American women. J Cardiovasc Risk. 2000;7:425–430. doi: 10.1177/204748730000700606. [DOI] [PubMed] [Google Scholar]
- 27.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 28.Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, Beck-Nielsen H, et al. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005;13:2139–2145. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- 29.He Q, Horlick M, Thornton J, Wang J, Pierson RN, Jr, Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002;87:2164–2170. doi: 10.1210/jcem.87.5.8452. [DOI] [PubMed] [Google Scholar]
- 30.Ma K, Kotler DP, Wang J, Thornton JC, Ma R, Pierson RN. Reliability of in vivo neutron activation analysis for measuring body composition: comparisons with tracer dilution and dual-energy x-ray absorptiometry. J Lab Clin Med. 1996;127:420–427. doi: 10.1016/s0022-2143(96)90058-x. [DOI] [PubMed] [Google Scholar]
- 31.Soriano JM, Ioannidou E, Wang J, Thornton JC, Horlick MN, Gallagher D, et al. Pencil-beam vs fan-beam dual-energy X-ray absorptiometry comparisons across four systems. J Clin Densitometry. 2004;7:281–289. doi: 10.1385/jcd:7:3:281. [DOI] [PubMed] [Google Scholar]
- 32.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 33.Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes. 2006;30:837–843. doi: 10.1038/sj.ijo.0803208. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 35.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord. 2002;26:978–983. doi: 10.1038/sj.ijo.0801982. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, et al. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 37.Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, Beck-Nielsen H, et al. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005;13:2139–2145. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- 38.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. PNAS. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novotny R, Daida YG, Grove JS, Marchand LL, Vijayadeva V. Asian adolescents have a higher truncal: peripheral fat ratio than whites. J Nutr. 2006;136:642–647. doi: 10.1093/jn/136.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]