Abstract
Reactive oxygen species (ROS) increase in the cardiovascular system during hypertension and in response to angiotensin II. As mitochondria contribute to ROS-generation we sought to investigate the role of thioredoxin-2, a mitochondria specific antioxidant enzyme.
Mice were created with overexpression of human thioredoxin-2 (TghTrx2 mice) and backcrossed to C57BL/6J mice for at least 6 generations. 12 week old male TghTrx2 or littermate wild-type mice were made hypertensive by infusion of angiotensin II (400ng/kg per min) for 14 days using osmotic minipumps. Systolic arterial blood pressure was not different between TghTrx2 and wild-type animals under baseline conditions (101±1 resp. 102±1 mmHg). The angiotensin II-induced hypertension in wild-type (145±2 mmHg) was significantly attenuated in TghTrx2 mice (124±1 mmHg, p<0.001). Aortic endothelium-dependent relaxation was significantly reduced in wild-type following angiotensin II infusion, but nearly unchanged in transgenic mice. Elevated vascular superoxide and hydrogen peroxide levels as well as expression of NADPH oxidase subunits in response to angiotensin II infusion were significantly attenuated in TghTrx2 mice. Mitochondrial superoxide anion levels were augmented after angiotensin II infusion in wild-type mice, this was blunted in TghTrx2 mice. Angiotensin II infusion significantly increased myocardial superoxide formation, heart weight and cardiomyocyte size in wild-type, but not in TghTrx2 mice.
These data indicate a major role for mitochondrial thioredoxin-2 in the development of cardiovascular alterations and hypertension during chronic angiotensin II infusion. Thioredoxin-2 may represent an important therapeutic target for the prevention and treatment of hypertension and oxidative stress.
Keywords: angiotensin II, endothelial function, hypertension, mitochondria, thioredoxin-2, reactive oxygen species
INTRODUCTION
Reactive oxygen species (ROS) play a key role for the development of vascular dysfunction and cardiac hypertrophy induced by hypertension.1 Increased vascular production of superoxide (O2·−) inactivates nitric oxide (NO) and thereby diminishes endothelium-dependent vasodilatation and promotes cardiac hypertrophy.2 Vascular O2·− and hydrogen peroxide (H2O2) increase in models of hypertension and in response to angiotensin II.3, 4 Various ROS-producing systems are stimulated by angiotensin II including NADPH oxidase, xanthine oxidase, uncoupling of endothelial NO synthase (eNOS) as well as mitochondria.
Mitochondrial ATP synthesis is a highly redox-active process as three of the five multiprotein complexes with central function in oxidative phosphorylation are redox-driven proton-pumps.5 Therefore, dysfunctional mitochondria generate excessive amounts of different ROS such as O2·−, H2O2, and peroxynitrite. Increased ROS production from mitochondria has been found in ischemia/reperfusion injury, aging and atherosclerosis.6 7, 8 Recently, Doughan et al. demonstrated that angiotensin II induces mitochondrial dysfunction via protein kinase C dependent activation of NADPH-oxidase and formation of peroxynitrite.9
Thioredoxin (Trx) together with glutathione and glutaredoxin form the thiol-reducing system. Trx has a redox-active site (sequence Cys-Gly-Pro-Cys).10 In mammals there are at least three different thioredoxins: Trx1 is present in the cytosol but can also translocate to the nucleus; Trx2 has a consensus signal for translocation to the mitochondria; and SP-Trx is found in spermatozoa. Trx2 plays an important role in the antioxidant defense system of mitochondria.5 Overexpression thereby should protect from mitochondrial oxidative stress and might be beneficial in hypertension. To test this hypothesis, we investigated the effects of chronic angiotensin II infusion on vascular function, blood pressure, cardiac hypertrophy and oxidative stress in transgenic mice overexpressing human Trx2 (TghTrx2).
METHODS
All animal experiments conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
The transgenic mouse model was designed by Dr. Jiyang Cai in collaboration with Dr. Dean P. Jones, Dr. Jason M. Hansen and Dr. W. David Martin, Emory University, Atlanta, Georgia. Human Trx2 cDNA11 was used for generation of the DNA construct that was inserted into mouse embryos and V5 epitope was engineered at C-terminus of human Trx2 for detection of transgene product.12 Mice overexpressing human Trx2 were backcrossed at least 6 times to C57BL/6J.
Treatment Groups
At the age of 12 weeks male TghTrx2 and littermate wild-type mice were anesthetized by inhalation of isoflurane (2%), oxygen (98%) and osmotic minipumps (Alzet Model 2002; Alzet Corp) were implanted to permit subcutaneous infusion of angiotensin II ([Val5]angiotensin II, infusion rate 400 ng/kg per min). Sham-operated animals underwent an identical surgical procedure, except that an empty osmotic pump was implanted. Blood pressure was measured by a tail-cuff system using a heated scanner unit (LE-5007, Foehr Medical Instruments). Before the osmotic pump was implanted, the mice were trained in the blood pressure device for at least 7 days to accustom them to the procedure. In some animals blood pressure was measured invasively in response to intravenous infusion of NO synthase inhibitor NG-nitro-L-arginine-methyl ester (L-NAME, 10 mg/kg body weight).13 For in vitro studies the animals were deeply anesthetized with isoflurane, and the aorta and heart were removed and dissected free of adherent tissues.
Vascular reactivity studies
Aortic ring segments were studied in organ chambers as previously described.14 Passive tension was adjusted to 1g, and vessels were preconstricted to equal levels with PGF2α. Relaxations to cumulative concentrations of acetylcholine and the NO donor, DEA-NONOate, were examined. In separate experiments a dose response curve to phenylephrine in the absence or presence of L-NAME (100µmol/l) was performed.
Determination of O2·− and H2O2
Vascular and myocardial O2·− was determined by the oxidation of dihydroethidium (DHE) to 2-hydroxyethidium using high-performance liquid chromatography (HPLC) analysis as described previously with some modifications.15 H2O2 was measured using a fluorometric HRP-linked assay (Amplex red assay, Invitrogen, Carlsbad, CA) as previously described.16 For assessment of mitochondrial O2·− , mitochondria were isolated from the left ventricle using the mitochondrial isolation kit (Sigma-Aldrich, Deisenhofen, Germany) according to the manufacturer’s directions. Isolated mitochondria were than incubated with DHE (10µmol/l) and the conversion to 2-hydroxyethidium monitored by HPLC.
Western blot and immunohistochemistry
The Trx2 antibody was from R&D (Minneapolis, MN) and the V5tag, Proliferating Cell Nuclear Antigen (PCNA) and Cytochrome Oxidase IV antibodies from Abcam (Cambridge, UK). p22phox antibody was from Santa Cruz (Santa Cruz, CA). p47phox, nox2, rac-1, eNOS and MnSOD antibodies were from BD Bioscience (San Jose, CA). β-actin and secondary antibodies were from Cell Signaling (Danvers, MA).
Assessment of cardiac hypertrophy
Mice were euthanized and body and left ventricular weight recorded. Transversely sectioned left ventricle frozen tissue sections (5µm) were stained with Alexa Fluor® 594 wheat germ agglutinin and blue-fluorescent Hoechst 33342 dye (Invitrogen, Carlsbad, CA). Four radially oriented microscopic fields from each section were photographed and the cross sectional area of at least 100 cells, in which the nucleus and a clear staining of the plasma membrane could be visualized, were averaged. The myocyte outlines were traced and the cell areas measured using “lasso” tool in Adobe Photoshop.
Statistical analysis
Values are presented as mean ± SEM. Comparisons between groups of animals or treatments were made by one-way ANOVA. Comparisons of dose-response curves were performed using one-way ANOVA for repeated measurements. When significance was indicated by ANOVA, the Student-Newman Keuls post hoc test was used to specify between group differences.
RESULTS
Characterization of mice overexpressing human thioredoxin-2
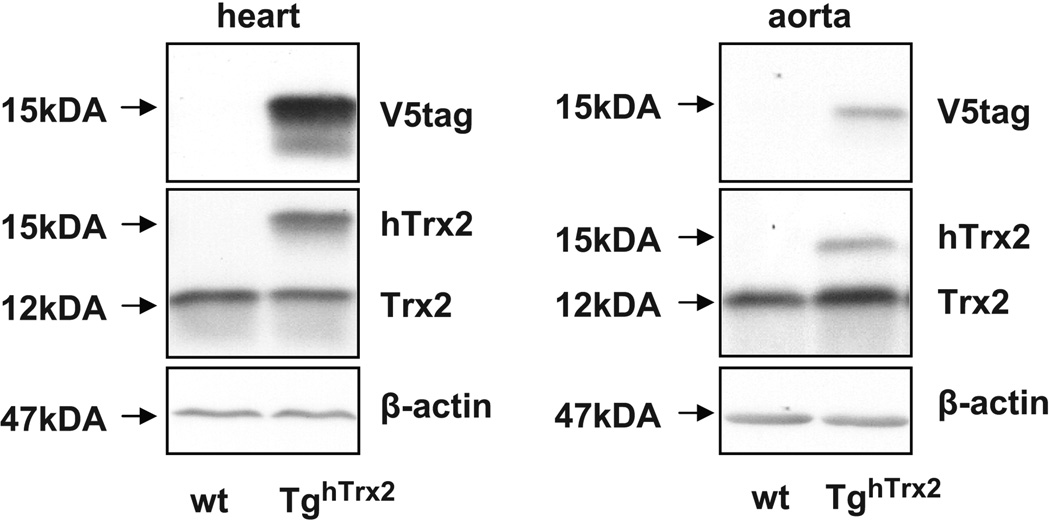
TghTrx2 mice showed no phenotype, had equal reproduction rates and mean arterial blood pressure at baseline (80±5 resp. 82±1 mmHg) or in response to L-NAME infusion (108±3 resp. 107±5 mmHg) as C57BL/6J animals. Expression of human Trx2 was confirmed in the heart and aorta via immunoblotting. We detected a specific band around 15kDA (human thioredoxin-2 12kDA and V5tag around 3 kDA) which was only present in tissue from TghTrx2 mice while blotting for Trx2 or V5tag (Figure 1).
Figure 1.
Representative Western blot of thioredoxin-2 and V5tag in aorta and heart from wild-type (wt) or transgenic mice overexpressing human thioredoxin-2 (TghTrx2). β-actin was used as a loading control.
Endothelium-dependent relaxation
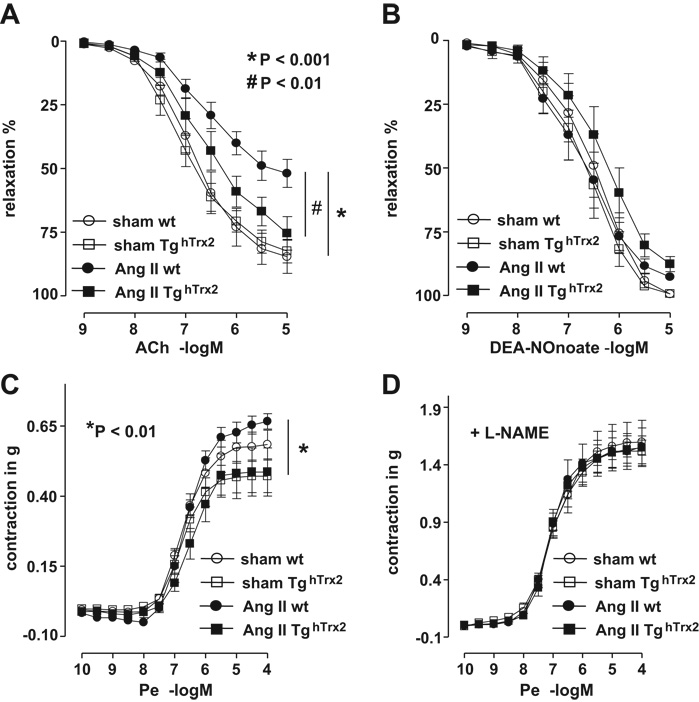
Hypertension is associated with diminished endothelium-dependent relaxation, the role of mitochondrial oxidative stress in this process, however, is yet unclear. In wild-type mice angiotensin II infusion caused a reduction in the acetylcholine-evoked endothelium-dependent relaxation as compared to control animals (Figure 2A). In contrast, in TghTrx2 mice, angiotensin II infusion had no significant effect on endothelium-dependent relaxation (Figure 2A). Endothelium-independent relaxation to DEA-NONOate was not different among the groups (Figure 2B). Under baseline conditions the contractile response to phenylephrine was slightly lower in TghTrx2 mice compared to wild-type animals and this difference was more pronounced after angiotensin II infusion (Figure 2C). Addition of L-NAME markedly increased the contraction induced by phenylephrine which was not different among the four groups (Figure 2D).
Figure 2.
Endothelium-dependent and endothelium-independent vasorelaxation in wild-type (wt) or transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion: Aortic segments (3mm) were mounted in isolated organ chamber baths and basal tension adjusted to 1 gram. Vascular relaxations to increasing concentrations of acetylcholine (A) and the nitric oxide donor, DEA-NONOate (B) were measured after preconstriction with PGF2α. The contractile response to increasing concentration of phenylephrine (Pe) is shown in C; previous addition of L-NAME (100µmol/, D) strikingly increased this response in all groups. Mean ± SEM, n = 4–7.
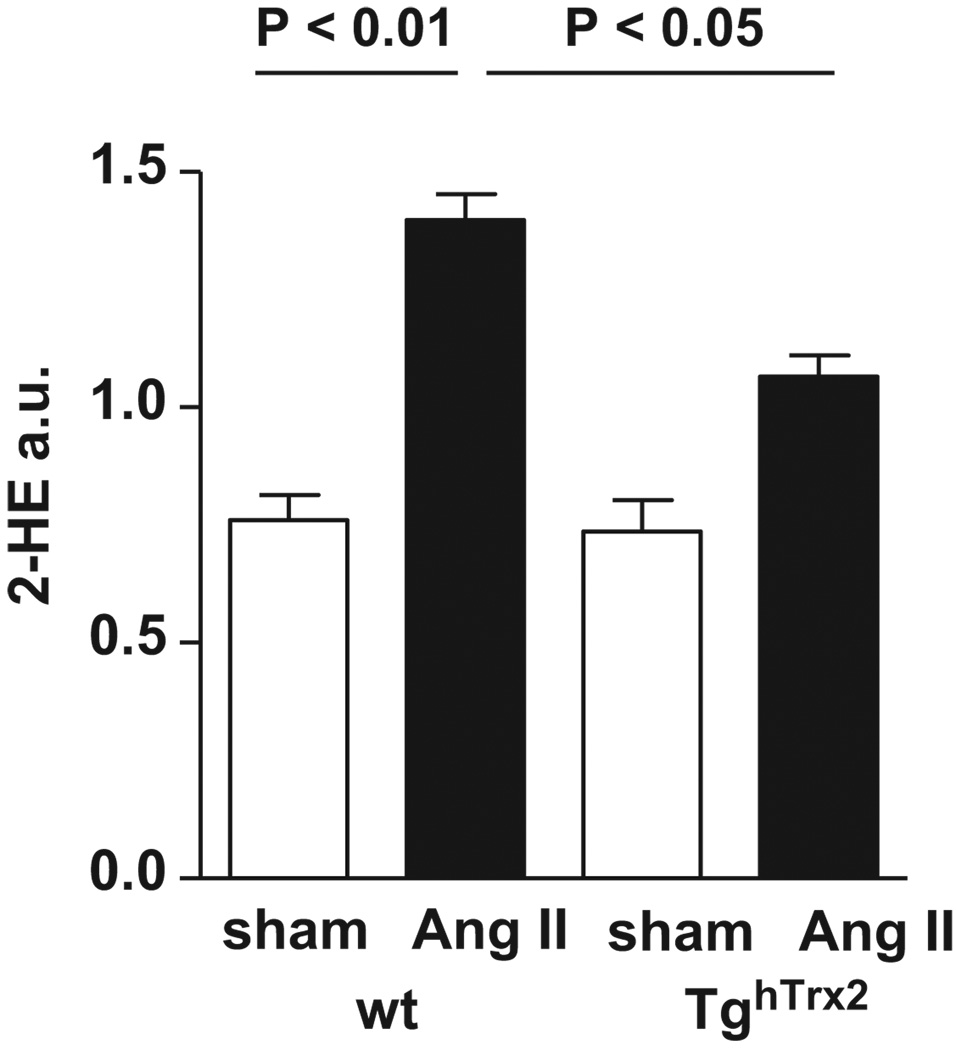
Vascular ROS production
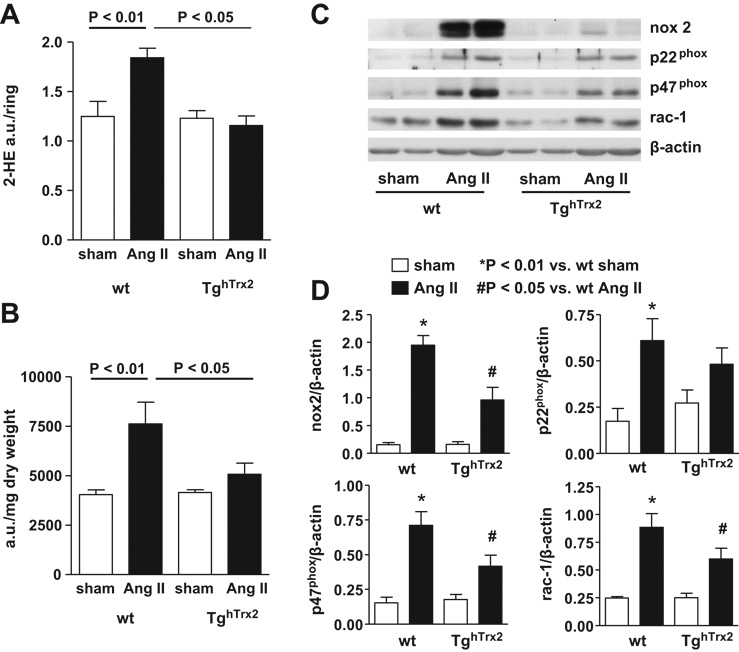
Modulation of vascular O2·− levels could explain the preservation of endothelial function in the TghTrx2 mice. At baseline, O2·− production, measured by monitoring the conversion of DHE to 2-hydroxyethidium was similar in wild-type and TghTrx2 mice. Chronic angiotensin II infusion led to an increase in aortic O2·− production in wild-type mice, as described previously.14, 17, 18 In contrast, in mice overexpressing Trx2, angiotensin II had no effect on O2·− production (Figure 3A). As H2O2 also contributes to vascular dysfunction we measured vascular H2O2 levels using the Amplex red assay. Compared to significantly increased H2O2 levels in wild-type mice with chronic angiotensin II infusion, angiotensin II had no effect on vascular H2O2 production in TghTrx2 mice (Figure 3B). As angiotensin II is a profound stimulator of NADPH oxidase; we investigated the aortic expression of the NADPH oxidase subunits p22phox, p47phox, nox2 and rac-1. Chronic angiotensin II infusion caused a striking increase in the expression of NADPH oxidase subunits in wildtype mice which was attenuated in animals overexpressing Trx2 (Figure 3C and D).
Figure 3.
Aortic superoxide anion and hydrogen peroxide in wild-type (wt) and transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion. A: Superoxide production was measured by monitoring the conversion of dihydroethidium to 2-hydroxyethidium (2-HE) with an HPLC-based method. B: Extracellular hydrogen peroxide measured by Amplex red assay. Mean ± SEM, n = 5–6. C: Representative Western blot and D: densitometry of aortic expression of NADPH oxidase subunits nox2, p22phox, p47phox and rac-1. β-actin was used as loading control. Mean ± SEM, n = 5–8.
Expression of the proliferation marker PCNA
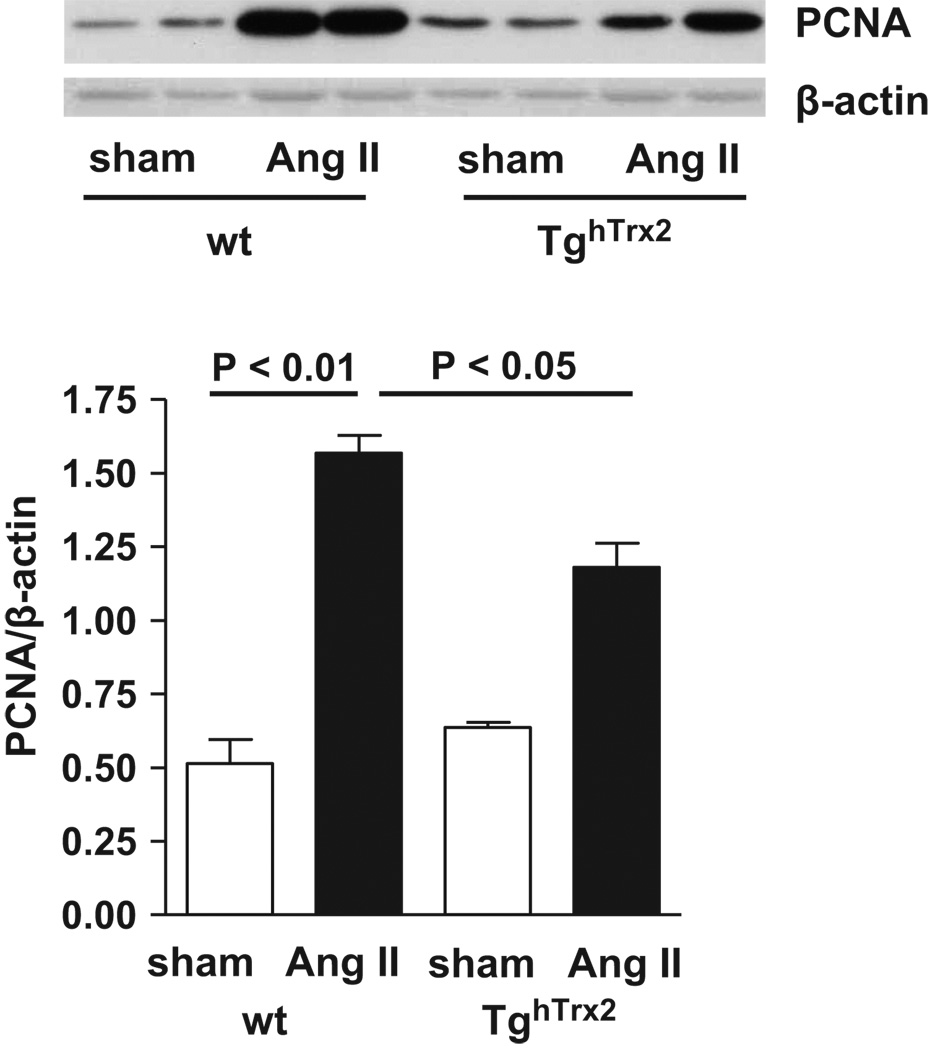
Angiotensin II is well known to stimulate cell proliferation. Chronic angiotensin II infusion markedly increased PCNA expression, a marker for cell cycle activity, in wild-type mice; this was blunted in TghTrx2 mice (Figure 4).
Figure 4.
Representative Western blot (top), and densitometry (bottom) of the proliferation marker PCNA (Proliferating Cell Nuclear Antigen) in the aorta from wild-type (wt) and transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion. β-actin was used as loading control. Mean ± SEM, n = 6–8.
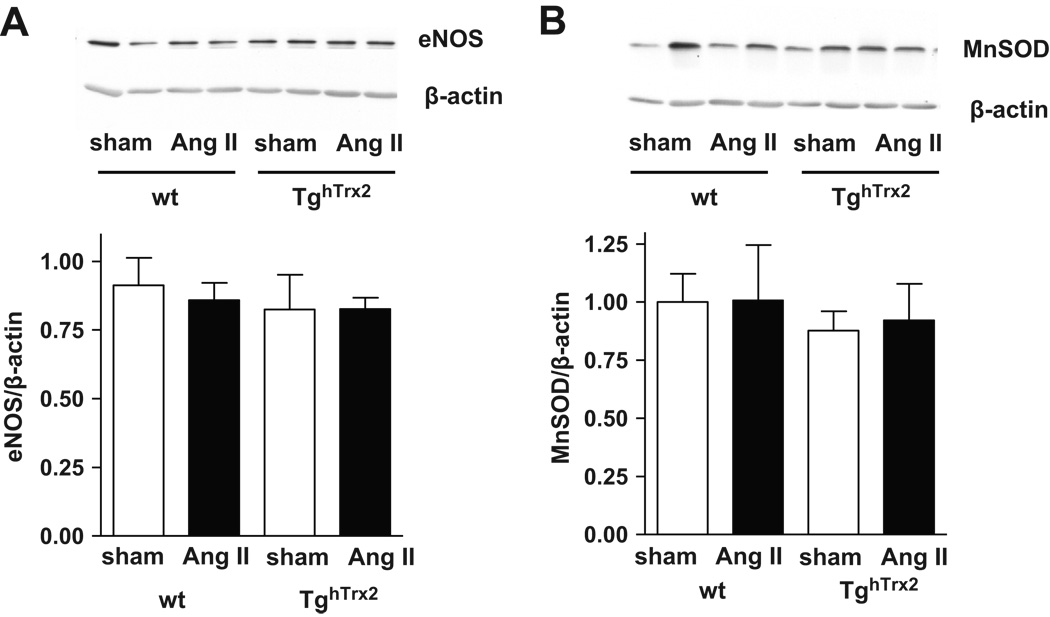
Expression of eNOS and antioxidant enzyme MnSOD
Neither the expression of eNOS nor MnSOD, a major mitochondrial superoxide anion scavenger, were influenced by chronic angiotensin II infusion or overexpression of human Trx2 (Figure 5).
Figure 5.
Representative Western blot and densitometry of endothelial nitric oxide synthase (eNOS) (A) and manganese superoxide dismutase (MnSOD) (B) in the aorta from wild-type (wt) and transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion. β-actin was used as loading control. Mean ± SEM, n = 5.
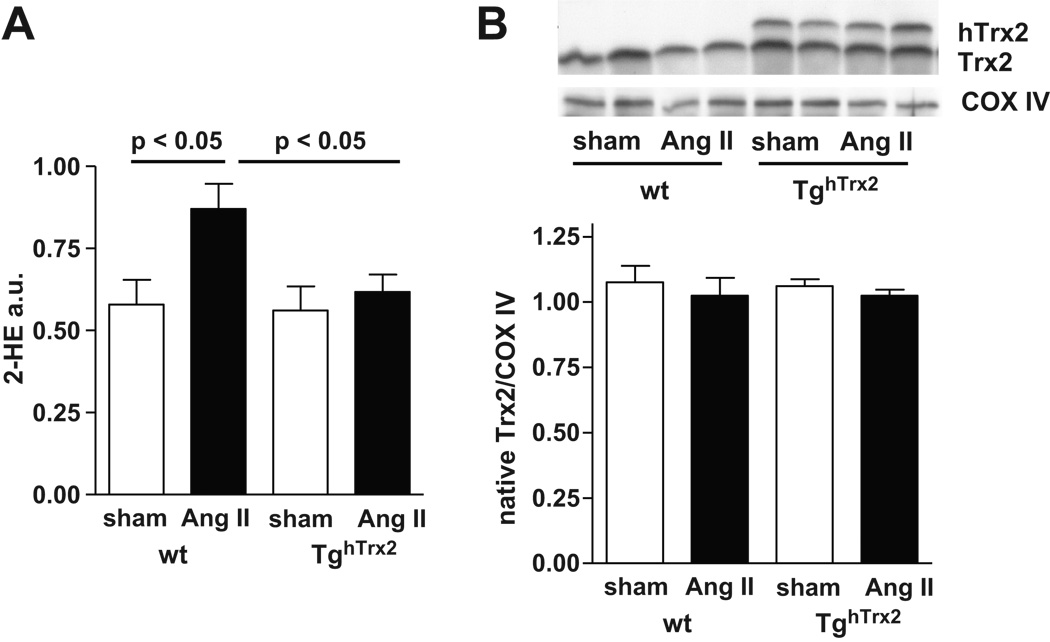
Mitochondrial ROS production
To further assess the role of mitochondria in angiotensin II mediated O2·− production and the effects of Trx2 overexpression we measured mitochondrial O2·− production (Figure 6A). Chronic angiotensin II infusion significantly increased O2·− levels in mitochondria in wild-type mice. In TghTrx2 mice receiving angiotensin II this increase was prevented (Figure 6A). Neither mitochondrial Trx2 expression nor hTrx2 expression was influenced by angiotensin II infusion (Figure 6B).
Figure 6.
A: Mitochondrial superoxide anion production of wild-type or transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion measured by monitoring the conversion of dihydroethidium to 2-hydroxyethidium (2-HE) with an HPLC-based method. Mean ± SEM, n = 4–6 B: Representative Western blot of thioredoxin-2 (Trx2) expression in isolated mitochondria and densitometry result for native Trx2. Cytochrome C Oxidase (COX) VI was used as loading control. Mean ± SEM, n = 6
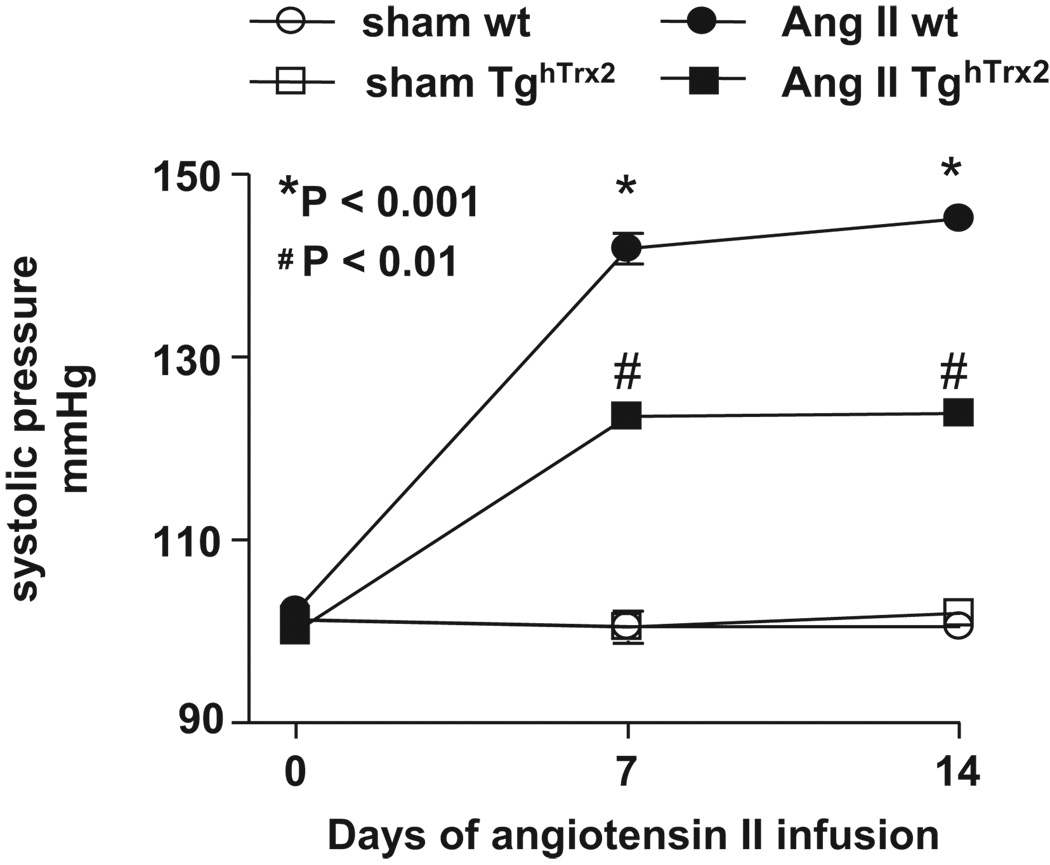
Role of Trx2 in modulating blood pressure
To determine if the preservation of endothelium-dependent relaxation and absence of an increase in vascular ROS production might be associated with an alteration in the hypertensive response to angiotensin II-infusion, we monitored blood pressure over time. While baseline systolic blood pressure was similar between wild-type and TghTrx2 mice, the increase in blood pressure caused by angiotensin II infusion was significantly greater in wild-type mice as compared to TghTrx2 mice (Figure 7).
Figure 7.
Blood pressure in wild-type (wt) or transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion measured before, on the 7th and 13th day of angiotensin II infusion. Mean ± SEM, n = 6–7.
Myocardial O2·− production
Similar as observed for vascular O2·− production chronic angiotensin II infusion increased myocardial O2·− production in wild-type mice. In TghTrx2 mice the increase in myocardial O2·− production was significantly blunted (Figure 8).
Figure 8.
Myocardial superoxide production in wild-type (wt) and transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion. Superoxide production was measured in left ventricular tissue by monitoring the conversion of dihydroethidium to 2-hydroxyethidium (2-HE) with an HPLC-based method. Mean ± SEM, n = 3.
Cardiac hypertrophy
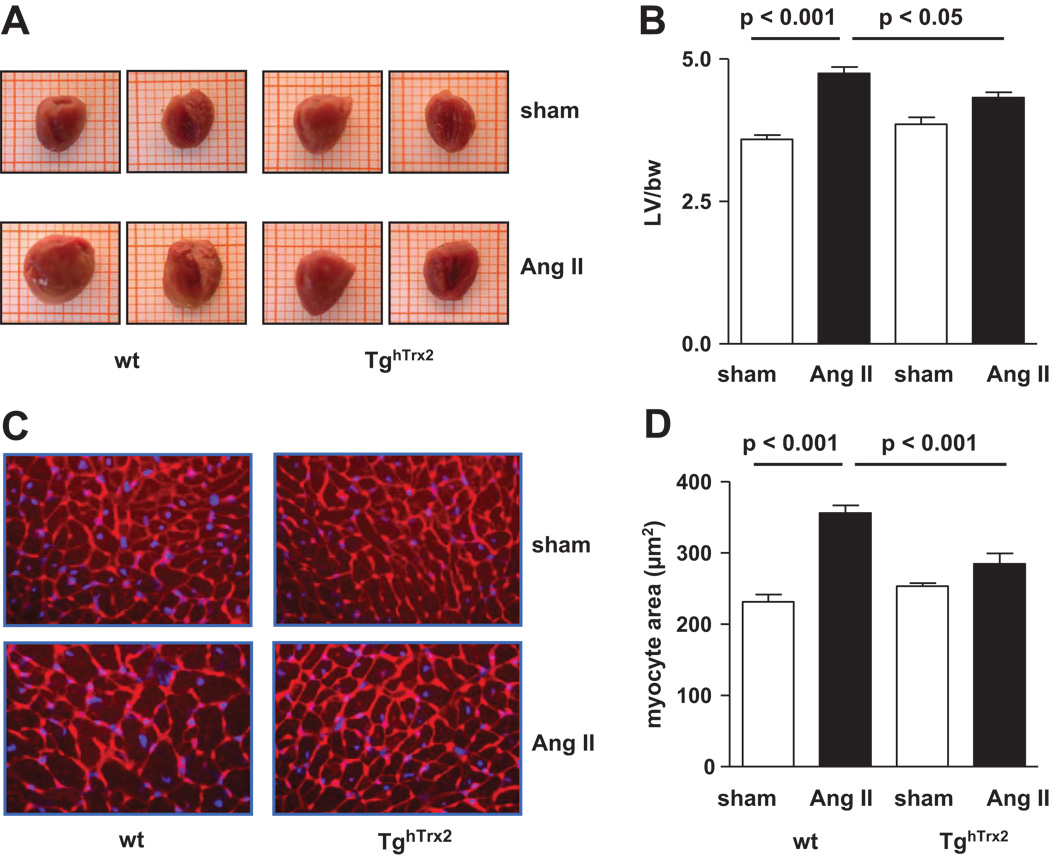
As both hypertension and angiotensin II cause cardiac hypertrophy, we investigated the effect of overexpression of human Trx2 on cardiomyocyte size and cardiac hypertrophy. Left ventricle to bodyweight ratio was significantly increased in wild-type animals receiving chronic angiotensin II infusion compared to control mice, however, in TghTrx2 mice this increase was significantly lower (Figure 9A and B). Cardiomyocyte size enlarged during chronic angiotensin II infusion in wild-type mice, while in mice overexpressing human Trx2 this enlargement was significantly attenuated (Figure 9C and D).
Figure 9.
Macroscopic view of representative hearts (A) and left ventricular to body weight ratio (B) in wild-type (wt) or transgenic mice overexpressing human thioredoxin-2 (TghTrx2) with or without chronic angiotensin II (Ang II) infusion. C: Representative slides stained with Alexa Fluor® 594 wheat germ agglutinin and blue-fluorescent Hoechst 33342 dye of left ventricular sections used for cardiomyocyte area determination (magnification x400). D: Cardiomyocyte cross-sectional area results. Mean ± SEM, n = 5–6.
DISCUSSION
The present study demonstrates that overexpression of the mitochondrial antioxidant Trx2 significantly improved endothelium-dependent vasorelaxation and prevented the increase in ROS-formation caused by chronic angiotensin II infusion. These favorable effects on vascular function were accompanied by a reduced blood pressure increase in response to chronic angiotensin II infusion in TghTrx2 mice. Further, angiotensin II-induced cardiomyocyte hypertrophy was blunted by overexpression of Trx2. These data indicate for the first time a role for Trx2 and mitochondrial ROS in angiotensin II-induced hypertension.
Increased ROS levels found in animal models of hypertension and in hypertensive humans contribute to endothelial dysfunction.1, 2, 19 Activation of the renin-angiotensin-system and elevated angiotensin II levels are major stimulators for vascular ROS production in hypertension. The source of ROS primarily studied in hypertension to date is NADPH oxidase. Modulation of NADPH oxidase expression influences endothelial function and blood pressure.17, 20, 21 Uncoupling of eNOS has been suggested as another source for angiotensin II-induced O2·− production14, 22, however in the present study using C57Bl/6J mice tetrahydrobiopterin levels as well as eNOS dimer to monomer ratio were not changed after chronic angiotensin II infusion (data not shown).
The mitochondrion is a potential source of superoxide anion, and dysfunctional mitochondria contribute to ROS production in diabetes, heart failure and ischemia/reperfusion injury.6–8 The role of mitochondrial ROS in hypertension, however, is understudied yet. We show that mitochondrial superoxide production was elevated in wild-type mice receiving chronic angiotensin II infusion, which is consistent with the observation that mitochondria isolated from spontaneously hypertensive rats treated with cyclosporine produce excess superoxide.23 In our study overexpression of Trx2 prevented this increase in mitochondrial superoxide production under chronic angiotensin II infusion indicating the major role of Trx2 for mitochondrial redox regulation.
Endothelium-dependent relaxation was preserved in mice overexpressing Trx2 after chronic angiotensin II infusion. In accordance with previous data we did not detect changes in vascular eNOS protein expression under chronic angiotensin II infusion in wildtype animals.14, 18 This was not different in TghTrx2 mice, indicating that overexpression of Trx2 rather influenced NO bioavailability than its source eNOS. Endothelial specific overexpression of Trx2 recently has been shown by Zhang et al. to cause lower resting blood pressure, diminished response to the vasoconstrictor phenylephrine, improved NO bioavailability and to reduce atherosclerotic lesion formation in apolipoprotein E-deficient mice.13 Our TghTrx2 mice had equal baseline blood pressure as wild-type C57BL/6J and only slightly blunted phenylephrine reponse. This difference is most likely explained by the much higher expression of the transgene in the animals used by Zhang et al. compared to our moderate overexpression. Further, these authors used male and female mice. Male differ from female mice in the response to chronic angiotensin II infusion with male displaying higher blood pressure, and NADPH-driven superoxide generation. Angiotensin II upregulates cardiac thioredoxin-1 in male mice, but not in females.24 In the present study in male C57BL/6J mice, endogenous mitochondrial thioredoxin-2 expression was unchanged by angiotensin II infusion; we did not address whether this is different in females.
Expression of MnSOD, a mitochondrial antioxidant enzyme, was neither influenced by chronic angiotensin II infusion as previously shown18 nor by Trx2 overexpression. However, O2·− and H2O2 production in the vessel wall was reduced in animals with Trx2 overexpression indicating that protecting mitochondria from excessive ROS production benefits overall ROS production in the vessel wall. Angiotensin II induced increase in NADPH oxidase subunit expression in wild-type mice was significantly attenuated in TghTrx2 mice. Doughan et al recently found that by activation of NAPDH oxidase angiotensin II triggers mitochondrial ROS formation which in turn is essential for sustained stimulation of NADPH oxidase activity.9 This crosstalk between mitochondrial and NADPH oxidase-derived ROS production was also found in nitroglycerin-triggered vascular dysfunction.25 Improved endothelial-dependent relaxation, blunted NADPH oxidase expression and decreased ROS-formation in TghTrx2 mice suggest that overexpression of Trx2 disrupts the crosstalk between mitochondrial and NADPH oxidase-derived ROS. A secondary effect of the limited overall ROS-production in the aorta of TghTrx2 mice after angiotensin II infusion is the reduced expression of the proliferation marker PCNA. Limiting mitochondrial ROS formation thus appears to be a novel and efficient approach to decrease overall cellular ROS production by inhibition of its stimulatory effects on NADPH oxidase.
This is further strengthened by the observation that overexpression of Trx2 significantly reduced blood pressure. Improving endothelial function and reducing vascular ROS production most likely underlies the prevention of blood pressure increase by overexpression of Trx2. Indeed numerous interventions improving vascular dysfunction and ROS production lead to reduced blood pressure.
Chronic elevation of angiotensin II levels causes cardiac hypertrophy, and ROS are essentially involved.26 Dysfunctional mitochondria seem to contribute to cardiac hypertrophy in heart failure as well as ischemia/reperfusion injury.8 The present study shows that overexpression of Trx2 diminished superoxide levels in the heart and prevented cardiomyocyte hypertrophy under chronic angiotensin II infusion. Vasoprotection and reduced blood pressure are likely to substantially contribute to the anti-hypertrophic effect seen in TghTrx2 mice. Nevertheless, blood pressure independent effects of angiotensin II on cardiac hypertrophy have been proposed27 (for review of this controversial issue see28). While thioredoxin-1 plays an important role in the development of cardiac hypertrophy independent of oxidative stress and blood pressure 10, 29, no such data existed for mitochondrial thioredoxin-2 yet. Our study provides first evidence that mitochondrial ROS production is involved in angiotensin II-induced myocardial hypertrophy in vivo.
Perspective
Reactive oxygen species play a major role in the pathophysiology of hypertension. In the present study overexpression of the mitochondrial specific antioxidant enzyme Trx2 attenuated angiotensin II-induced hypertension. TghTrx2 mice displayed diminished endothelial dysfunction, vascular and myocardial ROS production in response to angiotensin II as well as significantly lower blood pressure and reduced cardiomyocyte hypertrophy. These data provide first evidence that mitochondrial ROS production is essential for angiotensin II-induced sustained vascular ROS formation, vascular dysfunction and hypertension. Trx2 and mitochondrial ROS production therefore present a novel target for prevention and treatment of hypertension but also for other disease conditions associated with increased ROS formation, such as heart failure, diabetes and aging.
Acknowledgments
We gratefully acknowledge excellent technical support by Anette Berbner, Anna Schmitt, Susanne Schraut and Gabriele Riehl.
Sources of Funding
This work was supported in part by the Deutsche Forschungsgemeinschaft. Support for generation of the transgenic mouse model was provided by NIH grant ES009047.
Footnotes
Disclosures
NONE
Contributor Information
Julian D. Widder, Department of Internal Medicine I, University of Wuerzburg, Wuerzburg, Germany.
Daniela Fraccarollo, Department of Internal Medicine I, University of Wuerzburg, Wuerzburg, Germany.
Paolo Galuppo, Department of Internal Medicine I, University of Wuerzburg, Wuerzburg, Germany.
Jason M. Hansen, Department of Pediatrics, Division of Pulmonary, Asthma, Cystic Fibrosis and Sleep.
Dean P. Jones, Department of Medicine, Division of Pulmology, Emory University, Atlanta, Georgia.
Georg Ertl, Department of Internal Medicine I, University of Wuerzburg, Wuerzburg, Germany.
Johann Bauersachs, Department of Internal Medicine I, University of Wuerzburg, Wuerzburg, Germany.
REFERENCES
- 1.Lassegue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. Journal of the American Society of Hypertension. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 5.Go Y-M, Jones DP. Redox compartmentalization in eukaryotic cells. Biochimica et Biophysica Acta (BBA) - General Subjects. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 8.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract. 2008;38:137–155. doi: 10.1016/j.cvsm.2007.10.004. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 10.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med. 2006;84:997–1003. doi: 10.1007/s00109-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 12.He M, Cai J, Go Y-M, Johnson JM, Martin WD, Hansen JM, Jones DP. Identification of Thioredoxin-2 as a Regulator of the Mitochondrial Permeability Transition. Toxicol Sci. 2008;105:44–50. doi: 10.1093/toxsci/kfn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 15.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 17.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol. 2007;22:316–320. doi: 10.1097/HCO.0b013e3281ca710d. [DOI] [PubMed] [Google Scholar]
- 20.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 21.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 22.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louhelainen M, Merasto S, Finckenberg P, Lapatto R, Cheng ZJ, Mervaala EM. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J Hypertens. 2006;24:947–956. doi: 10.1097/01.hjh.0000222766.37971.9f. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 26.Morgan T. Renin, angiotensin, sodium and organ damage. Hypertens Res. 2003;26:349–354. doi: 10.1291/hypres.26.349. [DOI] [PubMed] [Google Scholar]
- 27.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 28.Reudelhuber TL, Bernstein KE, Delafontaine P. Is angiotensin II a direct mediator of left ventricular hypertrophy? Time for another look. Hypertension. 2007;49:1196–1201. doi: 10.1161/HYPERTENSIONAHA.106.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebrahimian T, Sairam MR, Schiffrin EL, Touyz RM. Cardiac hypertrophy is associated with altered thioredoxin and ASK-1 signaling in a mouse model of menopause. Am J Physiol Heart Circ Physiol. 2008;295:H1481–H1488. doi: 10.1152/ajpheart.00163.2008. [DOI] [PubMed] [Google Scholar]