Summary
In many taxa, germline precursors segregate from somatic lineages during embryonic development and are irreversibly committed to gametogenesis. However, in animals that can propagate asexually, germline precursors can originate in adults. Botryllus schlosseri is a colonial ascidian that grows by asexual reproduction, and on a weekly basis regenerates all somatic and germline tissues. Embryonic development in solitary ascidians is the classic example of determinative specification, and we are interested in both the origins and the persistence of stem cells responsible for asexual development in colonial ascidians. In this study, we characterized vasa as a putative marker of germline precursors. We found that maternally deposited vasa mRNA segregates early in development to a posterior lineage of cells, suggesting that germline formation is determinative in colonial ascidians. In adults, vasa expression was observed in the gonads, as well as in a population of mobile cells scattered throughout the open circulatory system, consistent with previous transplantation/reconstitution results. vasa expression was dynamic during asexual development in both fertile and infertile adults, and was also enriched in a population of stem cells. Germline precursors in juveniles could contribute to gamete formation immediately upon transplantation into fertile adults, thus vasa expression is correlated with the potential for gamete formation, which suggests that it is a marker for embryonically specified, long-lived germline progenitors. Transient vasa knockdown did not have obvious effects on germline or somatic development in adult colonies, although it did result in a profound heterochrony, suggesting that vasa might play a homeostatic role in asexual development.
Keywords: Asexual reproduction, Botryllus schlosseri, Blastogenesis, Heterochrony, Regeneration, Budding, Colonial ascidian, Coloniality, Germ cells, Germline, Stem cells, vasa
INTRODUCTION
Germline precursors in most metazoans segregate from somatic lineages specified during early stages of embryonic development and are irreversibly committed to gamete production (Santos and Lehmann, 2004). By contrast, studies in animals that can propagate asexually suggest that germline precursors can also originate in adults, from either pluripotent stem cells that can give rise to somatic or germline fate, or de- and/or transdifferentiation of cells and tissues (Blackstone and Jasker, 2003; Extavour et al., 2005; Nieuwkoop and Sutasurya, 1979; Nieuwkoop and Sutasurya, 1981).
We are studying germline precursors in the colonial ascidian Botryllus schlosseri. Ascidians are sessile marine invertebrate chordates that develop from swimming tadpole larvae with characteristic chordate features, and they are closely related to vertebrates (Bourlat et al., 2006; Delsuc et al., 2006). Embryogenesis in solitary ascidians is the textbook example of determinative or mosaic development, with most cell specification being due to the inheritance of cytoplasmic determinants (Conklin, 1905; Imai et al., 2006). Following a free-swimming phase, the larvae settle and metamorphose into a sessile, filter-feeding form, called an oozooid, wherein most chordate characteristics are lost. In most species, this initial metamorphosis is followed by growth and sexual maturity of the solitary individual. By contrast, colonial ascidian species grow by asexual propagation, resulting in a colony of genetically identical individuals (Swalla, 2006). This regenerative ability appears to be due to the presence of self-renewing progenitors (Laird et al., 2005; Sabbadin and Zaniolo, 1979), but the nature of these cells (e.g. pluripotent or lineage restricted), and the mechanisms by which they contribute to asexual reproduction are unknown.
To date, germline development has been studied in solitary ascidians, such as Ciona intestinalis, and progenitors have been found to segregate from somatic lineages early in development, resulting in maternally specified germ cells that migrate into the gonad rudiments during metamorphosis, and that later mature into gametes (Fujimura and Takamura, 2000; Shirae-Kurabayashi et al., 2006; Takamura et al., 2002; Yamamoto and Okada, 1999). Embryos in colonial species are brooded and lineage-tracing studies lag behind those in the more accessible free-spawning solitary species. Nevertheless, determinative development is likely to be a conserved characteristic in ascidians, and we hypothesize that colonial species retain long-lived germline progenitors specified during embryogenesis.
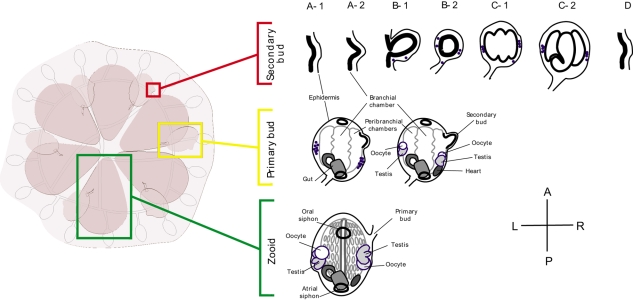
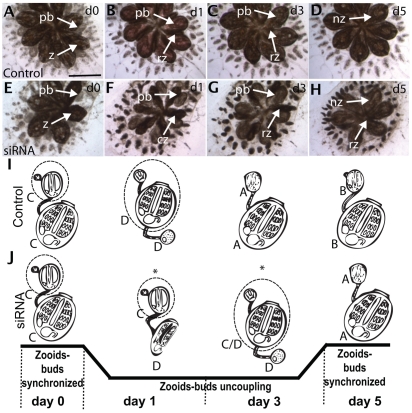
B. schlosseri is a colonial ascidian with three co-existing generations arranged spatially into a star-shaped group called a system, containing both adults (zooids) and buds, all connected by a common vasculature (Fig. 1). The center of each system is occupied by the zooids, which are actively feeding and capable of sexually reproducing. They are joined peripherally by `primary buds', which are completing their development of both somatic and germline tissues. In turn, these are connected to `secondary buds', which are in the initial stages of development. The zooid has a lifespan of only one week, after which it dies in a massive wave of apoptosis called `takeover' (Lauzon et al., 2002). Development is coordinated throughout the colony, and during takeover the zooid bodies undergo apoptosis and are removed via phagocytic cells in the blood, the primary bud migrates into the newly vacated region of the colony, opening its siphons and becoming a zooid, the secondary bud becomes the primary bud, and a new secondary bud begins to develop. Thus, the life history of Botryllus consists of a constant succession of individual zooids, each with a three-week lifespan (Lauzon et al., 2002).
Fig. 1.
Outline of asexual development (blastogenesis) in Botryllus schlosseri. Animals were staged according to Lauzon et al. (Lauzon et al., 2002). Each stage (A-D) represents 1 day under laboratory conditions. Drawings represent dorsal views of zooids (green frame), primary buds (yellow frame) and secondary buds (red frame). For simplification, each adult zooid carries only one set of buds. A secondary bud appears as a thickening of the epidermis and the peribranchial chamber leaflet of the primary bud (stage A1), which evaginates into a closed vesicle (stage B2), followed by organogenesis (stages C1-D). Gonadogenesis occurs in the secondary bud from mobile precursors (blue; stages B1-C2). During takeover (stages C2-D), the secondary bud becomes the primary bud and a new blastogenic cycle begins for the next secondary bud. After the second takeover event, the primary bud opens its siphons and becomes a functional adult (zooid). In fertile colonies (as illustrated here), the hermaphroditic gonad fully matures on both sides of the zooid.
Asexual development (blastogenesis) takes 14 days under laboratory conditions, and can be divided into seven distinct visual stages (Fig. 1; stages A-1 through D) (Lauzon et al., 2002). A new generation starts as a secondary bud, first visible as a thickening of the peribranchial epithelium of a primary bud (Fig. 1; stage A1), which evaginates and forms a closed vesicle (Fig. 1; stage A-2 through B-2). Next, a series of epithelial invaginations and protrusions (Fig. 1; stage C-1) differentiate into somatic tissues and organs (Fig. 1; stage C-2). After seven days (Fig. 1; stage D), the secondary bud transitions to a primary bud and continues to develop. At day 14, the siphons open and the primary bud becomes a filter-feeding adult zooid. Each zooid can generate multiple buds each week, so the colony will eventually expand asexually. While interconnected, the zooids and buds develop independently, and individuals can be separated from the colony without disturbing their growth (i.e. subcloning), thus multiple experiments can be done on a single genotype.
Following metamorphosis, colonies undergo at least 8-12 developmental cycles prior to the first appearance of gametes (sexual maturity). In addition, populations show seasonal fertility, and in the lab cycle in and out of reproductive (fertile) and non-reproductive (infertile) states. However when the colony is fertile development of the gametes is synchronized with somatic development (Mukai, 1977; Mukai and Watanabe, 1976; Sabbadin and Zaniolo, 1979). The first appearance of gonads occurs in the secondary bud (stage B), when mobile progenitors in the blood migrate to a region between the inner epithelium and the epidermis and begin to proliferate. Concurrently, oocytes at various stages of development also appear (Fig. 1). Over the next 10 days, the medial region of the blastema will differentiate into the lobular testis, while the lateral region will become the ovary (Sabbadin and Zaniolo, 1979). For the latter, one or several oocytes will become fixed on the epithelia of the peribranchial chamber, and an oviduct will form from the outer follicular layer. Upon transition to the adult zooid, mature eggs will immediately ovulate into the peribranchial chamber, be fertilized by exogenous sperm, and develop in situ. Several hours to days later the testes will complete development, and sperm will be released into the peribranchial chamber and flushed into the water column, fertilizing neighboring colonies (Johnson and Yund, 2004). The time lag between ovulation and sperm release (protogyny) prevents self-fertilization.
Given this plasticity, it appears that germline precursors are mobile, and can migrate to a niche in the secondary bud then expand and differentiate into gametes. Moreover, at least for oocytes, intermediate stages migrate between each successive asexual generation until maturity, where they become fixed and contribute to sexual reproduction in that generation (Izzard, 1968; Mukai, 1977; Sabbadin and Zaniolo, 1979). In summary, each asexual generation probably initiates gametogenesis of both male and female gametes, as well as harboring intermediate stages of oocytes.
The mobility of germline precursors has also been demonstrated independently. B. schlosseri undergoes a natural transplantation reaction that can result in vascular fusion, uniting the circulation of two individuals. In these chimeras, both long-lived germline and somatic chimerism has been observed, even months after surgical separation of the two individuals (Sabbadin and Zaniolo, 1979). Moreover, when stem cells of two genotypes mix, the populations will compete, and stem cells from one genotype will often replace the germline and somatic tissues of another, in a process called `stem cell parasitism' (Buss, 1982; Stoner and Weissman, 1996). Germline and somatic precursors can be prospectively enriched in B. schlosseri and show long-term reconstitution ability upon transplantation. In addition, limiting dilution experiments show that transplantation of single cells results in either somatic or germline chimerism, but not both, which suggests that both germline-committed and somatic-committed stem cell populations exist in adults (Laird et al., 2005). Our long-term goal is to isolate and study these stem cells in order to understand the cellular and molecular basis of asexual development (regeneration) and stem cell parasitism.
In this study, we characterized the expression and function of vasa as a putative lineage-specific marker for germline progenitors, and tested for the presence of functional germline progenitors in juveniles. vasa encodes an ATP-dependent RNA helicase, and its expression is restricted to primordial germ cells (PGCs) in most phyla studied to date (Sengoku et al., 2006). However, in some metazoans, vasa has also been implicated in aspects of somatic development (Dill and Seaver, 2008; Extavour et al., 2005; Mochizuki et al., 2001). In Botryllus, our results suggest that vasa labels long-lived, functional germline progenitors specified during embryogenesis that are also found in enriched stem cell populations, and these results correlate with functional reconstitution following transplantation (Laird et al., 2005). By contrast, transient siRNA-mediated knockdown of vasa had no effect on germline formation during blastogenesis, but did produce heterochronic shifts of somatic growth in the colony, suggesting that vasa-positive (vasa+) cells play a role in somatic regeneration as well.
MATERIALS AND METHODS
Animals
Botryllus schlosseri colonies were raised, staged, crossed and screened at 18-20°C according to Boyd et al. (Boyd et al., 1986). Embryos were isolated and prepared for in situ hybridization as previously described (Brown and Swalla, 2007). Following metamorphosis, colonies are considered juveniles until the first appearance of gametes (sexual maturity). Following this time, colonies with gametes are defined as fertile, those without are referred to as infertile.
Quantitative PCR
cDNA was synthesized from embryonic and adult stages as described (Tiozzo and De Tomaso, 2009). Quantitative PCR (qPCR) was carried out (Tiozzo et al., 2008) with the following set of Botryllus specific vasa primers: 5′-GGCGGATTTAGCGATGATGAG-3′ and 5′-TTCCCCCATAGCGACTGTTAGAC-3′. Analysis of q-PCR was performed using 2-ΔΔCt according to Livak and Schmittgen (Livak and Schmittgen, 2001). Fold change of mRNA was normalized to either actin (ActinF, 5′-CTATACGCTTCCGGCAGAAC-3′; ActinR, 5′-CAAGAGCGACATAGCACAGC-3′) or elongation factor 1 alpha (EFF, 5′-CGTGGTCATTGGCCACGTAGATTCCGGAAA-3′; EFR, 5′-ATGAAATCACGATGACCGGGAGCGTCGATG-3′) as specified. Individual experiments were then normalized to experiment-specific reference mRNA quantity (see Results). Each experiment was repeated at least three times from two different genotypes of every stage analyzed, with samples run in triplicate. Error bars were calculated using s.d. and statistical significance using paired t-tests (P<0.05).
Cell staining and sorting
Botryllus cells were labeled for sorting as outlined in Laird et al. (Laird et al., 2005). Following incubation, cells were washed, resuspended in Botryllus buffer containing 25 mM Verapamil and 1 μg/ml propidium iodine (Molecular Probes) to assay viability. FACS was carried out using a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA). Samples were compensated prior to sorting and dead cells were excluded by gating-out high propidium iodine signals. Analysis was performed using FlowJo software (Tree Star, San Carlos, CA, USA). Sorted cells were stained with 0.05% Trypan Blue to verify quantity and viability. SSClow and SSChigh gates were pooled for qPCR analysis.
In situ hybridization and histological analysis
In situ hybridization (ISH) was performed as described (Brown and Swalla, 2007). The sequence used for probes is shown in Fig. S1 in the supplementary material. Prehybridization proteinase K concentrations used were: 500 μg/ml for oozoid and adult whole mounts, 100 μg/ml for tadpole embryos and larvae, and 1 μg/ml for sections. Results are from at least three independent whole-mount ISH replicates, and at least five different colonies were sectioned for independent ISH replicates. Positive cells identified with either sense or antisense probes were compared in five independent ISH replicates (see Fig. S2 in the supplementary material) (Brown and Swalla (2007). Sectioning and image capture were performed as described previously (Tiozzo and De Tomaso, 2009).
siRNA delivery
siRNAs to vasa were generated as described (Tiozzo and De Tomaso, 2009; Tiozzo et al., 2008). Sequences targeted for siRNA are shown in Fig. S1 in the supplementary material. Samples were observed and collected for histology and RNA isolation, and results were correlated with vasa expression levels. Seven independent experimental replicates were done using different genotypes. For each experiment, a colony was separated into two subclones and vasa siRNA or control (GFP) siRNA treatment was initiated at the same stage in the blastogenic cycle. In all experiments, the level of vasa transcripts declined in the first 3 days and remained low during the treatment, as demonstrated by qPCR and RT-PCR (see Fig. S3 in the supplementary material).
Juvenile/adult transplantation and analysis of germline reconstitution
Genetically distinguishable genotypes were crossed, and progeny were fused into naïve subclones of the parents. We used six different genotypes in three independent crosses. At each time point, multiple testes were dissected from the colony and DNA isolated (Laird et al., 2005). Testes were tested for the presence of three PCR-based genetic markers (e18sp6, L19sscp and H9hdx), as previously described (De Tomaso and Weissman, 2003; De Tomaso and Weissman, 2004). Results were equivalent for each marker.
RESULTS
Spatiotemporal expression of vasa during embryogenesis, metamorphosis and in the adult colony
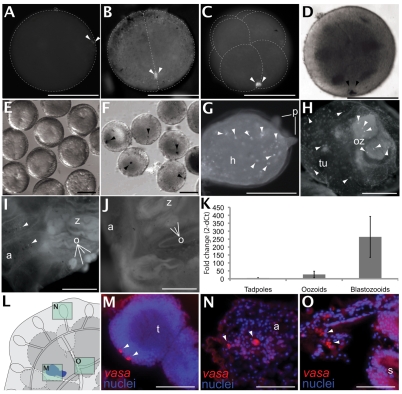
A B. schlosseri vasa cDNA clone was isolated, sequenced and annotated (see Fig. S1 in the supplementary material; GenBank Accession number FJ890989). Probes were designed to unique regions of the gene and expression analyzed in eggs and during embryogenesis (Fig. 2A-O). vasa mRNA concentrates in granular structures of the fertilized B. schlosseri egg cortex (Fig. 2A). During the first cleavage, vasa mRNA localized to the vegetal pole of the embryo (Fig. 2B); after the third cleavage, vasa segregated in the cleavage furrow of the posterior-most B4.1 pair of cells of the eight-cell embryo (Fig. 2C), and in the descendant B5.1 pair of cells of the 16-cell embryo (Fig. 2D). During gastrulation (Fig. 2E), vasa mRNA was expressed in a pair of cells found posterior to the blastopore that are likely to correspond to the precursor germline B7.6 pair (Fig. 2F) (Shirae-Kurabayashi et al., 2006).
Fig. 2.
vasa expression during embryogenesis, metamorphosis and in adult colonies as shown by ISH. (A) Fertilized egg with polar bodies in the animal pole and granule-like aggregation of maternal vasa (arrowheads) in the cortex. (B) A two-cell-stage embryo with vasa mRNA (arrowheads) aggregated on one side of the cleavage furrow. (C) An eight-cell-stage embryo with two proximate aggregates of vasa (arrowheads) at the posterior cortical region of the B4.1 blastomeres. (D) Sixteen-cell-stage embryo with vasa mRNAs (arrowheads) at the posteriormost cortical region of the B5.1 blastomeres. (E) Gastrula- and neural-plate-stage embryos (past the 110-cell stage) show invagination and blastopore formation on the vegetal side of the embryo. (F) Gastrula- and neural-plate-stage embryos with a single aggregate of vasa (arrowheads) in the posterior region of the embryos. (G) Detail of a larval head during metamorphosis. Anterior adhesive papillae (p) can be observed on the right; vasa+ cells (arrowheads) are scattered throughout the head (h). (H) vasa expression in a newly settled colony is seen in individual cells (arrowheads) in the oozooid (oz), and within the extracorporeal vasculature. (I) In a sexually mature adult, vasa expression is seen in small oocytes (o) at the site of the gonads of the zooid (z) and in individual cells (arrowheads) within the ampullae (a). (J) A sexually fertile adult shows general background levels of autofluorescence in blood and tunic cells, as shown by the sense probe negative control (cf. H and I; antisense probe). (K) Quantitative PCR analysis of vasa mRNA levels during metamorphosis. Larvae show the lowest mRNA levels, oozooids show a slight increase, and first-generation asexually derived zooids show a further increase after ten days of settlement. (L) Illustration of a colony (dorsal view) showing location of the testis, ampullae and zooid body (framed), corresponding to histological sections in M-O. (M) Fluorescence in situ hybridization shows two vasa expressing cells (red; arrowheads) at the periphery of a mature testis containing highly packed nuclei of sperm precursors (blue). (N) vasa expressing cells (red, arrowheads) in the ampullae. vasa+ cells are scattered throughout the vasculature and are surrounded by other hemocytes (blue, nuclei counterstained with DAPI). (O) Cells expressing vasa (red, arrowheads) are also seen in the sinuses and lacunae surrounding the epithelial folds of the stomach (s) in an adult zooid of the colony. a, ampullae; h, head; o, oocyte; oz, oozoid; p, papillae; s, stomach; t, testis; tu, tunic; z, zooid. Scale bars: 100 μm.
vasa localization was then characterized during metamorphosis, in oozooids, and in zooids derived after several rounds of asexual development, but prior to sexual maturity. During initiation of metamorphosis, zygotic expression of single vasa+ cells was scattered throughout the larval head (Fig. 2G). Immediately following metamorphosis, vasa+ cells were present in the mantle of the oozooid and within the peripheral vasculature of the tunic (Fig. 2H). In fertile colonies, vasa expression was observed in oocytes labeled with an antisense probe (Fig. 2I), but not in those labeled with a sense control (Fig. 2J). Histological analysis (Fig. 2L-O) showed vasa+ cells in the periphery of the lobules in developing testes (Fig. 2M), in single hemocytes in ampullae, throughout the peripheral vasculature (Fig. 2N), and in a region of mesenchyme surrounding the sinuses and lacunae of the zooid (Fig. 2O).
ISH in adult colonies is difficult due to the presence of the tunic, which shows medium to high non-specific background, but was necessary to comprehensively characterize vasa localization. Background was normalized as described (Brown and Swalla, 2007), and results were verified by qPCR. Expression was quantified in tadpoles, in post-metamorphosis oozooids and in first-generation asexually derived zooids. Tadpoles contained the lowest levels of vasa mRNA expression, oozooids showed a slightly higher expression, and zooids showed≥threefold higher levels, regardless of developmental stage (Fig. 2K).
Additionally, we confirmed that vasa is expressed in the extracorporeal circulation as well as within the zooid bodies (Fig. 3D). Colonies were dissected and vasa expression was quantified and compared between the extracorporeal vasculature and the zooid and bud tissues in fertile colonies (Fig. 3D). vasa+ cells were observed in the circulation both inside and outside of the zooid body; however, there were differences in the number of vasa+ cells in ampullae and in the zooid tissues between fertile and infertile colonies, most likely due to vasa expression in the gonads.
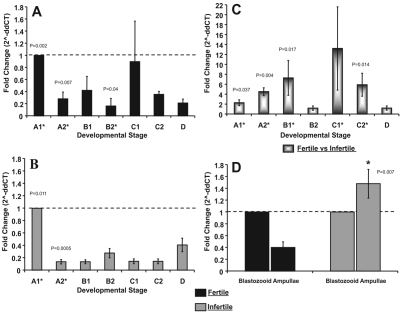
Fig. 3.
qPCR analysis of vasa expression levels. vasa expression levels were analyzed during asexual development (A-C) and in isolated tissues (D). (A) Fertile colonies (testes, oocytes and embryos present). Values were normalized as described in the Materials and methods. Resulting differences in gene expression are given in fold change with respect to the threshold (stage A1 normalized to self, dashed line). (B) Infertile colonies. Values were normalized and presented as in A. Asterisks indicate statistical significance (P-value displayed) with respect to the preceding developmental stage (see Results). (C) Comparative analysis of vasa expression in fertile versus infertile colonies. (D) vasa expression compared between the peripheral vasculature and zooid bodies in both fertile and infertile colonies.
Quantitative analysis of vasa expression during blastogenesis
Asexual somatic development occurs independently of gametogenesis, and adults will often fluctuate between fertile and infertile states in both laboratory-reared and natural populations. vasa expression was quantified and compared between fertile and infertile adult colonies across one complete cycle of asexual development (Fig. 3A-C). In fertile colonies, vasa expression was highest in stage A1 and fluctuated throughout the asexual cycle (Fig. 3A). Statistically significant changes (*) occurred at the transition from A1 to A2 (P=0.002), B1 to B2 (P=0.007) and D to A1 (P=0.04). Variability was highest in stage C1, the point in the cycle when germline formation is being initiated in secondary buds (Fig. 1, Fig. 3A). In infertile colonies (Fig. 3B), vasa expression was highest at stage A1 and fluctuated across successive stages of asexual development, with statistically significant changes detected between A1 and A2 (P=0.011), and D and A1 (P=0.0005). Fertile colonies were then compared with infertile colonies (Fig. 3C). In all stages, fertile colonies had higher levels of vasa expression (Fig. 3C). Changes in vasa expression most likely reflect cell proliferation owing to an increase in colony size following takeover in both fertile and infertile adults, as well as during gamete development in fertile colonies.
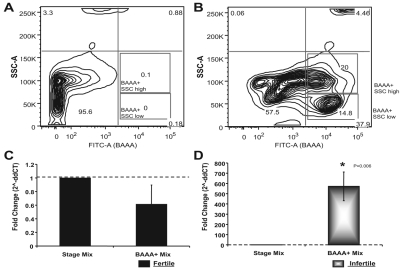
vasa enrichment in BAAA+ stem cell populations
Previous studies have enriched self-renewing germline and somatic progenitors 10-fold versus total cells based on ALDH activity using the reagent BAAA. We tested whether vasa expression was enriched in BAAA+ versus total cells from both fertile and infertile colonies by qPCR (Fig. 4). Botryllus cells were analyzed by FACS, comparing the unstained population (not treated with BAAA; Fig. 4A) with BAAA+ populations consisting of both side scatter (SSC) high and low populations (Fig. 4B). Both the BAAA+/SSClow and the BAAA+/SSChigh population were sorted, pooled and used for qPCR analysis of vasa gene expression. vasa expression was normalized first to the Elongation factor 1 alpha (EF1α) expression level, then to pooled cDNA consisting of total mRNA from each stage (Stage Mix), to determine average vasa expression during blastogenesis for both states of fertility (dashed threshold line). In BAAA+ cells from infertile colonies, vasa was highly enriched in comparison with the average expression (P=0.006; Fig. 4D). By contrast, BAAA+ cells from fertile colonies showed lower than average vasa expression (Fig. 4C). Results from experiments shown in Figs 2, 3, 4 suggest that the increased vasa expression in fertile colonies is likely to be due to differentiating gametes, which are not found in the BAAA+ pool, indicating that at least a proportion of the vasa+ cells in the BAAA+ population are long-lived progenitors.
Fig. 4.
vasa gene expression in BAAA+ cells from fertile and infertile colonies. FACS plots showing Botryllus cell populations based on Side Scatter (SSC-A, y-axis) and FITC-A emission (BAAA+, x-axis). (A) No treatment control showing autoflurescence only. (B) Cells treated with BAAA+ and PI for viability. Gates indicate populations sorted for qPCR analysis: BAAA+/SSC high and low (blue boxes) with total percentage indicated (×100). (C,D) qPCR analysis of vasa gene expression in BAAA+ populations from (C) fertile and (D) infertile colonies. Asterisk indicates statistically significant differences.
Rapid and long-term germline chimerism induced by juvenile progenitors
The identification of vasa+ cells during embryogenesis and in juvenile colonies suggested that germline progenitors are specified early in development. We independently tested for the presence of functional progenitors by assessing germline chimerism following the natural transplantation of cells between juveniles and fertile adults. We crossed two genetically distinct colonies and fused F1 oozooids back into naïve subclones of the parents (Table 1, Fig. 5). Three to four weeks following transplantation, we tested for the presence of donor markers in the testes of the recipient. As germline formation is initiated in the secondary bud at stage B1 (Fig. 1), this time point is the earliest in which chimerism in the testes could be detected. Eggs were not tested because they are surrounded by a somatic follicular layer, and because the time to maturation is not well defined (Sabbadin and Zaniolo, 1979).
Table 1.
Genetic analysis of chimeric testes
| Donor | Recipient | Time (weeks) | Number testes sampled | Number testes donor | Number testes recipient |
|---|---|---|---|---|---|
| 1025f | 1025c | 3 | 8 | 2 | 6 |
| 12 | 6 | 2 | 4 | ||
| 32 | 12 | 8 | 4 | ||
| Yw1519 | 921a | 3 | 6 | 2 | 4 |
| 12 | 5 | 1 | 4 | ||
| Sc6a-b | 3143j | 3 | 9 | 1 | 8 |
| 12 | 14 | 6 | 8 | ||
| 112 | 8 | 8 | 0 |
Donor and recipient (D and R in Fig. 5) genotypes were crossed and a single F1 individual was fused to a naïve subclone of the recipient parent. Three to four weeks later, individual testes were removed from the recipient and tested for the presence of donor markers.
Fig. 5.
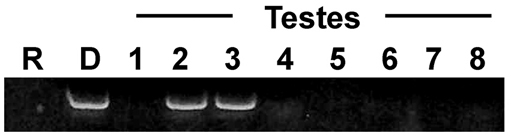
Genetic analysis of chimeric testes. F1 chimerism results from genotypes 1025f and 1025c (see also Table 1). 1025f was homozygous for a dominant marker (e18sp6, see Materials and methods). Donor and recipient genotypes were crossed and a single F1 individual was fused to a naïve subclone of the recipient parent. Four weeks later, eight testes were isolated from individual zooids throughout the subclone and tested for the presence of the donor marker. R, recipient (1025c); D, donor (1025f); 1-8 are individual testes from the donor subclone, 4 weeks following fusion, two show the presence of donor markers. Independent markers gave equivalent results.
When fertile, each zooid had one or two testes dissected and motile sperm isolated, free of somatic contamination (Laird et al., 2005; Stoner et al., 1999). Testes from individual zooids in the recipient were isolated (6-9 testes/experiment) and chimerism was assessed using several previously characterized genetic markers (De Tomaso and Weissman, 2003). In each cross, we observed that one or two testes from a group of 6-9 contained donor markers, whereas the rest were derived from the recipient (Table 1, Fig. 5). Control progeny from the same crosses did not become sexually mature until 10-12 weeks following metamorphosis, which is the normal time to sexual maturity under laboratory conditions.
On average, a B. schlosseri genotype has a lifespan of 3-9 months, whereas some laboratory-reared strains can live to be over two years (De Tomaso et al., 1998). To test whether these germline progenitors were long lived, we re-analyzed subclones of the same chimeric individuals at later time points. In all cases, chimerism was detected in the germline (Table 1, Fig. 5). In summary, sexually immature juveniles contain long-lived functional germline progenitors that can contribute to gamete formation immediately upon transplantation into a fertile colony, and that show long-term self-renewal.
Functional analysis of vasa during blastogenesis
Functional analysis of vasa was performed using siRNA-mediated genetic knockdown. It takes about 3 days to knockdown >95% of the mRNA for multiple genes (see Fig. S3 in the supplementary material), therefore in the first set of experiments siRNA treatment of fertile colonies began in stage C2, so that new buds would initiate and develop under near complete knockdown conditions, allowing us to follow an asexual generation throughout the life cycle.
In control experiments, subclones were unaffected, and takeover and the transition of asexual generations occurred normally (Fig. 6A-D). Experimental colonies also underwent takeover normally (Fig. 6F); however, vasa knockdown severely affected the timing of this process, extending it from 24 hours to around 4 days. Histological analysis showed that despite this delay, there were no abnormalities in the process (see Fig. S4 in the supplementary material). This delay can be easily visualized: adult zooids shrink and the primary buds grow much slower (compare Fig. 6G with 6C): However, the buds developed normally (Fig. 6H; see also Fig. S5 in the supplementary material). These experiments were repeated four times on different genotypes with no deviation.
Fig. 6.
Effects of vasa knockdown on asexual development. (A-D) Development of control colony, treated every day (d0-5) with GFP siRNA (d0; stage C). (E-H) Development of knockdown colony over a 5-day period. Development is delayed by 2 days compared with the control colony (compare G and B; H and C). (I,J) Schematic representation of zooid development in control and treated colonies: vasa knockdown causes a desynchronization in development between adult and buds, as illustrated on the black timeline. Asterisks indicate when the treated samples lost synchrony and the zooids underwent an early takeover. cz, contracted zooid; nz, new zooid; pb, primary bud; rz, regressing zooid; z, zooid. Scale bar: 1 mm.
vasa knockdown had no effect on the development of the germline in fertile colonies. Eggs and testes of the primary buds continued normal development (Fig. S6 in the supplementary material), and, in secondary buds, oocyte and gonad cell recruitment and blastema formation occurred similar to in untreated colonies (see Fig. S6E,F in the supplementary material). The adult gonads (egg and testis) presented no obvious defects. However, the germline developed in context of the delayed resorption during takeover and was delayed by four days (see Fig. S6A-D in the supplementary material).
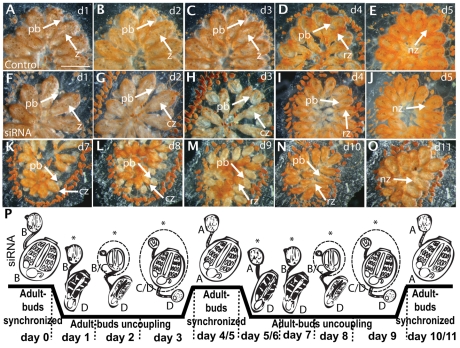
To further dissect the effect of vasa on the timing of blastogenesis, siRNA treatment was initiated at stage B2 or C1 and continued for two consecutive blastogenic cycles. vasa knockdown caused colonies to undergo an early takeover event, which began 24 hours following the initiation of siRNA treatment (about 24-48 hours before in the control). The phenotype was equivalent to that in the previous experiment: takeover was delayed, with resorbtion of the zooids and growth of the primary buds, as well as their transition to an adult delayed by about 48 hours (Fig. 7). Interestingly, forcing the colonies into early takeover via vasa knockdown coupled to a slower primary bud to zooid transition caused the transition to occur simultaneously in control and experimental subclones, and the two were re-synchronized at the same stage of development five days later (stage A; Fig. 7E,J). However, once vasa siRNA-treated subclones had completed this first takeover process (day 5; Fig. 7J), they immediately entered another takeover phase, around 6-7 days before the control (Fig. 7K). The zooids began to shrivel with the siphons closing, but in this case the takeover was further delayed, and the process took about 5-6 days (the entire cycle) to be completed (Fig. 7K-O). Despite this further delay, the process was again completed and the second generation (i.e. the secondary buds that were developing when siRNA treatment began) became adults, about 10 days from the start of the experiment, with no apparent defects (Fig. 7O). At this point, the colony immediately went into takeover for a third time, but rapidly became disorganized and died within two days. This was probably due to the fact that the early takeover phenotype caused the siphons to remain closed, preventing feeding for about two weeks. A schematic is shown in Fig. 7P. The same phenotype was observed regardless of the developmental stage of the colony at the onset of the siRNA treatment (day 0). Thus, it appears that knockdown of vasa causes the adult generation to undergo takeover within 24 hours, desynchronizing development between the zooids and buds. However, development of the primary and the secondary buds was not delayed, and the colonies re-synchronized at stage A, in step with the control subclones (Fig. 7E,J,O).
Fig. 7.
vasa knockdown during two consecutive blastogenic cycles. (A-E) Development of control colony treated every day (d0-11) with GFP siRNA (d0; stage B). (F-O) Development of knockdown colony over a two week period. Control colony develops and initiates takeover (stage D) normally (D; day 4), and the process is completed in about 24 hours (E, day 5). By contrast, the vasa knockdown colony goes into takeover within 24 hours (G), and disrupted development continues over the next 3 days (H,I), but terminates in a transient recovery of developmental synchrony (compare J with E). Although the control colony continues development, the vasa knockdown colony immediately goes back into takeover (K), which lasted the entire asexual cycle, but terminated development synchronously for a second time (compare O with E) in early stage A. (P) Schematic of blastogenic development during vasa knockdown showing the shifts in synchrony (shifts in black timeline) that occur between the zooids and buds. The asterisk indicates when the treated samples lost synchrony and the zooids underwent an early takeover, but synchrony was briefly reestablished after both takeover events. cz, contracted zooid; nz, new zooid; pb, primary bud; rz, regressing zooid; z, zooid. Scale bar: 500 μm.
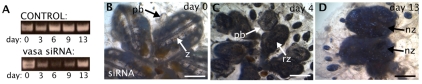
We next tested the reversibility of the observed phenotypes, as well as the potential effects of knockdown on succeeding blastogenic generations. vasa knockdown was initiated at stage B and the colony underwent early takeover (Fig. 8A,B). Six days later, vasa siRNA delivery was stopped and the colonies were allowed to continue development (Fig. 8C). Seven days later, vasa knockdown colonies had re-established normal coordination between takeover of the adult zooid and development of the bud, concurrent with vasa re-expression (Fig. 8D).
Fig. 8.
Release of vasa siRNA treatment has no effect on succeeding asexual generations. (A) vasa expression analyzed by RT-PCR at different timepoints in control and knockdown colonies; siRNA treatment was discontinued at day 6. (B) Colony at the onset of the vasa siRNA treatment; stage B of blastogenesis; (C) on day 4, zooids are in takeover (stage D), showing the typical vasa knockdown phenotype; (D) on day 13, zooids contain palleal buds that are synchronized with the zooids, showing that release of vasa siRNA treatment results in the re-synchronization of asexual development. nz, new zooid; pb, primary bud; rz, regressing zooid; z, zooid. Scale bars: 200 μm.
In summary, acute vasa knockdown in adults had no effect on germline development, but caused the colony to go into early takeover, and in addition the takeover event itself was delayed. The period of delay was not constant, but was proportional to when siRNA treatment began. By contrast, development of both primary and secondary buds was not affected and occurred with normal timing. Although we do not have the ability to carry out rescue with transgenic expression, experiments in which siRNA treatment was stopped revealed no long-term effects of knockdown on somatic or germline development. Thus, vasa appears to be functionally involved in the timing of takeover.
DISCUSSION
B. schlosseri is a basal chordate with the ability to regenerate all organs and tissues on a weekly basis, but the origins and persistence of the progenitors responsible are not understood. Previous studies had revealed three properties of germline progenitors in adults. First, Sabbadin and Zaniolo demonstrated that progenitors are mobile, and can naturally transplant between parabiosed individuals resulting in long-term germline chimerism and parasitism (Sabbadin and Zaniolo, 1979). Second, adults in both natural and laboratory-reared populations cycle between fertile and infertile states, demonstrating that germline progenitors that can be quiescent exist within an individual (Boyd et al., 1986; Sabbadin, 1953). Finally, prospective isolation studies suggested that both germline and somatic progenitors could be enriched about 10 times based on ALDH activity (Laird et al., 2005). These same studies also suggested that distinct germline and somatic lineages exist. First, single cell transplants from ALDHhi (BAAA+) populations resulted in germline or somatic chimerism, never both (Laird et al., 2005). In addition, following natural transplantation, parasitism of the germline is repeatable, hierarchical and appears to be genetically controlled. By contrast, somatic chimerism is random and does not correlate with germline results. In fact, following the fusion of two individuals, the resulting chimera is often composed of soma from one genotype, and germline from the other (Sabbadin and Zaniolo, 1979; Stoner et al., 1999), thus germline and somatic development seem to occur from independent populations of cells. The results presented here show that vasa expression is strongly correlated with germline precursors.
Early germline specification of long-lived germline progenitors
The constant development and turnover of zooids in colonial ascidians, coupled to the determinative development characteristic of the Tunicata, suggest that long-lived progenitors are specified during embryogenesis that contribute to development throughout life, but the nature of these cells (pluripotent or lineage-restricted) is not well understood. Our spatiotemporal localization of vasa in B. schlosseri embryos revealed expression patterns nearly equivalent to those found in solitary ascidians (Brown and Swalla, 2007; Shirae-Kurabayashi et al., 2006; Tanaka et al., 2000), with maternally deposited vasa segregating into a pair of posterior blastomeres, suggesting an early specification of germline-restricted progenitors. At later time points, vasa+ cells are seen scattered in the head of the tadpole, a region equivalent to the circulatory distribution in the adult body plan following metamorphosis. Independently, we found that cells in newly metamorphosed individuals are competent to reconstitute the germline following transplantation into fertile colonies, and that this chimerism is maintained for the life of the adult recipient (up to 112 weeks in one pairing). In both fertile and infertile adults, vasa is expressed in cells in the extracorporeal vasculature, as well as near lacunae surrounding the zooid body. Circulatory vasa+ cells have been observed in all botryllid species examined to date (Brown and Swalla, 2007; Rosner et al., 2009; Sunanaga et al., 2006; Sunanaga et al., 2008). vasa expression is also enriched in BAAA+ cells, which can reconstitute the germline (Laird et al., 2005).
Finally, in recent studies, we have analyzed the contribution of individual genotypes to germline and somatic tissues in chimeras made from juvenile individuals immediately following metamorphosis. We found that when these chimeras reached sexual maturity, patterns of reconstitution matched those of chimeras made from adults, and included complete germline parasitism and a lack of correlation between somatic and germline outcomes. Thus, even if chimeras were made months prior to sexual maturity, germline progenitors acted independently of somatic progenitors (M. C. Carpenter and A.W.D., unpublished).
Therefore, our data support the hypothesis that self-renewing, lineage-restricted germline progenitors are specified during embryogenesis that become functional immediately following metamorphosis and contribute to gametogenesis throughout the life of the colony. This suggests that fertility is based on developmental cues that cause these progenitors to move from the circulation to regions within the secondary bud and initiate germline formation.
vasa expression and enrichment of germline progenitors
We compared the enrichment of vasa in BAAA+ cells isolated from fertile and infertile adults to independently correlate vasa expression with long-lived progenitors. We found dramatic changes between the two. In infertile colonies, vasa expression was highly enriched in BAAA+ cells. By contrast, fertile colonies had lower vasa expression in BAAA+ versus total cells. This suggests that vasa expression is upregulated in cells of the developing gonads that are not part of the BAAA+ population. The BAAA+ populations from both fertile and infertile adults contain an enriched pool of stem cells (Laird et al., 2005), so our results provide strong evidence that a proportion of vasa+ cells in the BAAA+ population are germline progenitors.
However, these data do not rule out the existence of vasa-negative (vasa-) germline progenitors, as studies in two related species have revealed that surgical ablation of regions containing vasa+ cells did not affect germline development, and that new vasa+ cells appeared (Takamura et al., 2002; Sunanaga et at., 2006). These reports suggest that vasa-progenitors exist that become vasa+ at later stages of development. This would be analogous to the situation in mice, where PGCs are specified at day E6, but vasa expression does not turn on until day E9, when PGCs are migrating to the genital ridge (Lacham-Kaplam, 2004; Tanaka et al., 2000). However, in B. schlosseri, vasa+ cells are present at all times, as are functional germline progenitors.
Previous studies suggested that vasa+ somatic cells exist in ascidians. During Ciona embryogenesis, high vasa expression is detected in the head; however, the fate of these cells following metamorphosis is unknown, as in the adult vasa expression is restricted to the gonads (Takamura et al., 2002). Although vasa+ circulating somatic stem cells could exist in B. schlosseri, the only tissue outside of these circulatory cells in which vasa expression was seen is the gonad. In summary, our study cannot discount the possibility of vasa-germline progenitors nor of vasa+ somatic and/or pluripotent progenitors. However, the lack of congruity between somatic and germline chimerism following transplantation (either natural or experimental) suggests that a germline-committed population exists, and the distribution of vasa+ cells correlates exactly with previous limiting-dilution studies (Laird et al., 2005).
Effects of vasa knockdown in asexual development
Transient knockdown of vasa resulted in no obvious morphological defects in development of the germline but showed an effect on the synchrony of asexual development in the colony. One possible explanation for the absence of specific germline phenotypes is that only short-term knockdowns (2-3 weeks) were possible before the colonies degenerated. Testes require at least two weeks to develop, and oocytes can mature over several generations, therefore our treatment might not be long enough to assess the potential role of vasa in germline development. Conversely, vasa knockdown in different species shows disparate phenotypes, ranging from no defects [zebrafish (Braat et al., 2001) and flatworms (Pfister et al., 2008)] to sex-specific defects on male gamete formation, but not female [mice (Tanaka et al., 2000)], to defects in migration but not in the identity of the germ cell precursors [medaka (Li et al., 2009)]. Thus, it would not be surprising if vasa was shown not to be required for germline formation; however, its expression can still be used to identify precursors, as has been shown in other animals.
Given that vasa+ cells were scattered throughout the circulation, the global knockdown phenotype was unexpected. However, a somatic role for vasa would not be surprising. vasa is expressed in somatic cells and tissues of embryos and adults in species of different phyla, such as in the head of Ciona ascidian larvae (Fujimura and Takamura, 2000; Shirae-Kurabayashi et al., 2006), in the somatic stem cells of planarians and cnidarians (Shibata et al., 1999; Extavour et al., 2005; Rebscher et al., 2007), and in the embryonic mesodermal posterior growth zone and multiple somatic tissues of polychaetes (Rebscher et al., 2007; Dill and Seaver, 2008). Although ultimately there was no affect on somatic development, we speculate that the colony might regulate the number of progenitors as it asexually expands, and that vasa knockdown affects homeostasis. In previous experiments, it was found that the zooids appear to be monitoring growth of the buds, and, if delayed, they will undergo early takeover, presumably to transfer resources to the next generation (Tiozzo et al., 2008; Lauzon et al., 2002), so vasa+ cells might be involved in this process. This might also explain the dynamic expression of vasa in both fertile and infertile colonies, which in general resembles previous results that characterize telomerase activity during the budding cycle (Laird and Weissman, 2004).
In conclusion, given the experimental considerations mentioned above, we cannot yet rule out any germline defects. However, we did consistently observe a novel and robust phenotype of vasa knockdown: the breakdown of synchrony between zooid and buds, but the role vasa plays in these processes is not clear. Whether vasa is directly involved in regulating short- or long-range communication between cells and tissues (autocrine or endocrine) of the colony to trigger takeover, or whether vasa+ cell-specific defects, e.g. migration or determination defects, indirectly affect takeover remain open questions for future studies.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/20/3485/DC1
Supplementary Material
We would like to thank Karla Palmeri, Tanya McKitrick and Randy Will for their help and assistance. We thank Dr A. Sabbadin and Dr P. Burighel for valuable discussions for this manuscript. F.D.B. was supported by a predoctoral grant from the American Heart Association, by the Lerner Gray Fund for Marine Research from the American Museum of Natural History, and by the University of Washington Biology Department. S.T. was supported by a Stanford Dean's Fellowship. M.M.R. was supported by the NIH, individual NRSA (1F32GM086018-01). Part of this research was funded by a University of Washington Royalty Research Grant to B.J.S. This work was supported by the NIH (RO1A104588/R01DK405762) and the Ellison Medical Foundation to A.W.D. Deposited in PMC for release after 12 months.
References
- Blackstone, N. W. and Jasker, B. D. (2003). Phylogenetic considerations of clonality, coloniality, and mode of germline development in animals. J. Exp. Zoolog. B Mol. Dev. Evol. 297, 35-47. [DOI] [PubMed] [Google Scholar]
- Braat, A. K., van de Water, S., Korving, J. and Zivkovic, D. (2001). A zebrafish vasa morphant abolishes vasa protein but does not affect establishment of the germline. Genesis 30, 183-185. [DOI] [PubMed] [Google Scholar]
- Bourlat, S. J., Juliusdottir, T., Lowe, C. J., Freeman, R., Aronowicz, J., Kirschner, M., Lander, E. S., Thorndyke, M., Nakano, H., Kohn, A. B. et al. (2006). Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444, 85-88. [DOI] [PubMed] [Google Scholar]
- Boyd, H. C., Brown, S. K., Harp, I. L. and Weissman, I. L. (1986). Growth and sexual maturation of laboratory-cultured Monterey Botryllus schlosseri. Biol. Bull. 170, 91-109. [Google Scholar]
- Brown, F. D. and Swalla, B. J. (2007). Vasa expression in a colonial ascidian, Botrylloides violaceus. Evol. Dev. 9, 165-177. [DOI] [PubMed] [Google Scholar]
- Buss, L. W. (1982). Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. USA 79, 5337-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin, E. (1905). Mosaic development in ascidian eggs. J. Exp. Zool. 2, 145-223. [DOI] [PubMed] [Google Scholar]
- De Tomaso, A. W. and Weissman, I. L. (2003). Initial characterization of a protochordate histocompatibility locus. Immunogenetics 55, 480-490. [DOI] [PubMed] [Google Scholar]
- De Tomaso, A. W. and Weissman, I. L. (2004). Evolution of a protochordate allorecognition locus. Science 303, 977. [DOI] [PubMed] [Google Scholar]
- De Tomaso, A. W., Saito, Y., Ishizuka, K. I., Palmeri, K. K. and Weissman, I. L. (1998). Mapping the genome of a model urochordate. I. A low resolution genetic map encompassing the Fu/HC locus of Botryllus schlosseri. Genetics 149, 277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc, F., Brinkmann, H., Chourrout, D. and Philippe, H. (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965-968. [DOI] [PubMed] [Google Scholar]
- Dill, K. K. and Seaver, E. C. (2008). Vasa and nanos are coexpressed in somatic and germ line tissue from early embryonic cleavage stages through adulthood in the polychaete Capitella sp. I. Dev. Genes Evol. 218, 453-463. [DOI] [PubMed] [Google Scholar]
- Extavour, C. G., Pang, K., Matus, D. Q. and Martindale, M. Q. (2005). vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms. Evol. Dev. 7, 201-215. [DOI] [PubMed] [Google Scholar]
- Fujimura, M. and Takamura, K. (2000). Characterization of an ascidian DEAD-box gene, Ci-DEAD1: specific expression in the germ cells and its mRNA localization in the posterior-most blastomeres in early embryos. Dev. Genes Evol. 210, 64-72. [DOI] [PubMed] [Google Scholar]
- Imai, K. S., Levine, M., Satoh, N. and Satou, Y. (2006). Regulatory blueprint for a chordate embryo. Science 312, 1183-1187. [DOI] [PubMed] [Google Scholar]
- Izzard, C. S. (1968). Migration of germ cells through successive generations of pallial buds in Botryllus schlosseri. Biol. Bull. 135, 424. [Google Scholar]
- Johnson, S. L. and Yund, P. O. (2004). Remarkable longevity of dilute sperm in a free-spawning colonial ascidian. Biol. Bull. 206, 144-151. [DOI] [PubMed] [Google Scholar]
- Laird, D. J. and Weissman, I. L. (2004). Telomerase maintained in self-renewing tissues during serial regeneration of the urochordate Botryllus schlosseri. Dev. Biol. 273, 185-194. [DOI] [PubMed] [Google Scholar]
- Laird, D. J., De Tomaso, A. W. and Weissman, I. L. (2005). Stem cells are units of natural selection in a colonial ascidian. Cell 123, 1351. [DOI] [PubMed] [Google Scholar]
- Lauzon, R. J., Ishizuka, K. J. and Weissman, I. L. (2002). Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new: a model for development and regeneration. Dev. Biol. 249, 333-348. [DOI] [PubMed] [Google Scholar]
- Letunic, I., Copley, R. R., Pils, B., Pinkert, S., Schultz, J. and Bork, P. (2006). SMART 5, domains in the context of genomes and networks. Nucleic Acids Res. 34, D257-D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M., Hong, N., Xu, H., Yi, M., Li, C., Gui, J. and Hong, Y. (2009). Medaka vasa is required for migration but not survival of primordial germ cells. Mech. Dev. 126, 366-381. [DOI] [PubMed] [Google Scholar]
- Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402-408. [DOI] [PubMed] [Google Scholar]
- Mochizuki, K., Nishimiya-Fujisawa, C. and Fujisawa, T. (2001). Universal occurrence of the vasa-related genes among metazoans and their germline expression in Hydra. Dev. Genes Evol. 211, 299-308. [DOI] [PubMed] [Google Scholar]
- Mukai, H. (1977). Comparative studies on the structure of reproductive organs of four botryllid ascidians. J. Morphol. 152, 363-380. [DOI] [PubMed] [Google Scholar]
- Mukai, H. and Watanabe, H. (1976). Studies on formation of germ-cells in a compound ascidian Botryllus primigenus Oka. J. Morphol. 148, 337-362. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Sutasurya, L. A. (1979). Primordial germ cells in the chordates. Cambridge: Cambridge University Press.
- Nieuwkoop, P. D. and Sutasurya, L. A. (1981). Primordial Germ Cells in the Invertebrates: from epigenesis to preformation. Cambridge: Cambridge University Press.
- Pfister, D., De Mulder, K., Hartenstein, V., Kaules, G., Borgonie, G., Marx, F., Morris, J. and Ladurner, P. (2008). Flatworm stem cells and the germ line: developmental and evolutionary implications of macvasa expression in Macrostomum lignano. Dev. Biol. 319, 146-159. [DOI] [PubMed] [Google Scholar]
- Rebscher, N., Zelada-Gonzales, F., Banisch, T. U., Raible, F. and Arendt. D. (2007). Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev. Biol. 306, 599-611. [DOI] [PubMed] [Google Scholar]
- Rosner, A., Moiseeva, E., Rinkevich, Y., Lapidot, Z. and Rinkevich, B. (2009). Vasa and the germ line lineage in a colonial urochordate. Dev. Biol. 331, 113-128. [DOI] [PubMed] [Google Scholar]
- Sabbadin, A. (1953). Note preliminare sulle cellule del sangue di Botryllus schlosseri. Bulletin de l'istitut Oceanographique 50, 1-12. [Google Scholar]
- Sabbadin, A. and Zaniolo, G. (1979). Sexual differentiation and germ-cell transfer in the colonial ascidian Botryllus schlosseri. J. Exp. Zool. 207, 289-304. [Google Scholar]
- Santos, A. C. and Lehmann, R. (2004). Germ cell specification and migration in Drosophila and beyond. Curr. Biol. 14, R578-R589. [DOI] [PubMed] [Google Scholar]
- Sengoku, T., Nureki, O., Nakamura, A., Kobayashi, S. and Yokoyama, S. (2006). Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125, 287-300. [DOI] [PubMed] [Google Scholar]
- Shibata, N., Umesono, Y., Orii, H., Sakurai, T., Watanabe, K. and Agata, K. (1999). Expression of vasa(vas)-related genes in germline cells and totipotent somatic stem cells of planarians. Dev. Biol. 206, 73-87. [DOI] [PubMed] [Google Scholar]
- Shirae-Kurabayashi, M., Nishikata, T., Takamura, K., Tanaka, K. J., Nakamoto, C. and Nakamura, A. (2006). Dynamic redistribution of vasa homolog and exclusion of somatic cell determinants during germ cell specification in Ciona intestinalis. Development 133, 2683-2693. [DOI] [PubMed] [Google Scholar]
- Stoner, D. S. and Weissman, I. L. (1996). Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proc. Natl. Acad. Sci. USA 93, 15254-15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner, D. S., Rinkevich, B. and Weissman, I. L. (1999). Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. Natl. Acad. Sci. USA 96, 9148-9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanaga, T., Saito, Y. and Kawamura, K. (2006). Postembryonic epigenesis of Vasa-positive germ cells from aggregated hemoblasts in the colonial ascidian, Botryllus primigenus. Dev. Growth Differ. 48, 87-100. [DOI] [PubMed] [Google Scholar]
- Sunanaga, T., Satoh, M. and Kawamura, K. (2008). The role of Nanos homologue in gametogenesis and blastogenesis with special reference to male germ cell formation in the colonial ascidian, Botryllus primigenus. Dev. Biol. 324, 31-40. [DOI] [PubMed] [Google Scholar]
- Swalla, B. J. (2006). Building divergent body plans with similar genetic pathways. Heredity 97, 235-243. [DOI] [PubMed] [Google Scholar]
- Takamura, K., Fujimura, M. and Yamaguchi, Y. (2002). Primordial germ cells originate from the endodermal strand cells in the ascidian Ciona intestinalis. Dev. Genes Evol. 212, 11-18. [DOI] [PubMed] [Google Scholar]
- Tanaka, S. S., Toyooka, Y., Akasu, R., Katoh-Fukui, Y., Nakahara, Y., Suzuki, R., Yokoyama, M. and Noce, T. (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841-853. [PMC free article] [PubMed] [Google Scholar]
- Tiozzo, S. and De Tomaso, A. W. (2009). Functional analysis of Pitx during asexual regeneration in a basal chordate. Evol. Dev. 11, 152-162. [DOI] [PubMed] [Google Scholar]
- Tiozzo, S., Voskoboynik, A., Brown, F. D. and De Tomaso, A. W. (2008). A conserved role of the VEGF pathway in angiogenesis of an ectodermally-derived vasculature. Dev. Biol. 315, 243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M. and Okada, T. (1999). Origin of the gonad in the juvenile of a solitary ascidian, Ciona intestinalis. Dev. Growth Differ. 41, 73-79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.