Abstract
Vascular functions of PlGF remain poorly understood and controversial. Here, we show that tumor cell-derived PlGF-1 and PlGF-2 displayed significant remodeling effects on the tumor vasculature, leading to a normalized vascular phenotype and improved functions against leakage. In two murine tumor models, that is, T241 fibrosarcoma and Lewis lung carcinoma, stable expression of PlGF-1 and PlGF-2 in tumor cells resulted in significant reduction of tumor microvascular density and branch formation. Markedly, the vasculature in PlGF-expressing tumors consisted of relatively large-diameter microvessels with substantial improvement of pericyte coverage. Similarly, PlGF-induced vascular normalization and remodeling were also observed in a spontaneous human choriocarcinoma that expressed endogenous PlGF. Our findings shed light on functions of PlGF as a vascular remodeling factor that normalizes the tumor vasculature and thus may have conceptual implications of cancer therapy.
Keywords: vascular permeability, vascular remodeling
Malignant tissues produce multiple angiogenic factors to induce neovascularization, which significantly contributes to tumor growth and metastasis (1–6). Among known tumor angiogenic factors, VEGF-A remains one of the best-characterized angiogenic factors and as the key therapeutic target for antiangiogenic therapy against malignant and non-malignant diseases (7–13). VEGF-A displays a broad spectrum of biological functions including stimulation of angiogenesis, vasculogenesis, vascular permeability, neuroprotective activity, inflammation, and hematopoiesis (7, 14–16). VEGF-A displays these diverse biological functions by activation of its receptors distributed on endothelial and non-endothelial cells (7, 17). Although VEGF-A binds to VEGFR1 at a higher affinity, it is generally believed that VEGFR2 is the functional receptor that mediates both angiogenic and vascular permeability signals (18). In contrast to VEGFR2, accumulating evidence showed that VEGFR1 may mediate negative signals for angiogenesis and vascular leakage (7, 19). In addition to the tyrosine kinase receptors, VEGF-A also binds to neuropilin-1 and -2, which are involved in the guidance of vessel formation and modulate vascular functions mediated by the high affinity tyrosine kinase receptors (20–24).
In contrast to VEGF-A, PlGF together with VEGF-B belong to a subgroup of ligands within the VEGF family, which only binds to VEGFR1 (25–28). Consistent with the receptor binding patterns, PlGF and VEGF-B do not seem to display significant physiological functions. For example, plgf or vegf-b-null mice develop normally and lack obvious vascular and non-vascular defects during the adulthood (29, 30). However, recent studies show that PlGF might significantly contribute to pathological angiogenesis such as tumor neovascularization, vascular regeneration under tissue ischemia, and wound healing (31–34). Although molecular mechanisms underlying distinct roles of PlGF in regulation of pathological angiogenesis remain poorly understood, these studies suggest that PlGF might be an attractive target for cancer therapy (31, 33).
In addition to its positive roles in regulation of pathological angiogenesis, PlGF has also been reported as a negative regulator of tumor angiogenesis and tumor growth (7, 35–38). The mechanism of negative regulation of angiogenesis involves the formation of VEGF-PlGF heterodimers that do not display significantly angiogenic activity relative to VEGF homodimers (7, 39). Four isoforms of human PlGF and six isoforms of human VEGF-A generated by alternative splicing from the same genes could potentially form 24 different heterodimers with different affinities to heparan sulfate proteoglycans, and thus create complex gradients around their producing cells (7). In both xenograft and spontaneous tumor models, expression of PlGF in tumor cells leads to suppression of tumor angiogenesis and tumor growth (35–38). These findings support the notion of PlGF might negatively regulate tumor angiogenesis.
Vascular remodeling is crucial for controlling blood perfusion, vascular permeability, interstitial fluid pressure, tissue hypoxia, tumor growth, and metastasis (40–46). Regulation of vascular remodeling is an important approach for cancer therapy. For example, anti-VEGF agents can significantly normalize the tumor vasculature and may increase chemotherapeutic drug delivery when combined with cytostatic agents (41, 47). Similarly, the notch signaling pathway commits vessels to arterial differentiation and markedly regulates vascular remodeling (40, 42–45). In preclinical models, targeting notch ligands and their receptors have proven to be an effective approach for cancer therapy (40, 42–45). In the present study, we report that PlGF acts as a vascular remodeling factor that markedly normalizes the tumor vasculature. In both genetically modified and natural occurring tumors, PlGF significantly remodels the tumor vasculature toward large and well-organized blood vessels, which are virtually all coated with mural cells. Thus, modulation of PlGF-induced vascular remodeling activity in the tumor environment may be crucial for development of therapeutic agents in combination with currently-available anti-VEGF drugs for cancer treatments.
Results
PlGF-1 and PlGF-2 Promotes Vascular Normalization and Remodeling in a Murine Fibrosarcoma Model.
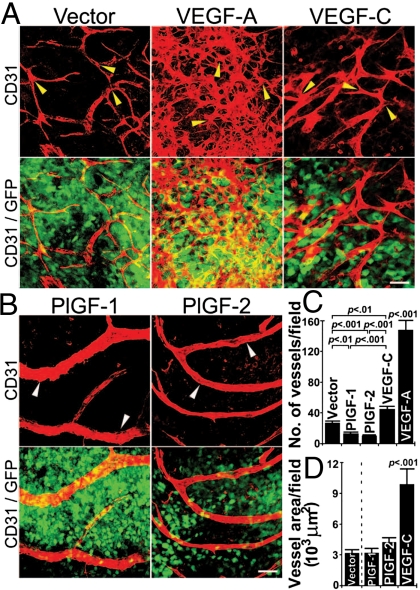
To study vascular remodeling effects of various members in the VEGF family in tumors, we generated murine T241 fibrosarcoma and Lewis lung carcinoma (LLC) cell lines that stably expressed human PlGF-1, PlGF-2, and VEGF165 and expression levels of these cytokines were equivalent (35, 36, 48, 49). Additionally, the mature form of VEGF-C was also overexpressed in T241 fibrosarcoma. Consistent with our previous reports, s.c. implantation of PlGF-1- and PlGF-2-expressing tumor cells resulted in delayed tumor growth rates (35, 36, 49). In contrast to PlGF tumors, implantation of both VEGF-A- and VEGF-C-expressing tumor resulted in significantly accelerated tumor growth rates (36, 48, 49). To study vascular remodeling effects, tumors from different groups were grown to a similar size and tumor vasculatures were detected using a whole-mount technique with anti-CD31 immunostaining. As expected, VEGF-A induced a robust angiogenic response in tumors and VEGF-A-induced tumor vasculature appeared as primitive and disorganized vascular plexuses owing to extensive sprouting and fusion of microvessels (Fig. 1 A and C). Similarly, VEGF-C also significantly increased tumor vessel density and tortuosity although these effects were not as pronounced as VEGF-A (Fig. 1 A and D).
Fig. 1.
Comparison of tumor vasculatures induced by members in the VEGF family. (A) Vector-, VEGF-A165- and VEGF-C-expressing tumor tissues were immunostained using anti-CD31 (red) and tumor cells expressed enhanced green flurorescent (green). Arrowheads point to representative tumor microvessels. (Scale bar, 50 μm.) (B) PlGF-1- and PlGF-2-expressing tumors were immunostained using anti-CD31 (red) and tumor cells expressed enhanced green flurorescent (green). Arrowheads point to representative tumor microvessels. (Scale bar, 50 μm.) (C) Quantification of microvessel density in T241-vector, -VEGF-A, -VEGF-C, -PlGF-1 and PlGF-2 tumors using 20× magnification (n = 4–16 per group). (D) Quantification of microvessel density in T241-vector, -PlGF-1, -PlGF-2, and -VEGF-C tumors using 20× magnification (n = 4–16 per group), and the data were presented as mean (± SEM.).
In marked contrast to VEGF-A and -C, both PlGF-1- and PlGF-2-induced tumor vasculatures appeared as normalized vascular networks that consisted of relatively large microvessels with fewer branches relative to vector-, VEGF-A- and VEGF-C-induced vasculatures (Figs. 1B and 2F). It should be emphasized that the total number of vascular branches in the VEGF-A-induced vasculature were under estimated owing to the high density and fusion of microvessels. Intriguingly, total numbers of PlGF-1- and PlGF-2-induced vessels were significantly decreased compared with vector tumors, suggesting that PlGFs significantly remodel tumor vessels (Fig. 1C). However, PlGF-stimulated vessels exhibited an arteriole-like phenotype, which contained relatively large lumens and fewer branching points (Fig. 1B). These findings demonstrate that PlGF significantly remodels and normalizes tumor vascultures.
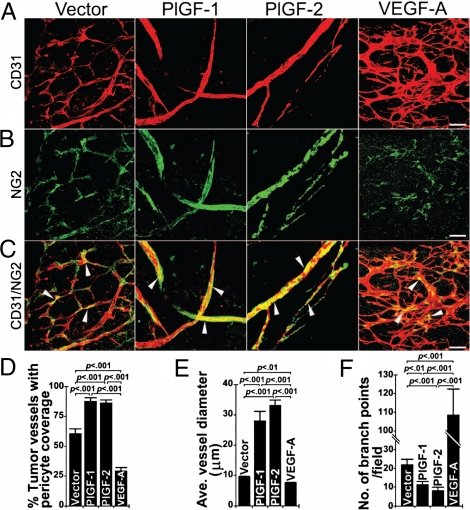
Fig. 2.
Pericyte coverage in tumor vasculatures. (A–C) T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A tumors were double immunostained using anti-CD31 (red) and anti-NG2 (green). Arrowheads indicate vasculature-associated pericytes. (Scale bar, 50 μm.) (D) Quantification of pericyte coverage in T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A tumor vasculatures (n = 13–17 per group). All quantifications were performed using the 20× magnification. (E) Quantification of average diameters of tumor microvessels in T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A groups (n = 6–10 per group). (F) Quantification of vascular branches of tumor microvessels in T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A groups (n = 13–14 per group). All quantifications were performed using the 20× magnification, and the data were presented as mean (± SEM.).
Improvement of Pericyte Association in the PlGF Tumor Vasculature.
Vascular remodeling effects of tumor-derived PlGF suggested structural and functional alterations of tumor vessels. Among several other unique features, tumor vasculatures usually lack appropriate coverage with pericytes (41, 46, 47). Consistent with this notion, immunohistological detection of pericytes by NG2 staining revealed that only less than 60% of the tumor vessels were covered by NG2+ pericytes in vector control tumors (Fig. 2 A–D). In contrast, pericyte coverage was significantly increased in PlGF-1 and PlGF-2 tumor vasculatures relative to the control group (Fig. 2 A–D). Inversely, the total number and coverage of pericytes were significantly reduced in VEGF-A-induced tumor vasculature. Increased average vessel diameters and pericyte coverage as well as reduction of branching formation further validate that PlGF acts as a vascular normalization factor in the tumor environment (Fig. 2 A–F).
Prevention of Vascular Leakage.
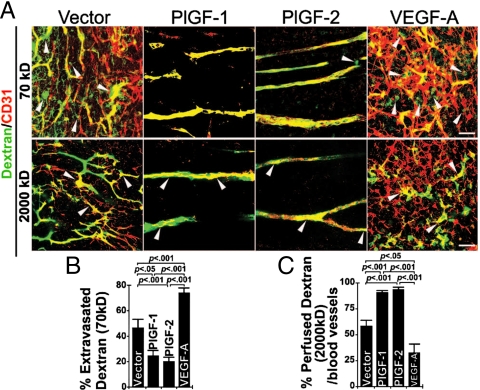
Improvement of pericyte coverage by tumor-derived PlGF may affect vascular perfusion and leakage. To study vascular perfusion and leakiness, we intravenously injected tumor-bearing mice with lysine-fixable tetramethylrhodamine dextran (LRD) of 2,000 kDa and 70 kDa, respectively. As expected, microvessels in vector control tumors were highly permeable and a significant amount of injected 70 kDa LRD were extravasated into the extravascular space 15 min after injection (Fig. 3 A and B). In striking contrast, PlGF-1- and PlGF-2-induced tumor vasculatures were protected against leakage and only negligible amounts of 70 kDa LRD were located in the extravascular space (Fig. 3 A and B). Inversely, the VEGF-A induced tumor vasculature were highly permeable, and approximately 80% of the injected 70 kDa LRD were extravasated 15 min after injection. These findings demonstrate that PlGF-1 and PlGF-2 protect tumor vessels against leakage and further support the notion that PlGF normalizes both structures and functions of the tumor vasculature.
Fig. 3.
Tumor vascular perfusion and leakiness. (A) T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A tumor-bearing mice were intravenously injected with LRD (lysinated rhodamine-labeled dextran, 70 kDa, or 2,000 kDa). Extravasation of 70 kDa LRD (green fluorescence) was analyzed at 5 or 15 min respectively after injection. Tumor blood vessels were revealed by immunostaining using anti-CD31 (red). Arrowheads indicate extravasation of LRD from the tumor vasculature. Overlapping yellow indicates intravascular LRD. (Scale bar, 50 μm.) (B) Quantification of LRD extravasation in T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A tumors using 20× magnification (n = 6–9 per group). (C) Quantification of 2,000 kDa LRD perfusion in T241-vector, T241-PlGF-1, T241-PlGF-2, and T241-VEGF-A tumors using 20× magnification (n = 4–6 per group), and the data were presented as mean (± SEM.).
To further study vascular functions, we injected 2,000 kDa LRD, which allowed us to study tumor vessel perfusion without leaking into the extravascular space (50). Interestingly, nearly 100% of microvessels in PlGF-1 and PlGF-2 tumors were perfused with 2,000 kDa LRD 5 min postinjection whereas less than 60% of the control vector tumor vascular network was perfused. In contrast, only less than 30% of blood vessels were perfused in VEGF-A tumors, suggesting that VEGF-A promoted non-productive vessel formation (Fig. 3 A and C).
Vascular Normalization in PlGF-Producing Mouse Lewis Lung and Human Tumors.
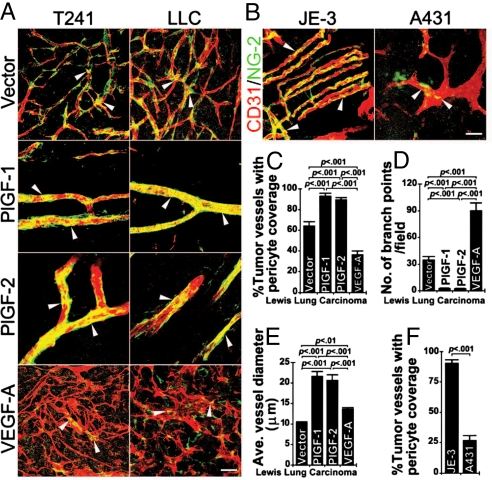
To exclude the possibility that vascular remodeling and normalization by tumor-derived PlGF only occurs in the murine fibrosarcoma model, we generated murine LLC cell lines that stably expressed PlGF-1 or PlGF-2. Similar to murine T241 fibrosarcomas, expression of PlGF-1 and PlGF-2 in LLC led to marked vascular normalization and remodeling activity, resulting in formation of relatively large and well-organized vascular networks with substantial improvement of pericyte coverage (Fig. 4 A and C). In addition, average diameters of the PlGF-1 and PlGF-2 LLC tumor vessels were significantly enlarged relative to the vector control group (Fig. 4 A and E). Inversely, highly disorganized and primitive tumor vascular networks were present in VEGF-A-LLC tumors, which contained NG2+ signals that remained dissociated from the tumor vasculature (Fig. 4A). These findings show that vascular normalization and remodeling effects of PlGF can be generalized in epithelial, mesenchymal and probably other cell type-originated tumors.
Fig. 4.
PlGF-indcued vascular remodeling and normalization in LLC and human tumors. (A) T241-vector, T241-PlGF-1, T241-PlGF-2, T241-VEGF-A, LLC-vector, LLC-PlGF-1, LLC-PlGF-2, and LLC-VEGF-A tumors were double immunostained using anti-CD31 (red) and anti-NG2 (green). Arrowheads point to representative pericyte coverage in tumor vessels. (Scale bar, 50 μm.) (B) Human PlGF-expressing JE-3 choriocarcinoma and PlGF-negative A431 squamous carcinoma were double immunostained using anti-CD31 (red) and anti-NG2 (green). Arrowheads point to representative pericyte coverage in tumor microvessels. (Scale bar, 50 μm.) (C) Quantification of pericyte coverage in LLC-vector, LLC-PlGF-1, LLC-PlGF-2, and LLC-VEGF-A tumor vasculatures (n = 6–10 per group). (D) Quantification of microvessel branches in LLC-vector, LLC-PlGF-1, LLC-PlGF-2, and LLC-VEGF-A tumors (n = 7–10 per group). (E) Quantification of average diameters of tumor microvessels in LLC-vector, LLC-PlGF-1, LLC-PlGF-2, and LLC-VEGF-A groups (n = 6–10 per group). (F) Quantification of pericyte coverage of microvessels in JE-3 choriocarcinoma and A431 squamous carcinoma (n = 12–13 per group). All quantifications were performed using the 20× magnification, and the data were presented as mean (± SEM.).
To further study pathophysiological relevance of our findings to human tumors, we analyzed naturally occurring high and low PlGF-producing human tumors. Our previous study showed that JE-3 choriocarcinoma produced 7.1 ng/mL PlGF homodimers, 0.82 ng/mL VEGF-A homodimers, and 8.9 ng/mL PlGF-VEGF-A heterodimers in 48 h conditioned medium (39). Under the identical condition, A431 squamous carcinoma barely produced detectable levels of PlGF homodimers (0.11 ng/mL), VEGF-A homodimers (6.0 ng/mL), and PlGF-VEGF-A heterodimers (0.025 ng/mL) (39). Intriguingly, the vasculature in JE-3 tumors appeared as relatively well-organized vascular networks that resembled those found in PlGF-1- and PlGF-2-transfected murine T241 and LLC tumors (Fig. 4B). In contrast, the A431 tumor vasculature appeared as disorganized, and primitive vascular networks often seen in a majority of other malignant tissues (Fig. 4B). Consistent with vascular architectures, a high numbers of JE-3 tumor vessels were covered by pericytes, whereas A431 tumor vasculatures lacked significant coverage of pericytes (Fig. 4 B and F). Similar to mouse tumors, PlGF-expressing JE-3 choriocarcinomas lacked obvious vascular branches as seen in PlGF negative A431 tumors (Fig. 4 A and F). These data demonstrate that PlGF-induced normalization and vascular remodeling occur in non-genetically manipulated human tumors.
Normalization of the Tumor Vasculature by an Intracellular PlGF.
To further study the role of PlGF in normalization of tumor vessels, we generated a genetically modified PlGF containing an endoplasmic reticulum (ER) retention signal at its C terminus (35). This ER-retaining PlGF could effectively form heterodimers with VEGF-A and thus antagonized VEGF-A-induced tumor angiogenesis and tumor growth (35). However, a significant amount of PlGF homodimers and PlGF-VEGF-A heterodimers were leaked from ER and eventually became secreted (35). Interestingly, vascular networks in PlGF-KDEL tumors also exhibited a normalized phenotype as seen in those of authentic PlGF-1- and PlGF-2-expressing tumors (Fig. S1 A–C). Consistent with the vascular normalization effect, diameters and pericyte coverage in PlGF-KDEL tumor vasculatures were significantly increased (Fig. S1 A–E). It should be emphasized that a significant increase of pericyte coverage was found in PlGF-KDEL tumor vasculature relative to PlGF-1 and PlGF-2 tumor vasculatures (Fig. S1D). Additionally, average vessel diameters in PlGF-KDEL-T241 tumor were significantly increased relative to those in vector tumors although they were indistinguishable from PlGF-1- and PlGF-2- induced tumor vasculature(Fig. S1E). Intriguingly, microvascular networks in PlGF-KDEL tumors contained a significantly reduced number of vascular branches relative to those in vector tumors (Fig. S1F).
VEGFR1 Blockade Affects Vessel Diameter But Not Vascular Remodeling.
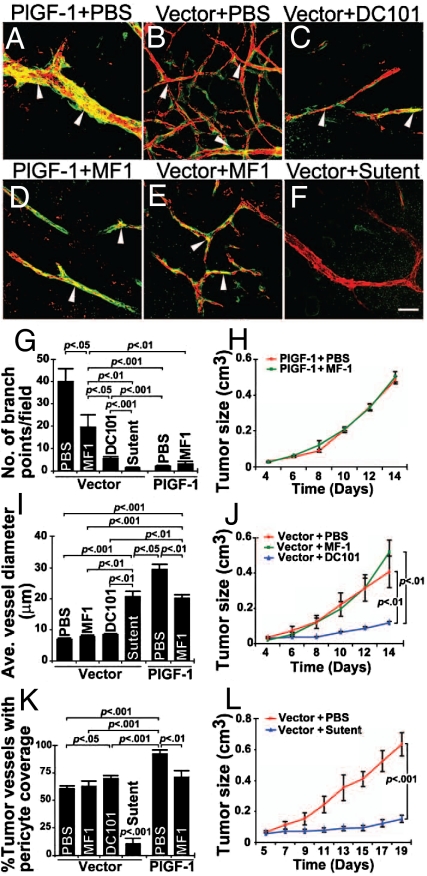
To further elucidate the role of VEGFR1 in mediating PlGF-induced vascular remodeling and normalization, T241-PlGF-1 tumor-bearing mice were systemically treated with a neutralizing antibody against mouse VEGFR1 (MF-1) (49, 51). Interestingly, VEGFR1 blockade did not significantly reduced tumor growth rates and branch formation (Fig. 5A, D, G, and J). However, average diameters of microvessels and pericyte coverage in VEGFR1 blockade-treated tumors were significantly smaller relative to those in PBS-treated group (Fig. 5 A, D, I, and K). Intriguingly, VEGFR1 blockade significantly inhibited sprouting of vector tumor vascular networks whereas pericyte coverage, tumor growth rates and vessel diameters remained unaffected (Fig. 5 B, E, G, and I–K). Inversely, treatments of vector tumors with a neutralizing antibody against mouse VEGFR2 (DC101) or sunitinib, a VEGFR2 tyrosine kinase inhibitor, resulted in significant normalization of tumor vasculatures and decreased tumor growth rates (Fig. 5 C, F, I, and K). Markedly, sunitinib but not DC101 treatment resulted in significant loss of vascular pericytes (Fig. 5 B, F, and K) probably due to its broad inhibitory effects on other membrane tyrosine kinase receptors such as platelet-derived growth factor receptor-β (PDGFR-β), which is known to mediate pericyte recruitment onto angiogenic vessels. Morphologically, VEGFR2 blockade-treated tumor vasculature and PlGF-induced vascular networks appeared similar. A marked difference of tumor vessel diameters were observed between the two groups (Fig. 5 A, C, and I), suggesting that PlGF might activate VEGFR1 that transduces active signals for vascular remodeling and dilation.
Fig. 5.
Treatment of vector-T241 and PlGF-T241 tumors with VEGFR blockades. (A and D) PlGF-1-T241 tumors were treated with or without anti-VEGFR1 and pericyte coverage in tumor vessels were detected by anti-CD31 (red) and anti-NG2 (green). (B, C, E, and F) Vector tumors were treated with PBS, anti-VEGFR1 (MF1), anti-VEGFR2 (DC101), or sunitinib (sutent) and tumor vasculatures were detected using anti-CD31 (red) and NG2 staining (green). Arrowheads indicate vasculature-associated pericytes. (Scale bar, 50 μm.) (G) Quantification of vascular branches of tumor microvessels in various treated and non-treated groups. (n = 5–9 per group). (H) Tumor growth rates of T241-PlGF-1 fibrosarcoma treated with MF-1 (green line) or PBS (red line) (n = 6 mice per group). (I) Quantification of average diameters of tumor microvessels in various treated and non-treated groups (n = 5–11 per group). (J) Tumor sizes of T241-vector fibrosarcoma treated with MF-1 (green line), DC101 (blue line), or PBS (red line) (n = 6 mice per group). (K) Quantification of pericyte coverage in various vector and PlGF tumor vasculatures treated with various anti-VEGF agents (n = 5–10 per group). All quantifications were performed using the 20× magnification. (L) Tumor sizes of T241-vector fibrosarcoma treated with sunitinib (blue line) or PBS (red line) (n = 8 mice per group) and the data were presented as mean (± SEM.).
Discussion
Despite its early identification in 1991, vascular functions of PlGF have remained largely uncharacterized owing to its specific binding to VEGFR1 but not VEGFR2 (7, 19, 28). VEGFR1 mediates both positive and negative signals for regulation of angiogenesis and deletion of the vegfr1 gene mice resulted in the formation of disorganized vasculature owing to uncontrollable overgrowth of endothelial cells into vascular lumens, leading to dysfunction of the vasculature and early embryonic lethality (52). This genetic study provides compelling evidence that VEGFR1-mediated signaling pathways are involved in negative regulation of angiogenesis and are committed to blood vessel organization and vascular remodeling. Consistent with these genetic findings, VEGFR1 blockade could further facilitate neovascularization. For example, inhibition of VEGFR1 in adipose tissues results in accelerated angiogenesis rather than suppression of neovascularization (51). These findings show the primary function of VEGFR1 as a negative modulator of angiogenesis.
Reconciling these data with the present study, we show that tumor-derived PlGF significantly inhibits tumor angiogenesis as reflected by reduction in tumoral vascular density. One of the possible mechanisms underlying suppression of tumor neovascularization involves the formation of angiogenically-inactive PlGF-VEGF heterodimers as previously reported (35–38). However, active secretion of significant amounts of PlGF homodimers and PlGF-VEGF-A heterodimers would still activate VEGFR1. If VEGFR1 mediates positive angiogenic signals, why do PlGF-expressing tumors have decreased vascular density? Our results suggest that activation of VEGFR1 by PlGF leads to negative regulation of angiogenesis despite of neutralization of VEGF-A by the formation of PlGF-VEGF-A heterodimers.
One of our most surprising findings in the present study is that PlGF expression in the tumor environment leads to extraordinary vascular remodeling by the formation of well-organized, large diameter, and pericyte-enriched vascular networks, which resemble those in healthy tissues. This PlGF-induced vascular remodeling effect resembles activation of the Dll-4-Notch signaling pathways, which define the arteriole identity and restrict vascular sprouting (40, 42–45, 53). Consequently, inhibition of Notch ligands and receptors resulted in generation of a disorganized tumor vasculature that remain poorly perfused and functionally non-productive (40, 42–44, 54). In contrast to Notch inhibitors, administration of a VEGFR1 blocking agent does not affect PlGF-induced vascular remodeling and normalization in tumors, suggesting different mechanisms of Notch- and PlGF-induced vascular remodeling and normalization. In addition to VEGFR1, PlGF-2 also binds to neuropilin-1, which may also be involved in vascular normalization. The fact that PlGF-1 displays indistinguishable vascular normalization from PlGF-2 argues against this possibility. Our findings are also physiopathologically relevant in that a natural occurring PlGF-producing choricarcinoma also display a normalized vascular phenotype, which may explain why this tumor is more sensitive to chemotherapy (55).
PlGF-induced vascular remodeling and normalization also resemble the tumor vascular phenotype primed by anti-VEGF-A treatment. Functional blockade of VEGF signaling pathways by administration of anti-VEGF-A or anti-VEGFR2 neutralizing antibodies in tumor-bearing mice results in normalization of disorganized and tortuous tumor vasculatures toward a normalized phenotype (41, 47, 56). In certain types of tumors, anti-VEGF-induced vascular normalization decreased permeability and blood perfusion in the tumor vasculature (41, 56). Increasing blood perfusion in normalized tumor vessels would in theory be translated into accelerated tumor growth rates. However, both preclinical and clinical data gained from anti-VEGF therapy showed the opposite effect, suggesting normalization of tumor vessels may not necessarily increase tumor growth. Inversely, anti-VEGF therapy has recently been reported to induce tumor tissue hypoxia, leading to invasive and metastatic phenotypes in experimental tumor models (57, 58). These paradoxical issues may only reflect a tiny part of the complex mechanisms underlying anti-VEGF therapy. Anti-VEGF drug-induced tissue hypoxia could further alter expression levels of other vascular modulators. For example, bevacizumab-induced tumor hypoxia could potentially upregulate the expression levels of Ang-2, which promotes torturosity rather than normalization of blood vessels. Based on clinical benefits of anti-VEGF drugs in combination with chemotherapy, it has been proposed that vascular normalization may increase cytostatic drug delivery (41). Thus, vascular normalization is one of the plausible mechanisms by which anti-VEGF drugs produce clinical benefits. If so, PlGF-normalized tumor vasculatures might also be prone to increased efficiency of chemotherapy and PlGF might be act as a chemo-sensitizer for cancer therapy. This possibility warrants further investigation.
In support of our findings, recently two independent studies showed that overexpression of PlGF in xenograft and spontaneous mouse tumor models leads to reduction of tumor neovascularization and tumor growth (37, 38). These results validate our early finding that PlGF downregulates VEGF-A-induced angiogenesis and tumor growth by formation of angiogenically inactive PlGF-VEGF heterodimers. In contrast to these findings, a recent report shows that PlGF significantly contributes to pathological angiogenesis and tumor growth, and PlGF blockade significantly inhibits tumor growth (31). Although the mechanisms responsible for the discrepancy between these studies is unclear, it is possible that PlGF and VEGF-A were produced in different cell populations and they do not form heterodimers. The heterodimeric issue was not investigated in that study (31).
Taken together, our results provide compelling evidence that tumor-produced PlGF displays significant effects on vascular remodeling and normalization, which may significantly alter tumor growth rates and probably drug sensitivity. Thus, modulation of PlGF levels and function in the tumor environment could be potentially be used as a therapeutic approach for cancer therapy.
Methods
Animals.
All animal studies were reviewed and approved by the Animal care and use committee of the North Stockholm Animal Board (Stockholm, Sweden).
In subsets of experiments, tumor-bearing mice were randomly divided into different groups (n = 6–8 per group) and received treatment with VEGFR1, VEGFR2 or VEGFR1/VEGFR2 blockades as previous described (49, 51). The treatment started at day 0 after tumor implantation and administration of anti-VEGFR1 (MF1, Imclone Inc., 600 μg/mouse) or anti-VEGFR2 (DC101, Imclone Inc., 600 μg/mouse) twice a week for a total of 2-week therapy. For sunitinib treatment, tumor-bearing mice daily received oral administration at the dose of 1.2 mg/mouse for a total of 18 days. At the end points of treatments, mice were killed and tumors were removed.
Vascular Permeability and Perfusion.
Lysine-fixable tetramethylrhodamine dextran (LRD) at molecular weight of 70 kDa or 2,000 kDa (100 μL, Invitrogen) was injected into the tail vein of each tumor-bearing mouse at the tumor size of approximately 0.8 cm3. Five to fifteen minutes after injection, mice were killed and tumor tissues were immediately fixed with 4% PFA overnight. Some of the fixed tissues were further immunostained with anti-CD31 for detection of tumor blood vessels. Extravasation of 70 kDa LRD and perfusion of 2,000 kDa LRD were detected by confocal microscopy (Nikon or Zeiss). The leaked and vascular-remaining LRD were quantified from 8 randomized fields (20×) of each group using a digital program. Data represent averages of mean determinants (± SEM).
Whole-Mount Staining.
Whole-mount immunostaining of tumor tissues were performed as previously described (48, 49, 51).
Statistical Analysis.
Statistical analyses of 3-D images were performed using two-tailed Student's t test in Microsoft Excel 2003. P < 0.05, P < 0.01, and P < 0.001 were deemed as significant, highly significant, and extremely significant, respectively.
Acknowledgments.
We thank Dr. Zhenping Zhu at the Imclone Inc. for providing anti-VEGFR1 and anti-VEGFR2 antibodies and Dr. Gianluca Canettieri at the University of Rome, Italy for providing JE-3 human cells. This work was supported by the laboratory of Y.C. through research grants from the Swedish Research Council, The Swedish Heart and Lung Foundation, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska gender foundation, and the Torsten and Ragnar Söderberg's Foundation. Y.C. is a Chang Jiang Scholar at the Shandong University, China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908026106/DCSupplemental.
References
- 1.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009 doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 5.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocak I, et al. The biologic basis of in vivo angiogenesis imaging. Front Biosci. 2007;12:3601–3616. doi: 10.2741/2337. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF. VPF/VEGF and the angiogenic response. Semin Perinatol. 2000;24:75–78. doi: 10.1016/s0146-0005(00)80061-0. [DOI] [PubMed] [Google Scholar]
- 9.Gragoudas ES, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrook R. The price of sight—ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–1412. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 14.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 16.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 20.Caunt M, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Favier B, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 22.Migdal M, et al. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 23.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Soker S, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Ji WR, Qi P, Rosin A. Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem Biophys Res Commun. 1997;235:493–498. doi: 10.1006/bbrc.1997.6813. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C, and VEGF-D. Int J Biochem Cell Biol. 2001;33:421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maglione D, et al. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aase K, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 31.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Hattori K, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RK, Xu L. alphaPlGF: A new kid on the antiangiogenesis block. Cell. 2007;131:443–445. doi: 10.1016/j.cell.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Luttun A, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 35.Bjorndahl M, Cao R, Eriksson A, Cao Y. Blockage of VEGF-induced angiogenesis by preventing VEGF secretion. Circ Res. 2004;94:1443–1450. doi: 10.1161/01.RES.0000129194.61747.bf. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson A, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 37.Schomber T, et al. Placental growth factor-1 attenuates vascular endothelial growth factor-A-dependent tumor angiogenesis during beta cell carcinogenesis. Cancer Res. 2007;67:10840–10848. doi: 10.1158/0008-5472.CAN-07-1034. [DOI] [PubMed] [Google Scholar]
- 38.Xu L, et al. Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res. 2006;66:3971–3977. doi: 10.1158/0008-5472.CAN-04-3085. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, et al. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 40.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 41.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 42.Noguera-Troise I, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 43.Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 44.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 45.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 46.Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci. 2009;14:3962–3973. doi: 10.2741/3504. [DOI] [PubMed] [Google Scholar]
- 47.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao R, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA. 2008;105:18513–18518. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Overholser KA, et al. Pulmonary vascular resistance distribution and recruitment of microvascular surface area. J Appl Physiol. 1994;77:845–855. doi: 10.1152/jappl.1994.77.2.845. [DOI] [PubMed] [Google Scholar]
- 51.Xue Y, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 53.Bhandarkar SS, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JL, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 55.Arbiser JL, Arbiser ZK, Majzoub JA. Regulation of gene expression in choriocarcinoma by methotrexate and hydroxyurea. Endocrinology. 1991;128:972–978. doi: 10.1210/endo-128-2-972. [DOI] [PubMed] [Google Scholar]
- 56.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]