Abstract
Peptides derived from exogenous proteins are presented by both MHC class I and II. Despite extensive study, the features of the endocytic pathway that mediate cross-presentation of exogenous antigens on MHC class I are not entirely understood and difficult to generalize to all proteins. Here, we used dendritic cells and macrophages to examine MHC class I and II presentation of hen egg-white lysozyme (HEL) in different forms, soluble and liposome encapsulated. Soluble HEL or HEL targeted to a late endosomal compartment only allowed for MHC class II presentation, in a process that was blocked by chloroquine and a cathepsin S (CatS) inhibitor; brefeldin A (BFA) also blocked presentation, indicating a requirement for nascent MHC class II. In contrast, liposome-encapsulated HEL targeted to early endosomes entered the MHC class I and II presentation pathways. Cross-presentation of HEL in early endosomal liposomes had several unique features: it was markedly increased by BFA and by blockade of the proteasome or CatS activity, it occurred independently of the transporter associated with antigen processing but required an MHC class I surface-stabilizing peptide, and it was inhibited by chloroquine. Remarkably, chloroquine facilitated MHC class I cross-presentation of soluble HEL and HEL in late endosomal liposomes. Altogether, MHC class I and II presentation of HEL occurred through pathways having distinct molecular and proteolytic requirements. Moreover, MHC class I sampled antigenic peptides from various points along the endocytic route.
Keywords: cross-presentation, endosome, liposomes
Dendritic cells (DCs) and macrophages (Mφs) present peptides derived from exogenous antigens on MHC class I and II (1). The subcellular localization of the antigen processing and MHC loading steps determines the molecular and proteolytic requirements for generation of a particular peptide (2, 3). In the MHC class II pathway, there is general agreement on the roles for protein synthesis, invariant chain, DM, and endosomal acidification for protein presentation, which differ from those of peptide presentation (1). Presentation by MHC class I (i.e., cross-presentation) involves unique antigen transport through the endocytic route that may or may not engage the classical MHC class I pathway (4). For example, the generation of Kb-SIINFEKL complexes from ovalbumin (Ova), the most frequently tested protein, required specific components of the MHC class I processing machinery contingent on the form of antigen and the nature of the presenting cell (5, 6); some forms of Ova entered the cytosol for proteasomal processing (6–10), whereas others did not (5, 11). Although several recent reports described endosomes/phagosomes facilitating both MHC class I and II presentation, it is unclear if these compartments are unique or even extant (12).
Previous studies by our laboratory demonstrated that protein encapsulation in liposomes enabled antigen targeting to specific endosomal compartments for processing and presentation on MHC class I and II (13, 14). Liposomes composed of dioleoyl-phosphatidylcholine (DOPC) and dioleoyl-phosphatidylserine (DOPS) were stable at acidic pH and released their contents via enzymatic degradation of the liposome membrane; these acid-resistant liposomes delivered antigen to late endosomes or lysosomes, facilitating efficient MHC class II presentation. In contrast, liposomes composed of dioleoyl-phosphatidylethanolamine (DOPE) and cholesteryl hemisuccinate (CHEMS) released their contents at pH <6.5; these acid-labile liposomes delivered antigen to early endosomes and allowed for presentation on both MHC class I and II. By targeting specific endosomes, liposome-encapsulated antigens allow us to examine several aspects of MHC class I and II presentation from different compartments: Is there a mechanistic difference between MHC class II presentation of the same epitope from early and late endosomal compartments? Does MHC class I cross-presentation occur from early or late endosomes, and, if so, what are the requirements? Here, we find distinct requirements for MHC class I and II presentation depending on the form of hen egg-white lysozyme (HEL) offered to the antigen-presenting cell (APC). In addition, HEL and Ova entered remarkably different cross-presentation pathways, implying mechanistic and anatomical heterogeneity among the endosomes that support cross-presentation.
Results
Dextrans in DOPC/DOPS and DOPE/CHEMS Liposomes Enter Distinct Endosomal Compartments.
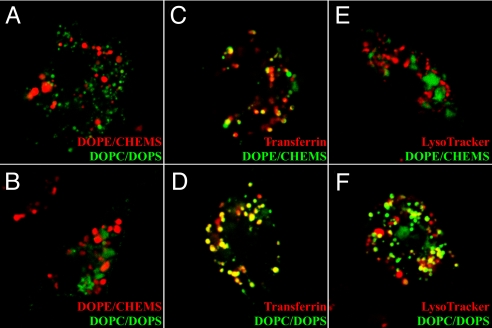
Based on previous studies, we used liposomes to target HEL to different vesicles (13). Liposomes containing fluorescently labeled dextrans were visualized in DCs and Mφs. After pulsing for several hours, leakage of fluorescent dextrans from either liposome formulation into the cytosol was not observed. Dextrans in DOPC/DOPS and DOPE/CHEMS liposomes resided in different endosomal compartments: FITC-dextran in DOPC/DOPS liposomes rarely colocalized with Texas Red-dextran in DOPE/CHEMS liposomes (Fig. 1 A and B). Dextrans in DOPE/CHEMS liposomes colocalized partially with transferrin-labeled endosomes but never colocalized with LysoTracker-labeled lysosomes (Fig. 1 C and E); thus, in agreement with previous studies, we concluded that these liposomes (henceforth referred to as “early endosomal liposomes”) rapidly associated with early recycling vesicles. Dextran in DOPC/DOPS colocalized with some transferrin-labeled vesicles and also with LysoTracker-labeled lysosomes (Fig. 1 D and F); accordingly, we concluded that these liposomes (henceforth referred to as “late endosomal liposomes”) entered late endosomes or lysosomes. Finally, dextrans in both liposome formulations colocalized with internalized MHC class I and II [supporting information (SI) Fig. S1].
Fig. 1.
DOPC/DOPS and DOPE/CHEMS liposomes enter distinct endosomal compartments. Splenic DCs (A) or peritoneal Mφs (B–F) were incubated with liposome-encapsulated FITC-dextran (A–F) or Texas Red-dextran (A and B) with Texas Red-transferrin (C and D) or LysoTracker Red (E and F). Confocal images were acquired 12 h after pulsing. Similar results were obtained 1–2 h after pulsing. (Magnification: ×63 oil, ×4 zoom.)
Nascent MHC Molecules Are Required for Class II but Not Class I Presentation of HEL.
We recently found that immunization of H-2g7 mice with HEL in Freund's adjuvants primed I-Ag7–restricted CD4 T cells as well as Db-restricted CD8 T cells; on the H-2g7 background, the CD4 T-cell epitope, HEL 11–25, lies adjacent to the CD8 T-cell epitope, HEL 23–31. To elucidate the mechanism(s) by which these 2 epitopes are generated, CD4 and CD8 T-cell hybridomas directed to these 2 peptides were used to measure processing and presentation of exogenous HEL in soluble form or encapsulated in liposomes.
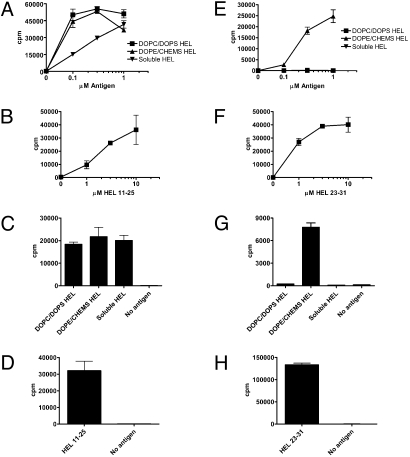
HEL encapsulated in late endosomal liposomes was efficiently processed and presented by MHC class II but not by MHC class I. In contrast, HEL encapsulated in early endosomal liposomes was presented on both MHC molecules (Fig. 2A, C, E, and G). Soluble HEL was presented solely on MHC class II. The same results were obtained for peritoneal Mφs (Fig. 2 A and E) and splenic DCs (Fig. 2 C and G). These results agree with previous work from our laboratory demonstrating MHC class I cross-presentation of Ova encapsulated in early (but not late) endosomal liposomes (14).
Fig. 2.
Presentation of soluble and liposome-encapsulated HEL on MHC class I and II. (A, B, E, and F) Peritoneal Mφs from NOD mice were cultured for 12–16 h with soluble HEL, HEL in DOPC/DOPS liposomes (targeted to late endosomes or lysosomes), HEL in DOPE/CHEMS liposomes (targeted to early endosomes), or HEL peptide. (C, D, G, and H) Splenic DCs from NOD mice were cultured for 4–6 h with 10 μM antigen. Cells were washed and cultured with HEL 11–25–specific CD4 (A–D) or HEL 23–31–specific CD8 (E–H) T-cell hybridomas overnight. IL-2 in culture supernatants was measured by CTLL-2 incorporation of 3H-thymidine. These and all subsequent experiments presented show the mean and SD of triplicate wells.
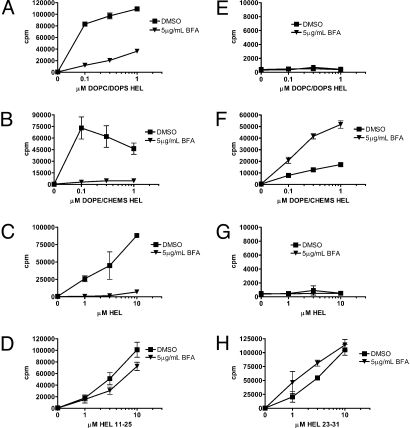
Processing and presentation of both endogenous and exogenous antigens often requires nascent MHC molecules (1). To determine if presentation of liposome-encapsulated HEL by MHC class I and II used newly synthesized molecules, brefeldin A (BFA) was used to block protein transport from the Golgi apparatus. BFA treatment completely blocked MHC class II presentation of HEL encapsulated in early and late endosomal liposomes as well as soluble HEL (Fig. 3 A–C). In striking contrast, cross-presentation of HEL in early endosomal liposomes was enhanced by BFA treatment (Fig. 3F); in 3 independent experiments, BFA increased presentation of 1 μM antigen 3.7-fold, 4.5-fold, and 3-fold. As a control, the effect of BFA on cross-presentation of soluble Ova was tested. Consistent with previous reports, BFA-treated APCs did not present soluble Ova on MHC class I (Fig. S2A). Together, the BFA-mediated blockade of HEL presentation on MHC class II and Ova presentation on MHC class I indicated that removal of the inhibitor did not allow for de novo antigen processing to normalize presentation to control levels.
Fig. 3.
Nascent MHC molecules are required for MHC class II, but not MHC class I, presentation of HEL. Peritoneal Mφs from NOD mice were preincubated for 30 min with 5 μg/mL BFA before addition of antigen dose curves for 12–16 h. Cells were washed and cultured with CD4 (A–D) or CD8 (E–H) T-cell hybridomas overnight, and IL-2 was measured in culture supernatants.
Proteasome Inhibitors Augment Cross-Presentation of HEL in Early Endosomal Liposomes.
In the classical MHC class I pathway, cytosolic proteins are catabolized by the proteasome before MHC class I loading. Cross-presentation of exogenous antigens on MHC class I can also involve transport into the cytosol followed by proteasomal processing (4). Recent data suggest that peptides generated by the proteasome can be loaded on MHC class I in either the endoplasmic reticulum (ER) or endosomes (6); loading in the ER is BFA-sensitive, whereas loading in endosomes is BFA-insensitive.
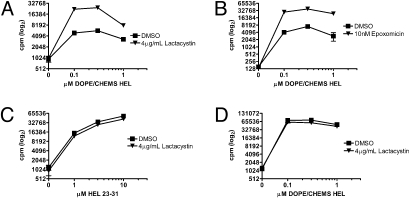
The BFA-mediated enhancement in cross-presentation of HEL in early endosomal liposomes indicated that MHC class I loading with HEL peptide did not occur in the ER. To determine if the proteasome was required for processing of HEL in early endosomal liposomes, Mφs were treated with lactacystin before pulsing with HEL. Treatment with lactacystin markedly increased cross-presentation of HEL in early endosomal liposomes (Fig. 4A); in 3 independent experiments, lactacystin enhanced presentation of 1 μM antigen 4.3-fold, 7.7-fold, and 8.1-fold. Epoxomicin produced a similar effect (Fig. 4B). Presentation of all other forms of HEL on MHC class I and II was unaffected by proteasome inhibition (Fig. 4 C and D). In contrast, lactacystin inhibited cross-presentation of soluble Ova, demonstrating that proteasome inhibition was effective under our experimental conditions (Fig. S2A). In addition to the proteasome, the cytosolic protease tripeptidyl peptidase II (TPPII) has been implicated in MHC class I presentation (15). Experiments using a chemical inhibitor of TPPII did not show an effect on cross-presentation of HEL in early endosomal liposomes (Fig. S3A). Overall, these data indicate that HEL in early endosomal liposomes does not require cytosolic proteolysis for cross-presentation.
Fig. 4.
Proteasome inhibition by lactacystin enhances MHC class I presentation of HEL in early endosomal liposomes. Peritoneal Mφs from NOD mice were preincubated for 30 min with 4 μg/mL lactacystin (A, C, and D) or 10 nM epoxomicin (B) before addition of antigen for 12–16 h. Cells were washed and cultured with CD8 (A–C) or CD4 (D) T-cell hybridomas overnight, and IL-2 was measured in culture supernatants. Note that ordinate values are plotted on a log2 scale.
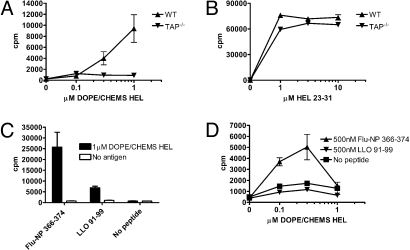
Cross-Presentation of HEL in Early Endosomal Liposomes Does Not Require the Transporter Associated with Antigen Processing (TAP).
Cytosolic peptides can be transferred into the ER (4) or endosomes (6) via TAP. Cross-presentation of HEL in early endosomal liposomes was completely abolished in APCs from TAP1−/− mice (Fig. 5A). Nonetheless, TAP1−/− APCs presented HEL 23–31 peptide, demonstrating that MHC class I could traffic to the cell surface (Fig. 5B). This result contradicted the previous finding that cytosolic processing was not required. However, it is possible that the presentation defect in TAP1−/− APCs reflected aberrant or limited trafficking of MHC class I rather than a requirement for HEL peptide transport from the cytosol (16). We tested this possibility by incubating TAP1−/− APCs with HEL in early endosomal liposomes and a Db-binding peptide from the influenza nucleoprotein (Flu-NP 366–374, ASNENMETM). We reasoned that Flu-NP 366–374 binding to Db would facilitate traffic to early endosomes for peptide exchange. In 2 independent experiments, addition of Db-binding peptide Flu-NP 366–374 rescued presentation of HEL in early endosomal liposomes by TAP1−/− APCs (Fig. 5 C and D). In experiment 1 using Mφs, a control peptide derived from listeriolysin O (LLO 91–99, GYKDGNEYI) weakly enabled MHC class I presentation of HEL in early endosomal liposomes (Fig. 5C); we attribute this result to a weak Db-binding motif within LLO 91–99 (17). In experiment 2 using DCs, LLO 91–99 failed to enable MHC class I presentation (Fig. 5D). When no exogenous peptide was added, we observed no cross-presentation by TAP1−/− APCs. Altogether, these data imply that traffic of MHC class I, and not peptide transport into the ER, accounted for the apparent TAP dependence for cross-presentation of HEL in early endosomal liposomes.
Fig. 5.
TAP is not required for MHC class I presentation of HEL in early endosomal liposomes. (A and B) Peritoneal Mφs from WT and TAP−/− B6 mice were incubated with antigen and CD8 T-cell hybridomas. Peritoneal Mφs (C) and splenic DCs (D) from TAP−/− mice were incubated with Flu-NP or LLO peptide at 26 °C. After 12–16 h, HEL in DOPE/CHEMS liposomes and CD8 T-cell hybridomas were added overnight at 37 °C and IL-2 was measured in culture supernatants. Hybridoma responses to HEL 23–31 peptide were unaffected by addition of Flu-NP or LLO peptide.
Endosomal Acidification Differentially Affects MHC Class I and II Presentation of HEL.
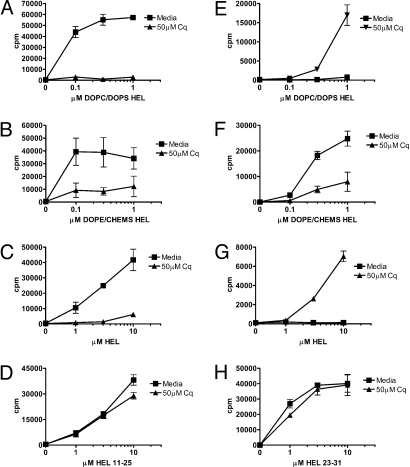
Agents that prevent endosomal acidification (e.g., chloroquine) can hinder proteases required for processing and presentation on both MHC class I and II (18, 19). Pretreatment of Mφs with chloroquine inhibited MHC class II presentation of HEL encapsulated in either early or late endosomal liposomes; chloroquine also inhibited MHC class II presentation of soluble HEL (Fig. 6 A–C). MHC class II presentation of soluble peptide was unaffected by chloroquine (Fig. 6D).
Fig. 6.
Blockade of endosomal acidification affects MHC class I and II presentation differentially. Peritoneal Mφs from NOD mice were preincubated for 30 min with 50 μM chloroquine (Cq) before addition of antigen dose curves for 12–16 h. Cells were washed and cultured with CD4 (A–D) or CD8 (E–H) T-cell hybridomas overnight, and IL-2 was measured in culture supernatants.
Chloroquine treatment blocked cross-presentation of HEL in early endosomal liposomes (Fig. 6F); in 3 independent experiments, chloroquine reduced presentation by 65–80%. To rule out the possibility that chloroquine was preventing the release of HEL from liposomes, we also examined MHC class I presentation of peptide encapsulated in early endosomal liposomes; chloroquine treatment had no effect on MHC class I presentation of liposome-encapsulated peptide (Fig. S4A). Together, these results imply that proteolysis within an endosomal compartment generated the MHC class I epitope. Remarkably, chloroquine enabled cross-presentation of soluble HEL and HEL encapsulated in late endosomal liposomes (Fig. 6 E and G), suggesting that proteolytic destruction of the MHC class I epitope precluded cross-presentation; we observed similar results in 4 independent experiments.
In summary, endosomal acidification is an essential step in HEL processing for MHC class II presentation, regardless of the manner in which HEL is offered to the APCs. Although acidification is required for cross-presentation of HEL in early endosomal liposomes, this process hinders cross-presentation of HEL directed to late endosomal compartments or HEL taken up in soluble form.
Cathepsin Proteases Differentially Affect MHC Class I and II Presentation of HEL.
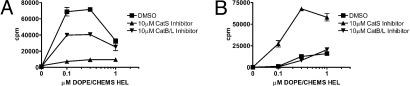
Previous studies implicated cathepsin (Cat) proteases in both MHC class I and II antigen processing and presentation (20). To determine if particular Cats were involved in processing HEL epitopes for MHC class I and II presentation, inhibitors specific for cysteinyl proteases CatS and CatB/L were tested. MHC class II presentation of soluble HEL and HEL in early and late endosomal liposomes was blocked by CatS inhibition (Fig. 7A and Fig. S5 A and B); in 2 independent experiments, CatS inhibition reduced presentation of 1 μM antigen by 60–80%. Presentation of exogenous peptide was unaffected by CatS inhibition (Fig. S5C), indicating that the effect on HEL liposome presentation was not attributable to a generalized impairment in MHC class II presentation. CatB/L inhibition had no effect on MHC class II presentation of soluble HEL or HEL in late endosomal liposomes but reduced presentation of HEL in early endosomal liposomes (Fig. 7A and Fig. S5 A and B); in 2 independent experiments, CatB/L inhibition reduced presentation by 50%. In summary, all forms of HEL required CatS activity for MHC class II presentation. CatB/L inhibition selectively reduces MHC class II presentation of HEL in early endosomal liposomes, indicating that different endosomal compartments use unique proteases for HEL processing.
Fig. 7.
CatS proteolytic activity affects MHC class I and II presentation differentially. Peritoneal Mφs from NOD mice were preincubated for 30 min with 10 μM CatS or CatB/L inhibitor before addition of HEL in DOPE/CHEMS liposomes. After incubation for 12–16 h with antigen, cells were washed and cultured with CD4 (A) or CD8 (B) T-cell hybridomas overnight, and IL-2 was measured in culture supernatants.
Surprisingly, cross-presentation of HEL in early endosomal liposomes was augmented by CatS inhibition, whereas CatB/L inhibition had no effect (Fig. 7B); in 3 independent experiments, CatS inhibition enhanced presentation of 1 μM antigen 2.9-fold, 3.7-fold, and 2.7-fold. We also examined cross-presentation by Mφs and DCs from CatS−/− B6 mice. Cross-presentation of HEL in early endosomal liposomes was enhanced in the absence of CatS, although to a lesser extent compared with chemical inhibition of CatS.
Discussion
We derive 3 general conclusions from the experiments presented here: (i) MHC class I and II are available to bind HEL peptides in diverse endosomal compartments; (ii) the proteolytic environment dictates the capacity of different endosomal compartments to mediate MHC class I and II presentation of exogenous HEL; and (iii) the nature of the protein, perhaps through its mechanism of uptake, is important in determining the features of cross-presentation. Specifically, our data support the conclusion that soluble HEL and HEL in late endosomal liposomes entered endosomes that rapidly acidified en route to lysosomes equipped only for MHC class II presentation; antigen catabolism precluded cross-presentation in this pathway. Early endosomal liposomes targeted HEL to a slowly acidifying and minimally degradative endosome that permitted antigen processing for MHC class I and II presentation; cross-presentation from this compartment did not require nascent MHC molecules, cytosolic processing, or TAP.
Cross-presentation of HEL in early endosomal liposomes showed a number of unique properties that distinguished it from previously studied proteins and gave us clues on the nature of the processing endosomes (Table S1). BFA and proteasome inhibition consistently enhanced cross-presentation; these treatments increased presentation independent of antigen processing, because presentation of liposome-encapsulated peptide was also enhanced (Fig. S5A). In addition to the Golgi apparatus, endosomal membrane traffic is susceptible to BFA (21). Hence, it is possible that the BFA-mediated enhancement in cross-presentation resulted from the formation or stabilization of a specialized endosomal compartment. The endosomal tubules that form with BFA treatment (21) resemble tubules involved in recycling of MHC class I (22), suggesting that this compartment may facilitate cross-presentation. It is unclear how proteasome inhibition could enhance processing and presentation of a noncytosolic antigen. It is possible that regulation of MHC class I traffic by ubiquitination (23, 24) in conjunction with proteasome-mediated deubiquitination for proper endosomal sorting (25) could affect certain cross-presentation pathways. Ongoing imaging experiments should clarify the relation between endosomal membrane traffic, MHC class I localization, and cross-presentation.
A relation between endosomal acidification and cross-presentation was raised by Amigorena's group (26), who showed that Ova cross-presentation was favored by neutral pH and low proteolytic activity. Along these lines, we observed cross-presentation of soluble HEL and HEL in late endosomal liposomes only when acidification was blocked by chloroquine; on the other hand, cross-presentation of HEL in early endosomal liposomes required acidification, suggesting productive antigen processing by acid-dependent proteases. Taken together, these results are not contradictory. We speculate that APCs contain a heterogeneous set of endosomes whose proteolytic environments dictate their capacity for MHC class I cross-presentation. The proteolytic environment encountered by soluble HEL and HEL in late endosomal liposomes is biased toward epitope destruction; we are currently investigating if this is the result of more proteolysis in general or exposure to particular proteases that specifically destroy the HEL epitope. In contrast, soluble Ova and HEL in early endosomal liposomes naturally enter compartments where the low proteolytic environment favors cross-presentation (via 2 different mechanisms). For soluble Ova, this entails escape into the cytosol for proteasomal processing; for HEL in early endosomal liposomes, this entails processing and MHC class I loading that takes place entirely within the endosome.
Previous reports using a variety of antigens described cross-presentation pathways involving acid-dependent proteases (19, 27, 28), especially cysteinyl proteases (5, 29, 30). In both Mφs and DCs, the bulk of cysteinyl protease activity increased with endosomal maturation (31). Interestingly, the cysteinyl protease CatS exhibited activity at both acidic and neutral pH (32) and localized to endosomes and lysosomes (33). In our experiments, CatS activity hindered cross-presentation of HEL in early endosomal liposomes, which contrasts with the findings in the report from Rock's group (5) examining cross-presentation of Ova and viral antigens. Altogether, this implies that CatS is poised to generate or destroy antigenic peptides in several endosomal compartments, including those involved in cross-presentation.
The cross-presentation pathways of soluble HEL and Ova are strikingly dissimilar (Table S1), likely attributable to different uptake mechanisms. Kurts and colleagues (34) demonstrated that the mannose receptor delivered soluble Ova to an endosomal compartment equipped for cross-presentation; in contrast, macropinocytosis of Ova allowed for MHC class II presentation but not cross-presentation. HEL is not glycosylated and is presumably taken up by macropinocytosis when offered in soluble form; HEL can bind to constituents of plasma membranes, such as phospholipids (35) and glycosaminoglycans (36), but receptor-mediated uptake has not been shown. Our data indicate that soluble HEL rapidly enters late endosomes and does not remain in early endosomes for enough time to allow processing and cross-presentation; this feature of soluble HEL trafficking also hindered MHC class II presentation of type B epitopes, which are generated exclusively in early endosomes lacking the accessory molecule DM (37).
It is unlikely that all endocytosed proteins will cross into the cytosol from endosomes or, if they do cross, whether they are processed by the MHC class I system. Whether Ova's traffic represents the rule or an exception needs to be evaluated. We have found no evidence that any form of HEL enters the cytosol for cross-presentation. It is unclear if this feature of HEL cross-presentation is antigen- or compartment-specific; that is, transport of HEL protein into the cytosol may be impossible, or HEL may not enter a compartment where translocation into the cytosol is an option. Interestingly, our CD8 T-cell hybridomas do not recognize APCs from mice expressing membrane-bound HEL, even though it is likely that a fraction of membrane-bound HEL enters the cytosol via the ER-associated degradation pathway. Thus, it is possible that HEL processing in the cytosol and/or ER is not conducive to peptide-MHC generation in our system.
Multiple studies indicated that CD8α+ DCs are the principal cross-presenting APCs in vivo (38, 39). Moreover, many of the specialized receptors that deliver exogenous antigen to cross-presenting endosomal compartments are highly enriched on or unique to CD8α+ DCs (40, 41). The trafficking and biochemical features of these particular endosomes are unknown, but it is likely that specific receptors deliver exogenous antigens to endosomes with low degradative activity. In preliminary experiments, we observed cross-presentation of HEL in early endosomal liposomes by CD8α-depleted splenic DCs; this is consistent with previous reports (42) and implies that the antigen uptake and processing functions specific to CD8α + DCs are dispensable for some modes of cross-presentation. In addition, we and others observed robust cross-presentation by Mφs in vitro (14, 34) and in vivo (43); notably, 2 recent reports demonstrated that Mφs reduced their proteolytic activity and altered their membrane trafficking after exposure to lipopolysaccharide and IFN-γ, insinuating that these cells boost their cross-presenting capacity on activation (44, 45). Considering all these data, it is likely that specialized receptors, but not specialized endosomal compartments, endow certain APCs with robust cross-presenting ability. We speculate that all cells contain endosomal compartments suitable for cross-presentation; in fact, Cresswell and colleagues (46) recently showed that 293T cells are capable of cross-presentation. Further characterization of the antigen processing and MHC class I loading machinery as well as the types of antigens contained within these endosomal compartments should clarify the mechanisms of cross-presentation.
Materials and Methods
Mice.
Nonobese diabetic (NOD), C57BL/6 (B6), B6.H-2g7, and B10.BR mice were obtained from The Jackson Laboratory. TAP1−/− mice on the B6 background were obtained from Ted Hansen (Washington University, St. Louis, MO). CatS-deficient mice on the B6 background were obtained from Harold Chapman (University of California, San Francisco, CA). Nondiabetic NOD male mice (8–10 weeks old) were used for most experiments. B6.H-2g7 mice yielded identical results. All mice were bred under specific pathogen-free conditions at Washington University in accordance with institutional animal care guidelines.
Antigens.
HEL protein was from Sigma and was purified by affinity chromatography to remove contaminants and degraded protein. Purified HEL contained <0.1 EU/mg LPS. Mass spectrometry analysis indicated no evidence of degraded protein. Ova protein was from Worthington Biochemical. The following peptides were synthesized by Fmoc techniques and verified by mass spectrometry: HEL 11–25 (AMKRHGLDNYRGYSL), HEL 23–31 (YSLGNWVCA), LLO 91–99 (GYKDGNEYI), and Ova 257–264 (SIINFEKL). Flu-NP 366–374 (ASNENMETM) was obtained from Ted Hansen (Washington University, St. Louis, MO). DOPC, DOPS, and DOPE were from Avanti Polar Lipids and were dissolved in chloroform. CHEMS was from Sigma and was dissolved in chloroform. All lipids were stored under nitrogen at −20 °C. Liposome-encapsulated HEL was prepared as previously described (47).
Chemical Reagents.
BFA, chloroquine, epoxomicin, and lactacystin were obtained from Sigma. Inhibitors of CatB/L (ZZ-FF-FMK), CatS (Z-FL-COCHO), and TPPII (H-AAF-CMK) were obtained from Calbiochem. LysoTracker Red, Texas Red-conjugated transferrin, FITC- and Texas Red-conjugated 10-kD dextran, and Alexa555-conjugated streptavidin were obtained from Invitrogen. Biotinylated Kk antibody was obtained from Caltag. Biotinylated I-Ak antibody (40F) was generated in our laboratory.
T-Cell Hybridoma Generation.
HEL-specific CD4 and CD8 T cells from NOD mice immunized with HEL emulsified in complete Freund's adjuvant (Difco) were fused to BW5147 partner cells to generate T-cell hybridomas (48). A similar protocol was used for generation of SIINFEKL-specific CD8 T-cell hybridomas from B6 mice immunized with Ova emulsified in complete Freund's adjuvant. Hybridomas were subcloned before use in antigen presentation assays.
Antigen Presentation Assays.
Unless otherwise noted, cells were cultured at 37 °C/5% CO2 (vol/vol) in DMEM containing antibiotics and 10% (vol/vol) FCS. CD11c+ DCs were obtained from spleens of mice injected with Flt3L-Fc protein using CD11c MACS beads (Miltenyi). Splenic DCs from untreated mice were also used and yielded similar results. Peritoneal Mφs were obtained from mice injected with thioglycollate 4 days previously. T-cell assays were performed in 96-well V-bottom (for DCs) or flat-bottom (for Mφs) plates. Briefly, 5 × 104 DCs per well or 105 Mφs per well were incubated with or without chemical inhibitors for 30 min before the addition of antigen dose curves. DCs were pulsed with antigen for 4–6 h, and Mφs were pulsed for 12–16 h. Cells were subsequently washed with DMEM, and 5 × 104 T-cell hybridomas per well were added. After overnight culture, IL-2 in the supernatant was measured by CTLL-2 3H-thymidine incorporation.
Confocal Microscopy.
DCs and Mφs were obtained as described previously and cultured on glass coverslips. To visualize both types of liposomes simultaneously, cells were incubated with FITC-dextran in DOPC/DOPS and Texas Red-dextran in DOPE/CHEMS (50–100 μg/mL each) for 12 h. For colocalization experiments, cells were incubated with 100 μg/mL Texas Red-transferrin or 10 nM LysoTracker Red in the presence of liposome-encapsulated FITC-dextran (50–100 μg/mL) for 12 h. For MHC class I and II internalization experiments, cells were incubated at 4 °C with 200 ng of biotinylated anti-Kk, anti-I-Ak, or control IgG for 1 h, followed by Alexa555-conjugated streptavidin for 1 h. After washing with cold PBS, cells were incubated at 37 °C with FITC-dextran in DOPC/DOPS or DOPE/CHEMS liposomes (50–100 μg/mL) for 12 h. In all experiments, cells were washed with PBS and mounted on slides; images were acquired using a Zeiss 510 laser scanning confocal microscope.
Supplementary Material
Acknowledgments.
We thank Jeremy Herzog and Shirley Petzold for technical assistance; Kathy Frederick for animal husbandry; and Drs. Ted Hansen, Robert Lindner, Javier Carrero, and Boris Calderon for comments on the manuscript. This publication was made possible by Grant AI022033 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908583106/DCSupplemental.
References
- 1.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 2.Lindner R, Unanue ER. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO J. 1996;15:6910–6920. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- 4.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 5.Shen LJ, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 7.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 9.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 10.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeifer JD, et al. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 12.Groothuis TAM, Neefjes J. The many roads to cross-presentation. J Exp Med. 2005;202:1313–1318. doi: 10.1084/jem.20051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 14.Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–2863. [PubMed] [Google Scholar]
- 15.Seifert U, et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat Immunol. 2003;4:375–379. doi: 10.1038/ni905. [DOI] [PubMed] [Google Scholar]
- 16.Chefalo PJ, Grandea AG, Van Kaer L, Harding CV. Tapasin−/− and TAP1−/− macrophages are deficient in vacuolar alternate class I MHC (MHC-I) processing due to decreased MHC-I stability at phagolysosomal pH. J Immunol. 2003;170:5825–5833. doi: 10.4049/jimmunol.170.12.5825. [DOI] [PubMed] [Google Scholar]
- 17.Rammensee HG, Bachmann J, Emmerich NPN, Bachor OA, Stevanovic S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schirmbeck R, Melber K, Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995;25:1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 20.Rock KL, Shen L. Cross-presentation: Underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 21.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehner PJ, Hoer S, Dodd R, Duncan LM. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol Rev. 2005;207:112–125. doi: 10.1111/j.0105-2896.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZQ, et al. Activation of CXCR4 triggers ubiquitination and down-regulation of major histocompatibility complex class I (MHC-I) on epithelioid carcinoma HeLa cells. J Biol Chem. 2008;283:3951–3959. doi: 10.1074/jbc.M706848200. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo ME, Jung JU, Ploegh HL. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class I major histocompatibility complexes to late endocytic compartments. J Virol. 2002;76:5522–5531. doi: 10.1128/JVI.76.11.5522-5531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Bertholet S, et al. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 28.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez D, Del Val M. Cutting edge: Selective involvement of proteasomes and cysteine proteases in MHC class I antigen presentation. J Immunol. 1997;159:5769–5772. [PubMed] [Google Scholar]
- 30.Campbell DJ, Serwold T, Shastri N. Bacterial proteins can be processed by macrophages in a transporter associated with antigen processing-independent, cysteine protease-dependent manner for presentation by MHC class I molecules. J Immunol. 2000;164:168–175. doi: 10.4049/jimmunol.164.1.168. [DOI] [PubMed] [Google Scholar]
- 31.Lennon-Dumenil AM, et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. 2002;196:529–539. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirschke H, Wiederanders B, Bromme D, Rinne A. Cathepsin S from bovine spleen: Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989;264:467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutzner N, Kalbacher H. Quantifying cathepsin S activity in antigen presenting cells using a novel specific substrate. J Biol Chem. 2008;283:36185–36194. doi: 10.1074/jbc.M806500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 35.Gorbenko GP, Ioffe VM, Kinnunen PKJ. Binding of lysozyme to phospholipid bilayers: Evidence for protein aggregation upon membrane association. Biophys J. 2007;93:140–153. doi: 10.1529/biophysj.106.102749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemansky P, Hasilik A. Chondroitin sulfate is involved in lysosomal transport of lysozyme in U937 cells. J Cell Sci. 2001;114:345–352. doi: 10.1242/jcs.114.2.345. [DOI] [PubMed] [Google Scholar]
- 37.Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. doi: 10.1111/j.0105-2896.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 38.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 39.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sancho D, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin ML, Zhan YF, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 43.Pozzi LAM, Maciaszek JW, Rock KL. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005;175:2071–2081. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- 44.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–250. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 45.Trost M, et al. The phagosomal proteome in interferon-γ-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci USA. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–476. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 48.Velazquez C, Vidavsky I, van der Drift K, Gross ML, Unanue ER. Chemical identification of a low abundance lysozyme peptide family bound to I-Ak histocompatibility molecules. J Biol Chem. 2002;277:42514–42522. doi: 10.1074/jbc.M202316200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.