SUMMARY
Neurodegenerative disorders are pathologically characterized by the deposition of abnormal proteins in the brain. It is likely that future treatment trials will target the underlying protein biochemistry and it is therefore increasingly important to be able to distinguish between different pathologies during life. The aim of this study was to determine whether rates of brain atrophy differ in neurodegenerative dementias that vary by pathological diagnoses and characteristic protein biochemistry. Fifty-six autopsied subjects were identified with a clinical diagnosis of dementia and two serial head MRI. Subjects were subdivided based on pathological diagnoses into Alzheimer's disease (AD), dementia with Lewy bodies (DLB), mixed AD/DLB, frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes (FTLD-U), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). Twenty-five controls were matched by age, gender, and scan interval, to the study cohort. The boundary-shift integral was used to calculate change over time in whole brain (BBSI) and ventricular volume (VBSI). All BSI results were annualized by adjusting for scan interval. The rates of whole brain atrophy and ventricular expansion were significantly increased compared to controls in the AD, mixed AD/DLB, FTLD-U, CBD and PSP groups. However, atrophy rates in the DLB group were not significantly different from control rates of atrophy. The largest rates of atrophy were observed in the CBD group which had a BBSI of 2.3% and VBSI of 16.2%. The CBD group had significantly greater rates of BBSI and VBSI than the DLB, mixed AD/DLB, AD and PSP groups, with a similar trend observed when compared to the FTLD-U group. The FTLD-U group showed the next largest rates with a BBSI of 1.7% and VBSI of 9.6% which were both significantly greater than the DLB group. There was no significant difference in the rates of atrophy between the AD, mixed AD/DLB and PSP groups, which all showed similar rates of atrophy; BBSI of 1.1, 1.3 and 1.0% and VBSI of 8.3, 7.2 and 10.9% respectively. Rates of atrophy therefore differ according to the pathological diagnoses and underlying protein biochemistry. While rates are unlikely to be useful in differentiating AD from cases with mixed AD/DLB pathology, they demonstrate important pathophysiological differences between DLB and those with mixed AD/DLB and AD pathology, and between those with CBD and PSP pathology.
Keywords: magnetic resonance imaging, Alzheimer's disease, dementia with Lewy bodies, frontotemporal lobar degeneration, progressive supranuclear palsy
INTRODUCTION
Neurodegenerative disorders are defined pathologically by the deposition of abnormal proteins in the brain. The most common proteins are β-amyloid (Glenner and Wong, 1984), the microtubule-associated protein tau (Goedert et al., 2001), the synaptic vesicle protein α-synuclein (Spillantini et al., 1997), and the proteasomal protein ubiquitin (Hershko and Ciechanover, 1998). The protein TDP-43 has also recently been reported in association with ubiquitin inclusions however its specificity needs to be confirmed (Neumann et al., 2006). The most common neurodegenerative disease is Alzheimer's disease (AD), which is characterized by the presence of β-amyloid plaques and tau-positive neurofibrillary tangles (NFT) (NIA, 1997). Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia and is characterized by the presence of α-synuclein-positive Lewy Bodies (McKeith et al., 2005; Spillantini et al., 1997). While AD and DLB account for the majority of cases of dementia over the age of 65 frontotemporal lobar degeneration (FTLD) has been shown to be as common as AD in subjects under the age of 65 (Ratnavalli et al., 2002). The pathologies underlying FTLD are heterogeneous, however the most common variant, which accounts for almost 60% of cases of FTLD in some series (Josephs et al., 2004a), is frontotemporal lobar degeneration with ubiquitin-only-immunoreactive neuronal changes (FTLD-U). In FTLD-U ubiquitin-positive inclusions are found in the frontotemporal cortices and the dentate cell layer of the hippocampus (Josephs et al., 2006c; McKhann et al., 2001). Other pathological variants of FTLD are characterized by the presence of tau-positive inclusions. These include disorders such as corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) (Dickson, 1999), and Pick disease (PiD).
It is likely that future treatment trials will target the biochemistry underlying each of these diseases and it is therefore increasingly important to be able to distinguish between these different pathologies. In fact, a phase IIa treatment trial aimed at reducing the burden of β-amyloid was recently undertaken (Gilman et al., 2005). While there is a relatively good correlation between the clinical and pathological diagnoses of AD, with 80–90% of pathologically confirmed AD accurately diagnosed during life (Galasko et al., 1994), misdiagnosis is common between cases with AD, DLB and those with mixed AD/DLB pathology (Luis et al., 1999; McKeith et al., 2000; Merdes et al., 2003). In addition, the different pathological subtypes of FTLD do not map neatly onto the various clinical syndromes of frontotemporal dementia, making it difficult to predict pathology during life (Forman et al., 2006; Hodges et al., 2004; Josephs et al., 2006a; Josephs et al., 2006c; Kertesz et al., 2005). Hence, there is a need for sensitive and specific biomarkers to aid in the differentiation of these diseases.
Magnetic resonance imaging (MRI) has become increasingly important in the assessment of brain atrophy in neurodegenerative diseases. The majority of studies have been cross-sectional in design assessing patterns of whole brain and regional atrophy in subjects with dementia. These studies have provided valuable information concerning patterns of atrophy in the different neurodegenerative dementias (Barber et al., 1999; Burton et al., 2002; Jack et al., 1992; Whitwell et al., 2006; Whitwell et al., 2005), but are limited both by the large degree of inter-individual variability in brain morphology and by the fact that most studies assess clinically defined subjects without pathological confirmation. Longitudinal studies allow the assessment of brain changes over time in individual subjects using multiple serial MRI scans and therefore have the advantage of reducing the problem of inter-individual variation. They can also provide information about the trajectory of the disease (Chan et al., 2003). Automated registration and subtraction techniques have been developed that provide quick and reproducible measurements of brain volume loss and ventricular expansion over time. One such technique is the boundary shift integral (BSI) which calculates the integral change in the brain/CSF boundary over the interior and exterior surfaces of the brain (Freeborough and Fox, 1997; Gunter et al., 2003). Rates of atrophy have been shown to be excellent biomarkers of disease progression in clinical cohorts and are already routinely being used as outcome measure in clinical trials (Fox et al., 2005; Jack et al., 2003).
The aim of this study was therefore to assess and compare the rates of whole brain atrophy and ventricular expansion in pathologically confirmed subjects with AD, DLB, FTLD-U, CBD, PSP and PiD.
METHODS
Subjects
The neuropathology files of the Mayo Clinic were used to identify all cases that had come to autopsy and had been given a diagnosis of AD, DLB, FTLD-U, CBD, PSP, or PiD and that had at least two volumetric head MRI scans of adequate quality for the BSI measurements. Cases with mixed AD and DLB were also included. The pathological criteria used to define these groups are outlined in detail below.
All subjects had been studied prospectively. The medical records of all cases were reviewed by a behavioral neurologist (KAJ). In order to be included in the study all subjects had to fulfill strict clinical criteria: all pathologically defined AD cases had to have fulfilled clinical criteria for AD (McKhann et al., 1984) at the time of scan. All pathologically defined DLB cases had to have fulfilled recent clinical criteria for dementia with Lewy Bodies (DLB) or Parkinson's disease with dementia (McKeith et al., 2005) at the time of scan. The cases with mixed AD and DLB pathology had to have fulfilled criteria for either AD or DLB at the time of the scans. All FTLD-U, CBD, PSP and PiD cases must have fulfilled clinical criteria for one of the disorders considered to lie under the frontotemporal dementia spectrum. These include behavioral variant frontotemporal dementia, semantic dementia, progressive non-fluent aphasia, PSP, corticobasal syndrome (Boeve et al., 2003), or apraxia of speech (Josephs et al., 2006c; Neary et al., 1998). Cognitive ability across groups was assessed using the Mini-Mental Status Examination (MMSE) (Folstein et al., 1975) and the Clinical Dementia Rating scale (CDR) (Hughes et al., 1982).
The baseline scan was selected as the first scan of adequate quality performed after each subject fulfilled criteria for the clinical diagnoses described above. In most cases, repeat scans were performed between one and two years later. Subjects were rejected if the scan interval was less than six months or greater than four years. All MRI scans were visually assessed (JLW and CRJ). Scans were rejected for poor quality (such as motion artifact) or the presence of other pathologies (such as stroke, tumor or other structural lesion) that may influence either the structural analysis or clinical presentation.
A total of 133 autopsy confirmed cases with a pathological diagnosis of AD, DLB, mixed AD/DLB, FTLD-U, CBD, PSP, and PiD were identified. Of these, 56 cases fulfilled the imaging inclusion criteria of having two volumetric head MRI scans: 12 AD, nine DLB, 13 mixed AD/DLB, 12 FTLD-U, five CBD and five PSP. No cases of PiD identified had two usable head MRI. A group of 25 healthy living controls with two volumetric MRI scans were then matched by age, gender, and scan interval, to the study cohort. All the healthy control subjects were initially recruited into the Mayo Clinic Alzheimer Disease Research Center (ADRC), or the Alzheimer Disease Patient Registry (ADPR), and were identified from the ADRC/ADPR database. Controls were identified as individuals who a) were independently functioning community dwellers, b) did not have active neurologic or psychiatric conditions, c) had no cognitive complaints, d) had a normal neurological and neurocognitive examination, and e) were not taking any psychoactive medications in doses that would affect cognition.
Pathological Assessment
Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for Alzheimer's Disease (Mirra et al., 1991). In all cases pathological assessment and diagnosis was conducted by one of two expert neuropathologists (DWD or JEP). After removal, the brain was divided into right and left hemibrains. One hemibrain was fixed in 10% buffered formaldehyde for 7 to 10 days, and then sectioned. Routinely sampled brain areas included: middle frontal gyrus (Brodmann area [BA] 9), inferior parietal lobule (BA 39), superior temporal gyrus (BA 22), calcarine cortex (BA 17), anterior cingulate gyrus (BA 24), hippocampus at the level of the lateral geniculate body, amygdala, transentorhinal and entorhinal cortices at the level of the mamillary bodies, nucleus basalis, cerebellum, dorsomedial thalamus with subthalamic nucleus, midbrain with substantia nigra, pons, and medulla. Samples were processed in paraffin and stained with hematoxylin and eosin, stained with modified Bielschowsky silver impregnation, and immunostained with antibodies to β-amyloid (clone 6F/3D, 1:10 dilution; Novocastra Vector Labs, Burlingame, CA), τ(clone AT8, 1:1,000 dilution; Endogen, Woburn, MA), α-synuclein (clone LB509, 1:200 dilution; Zymed, San Francisco, CA), neurofilament (DAKO clone 2F11, 1:75 dilution; DAKO, Carpinteria, CA), and ubiquitin (DAKO polyclonal, 1:100 dilution).
Pathological Criteria
Alzheimer's disease
Subjects were classified as AD if on semiquantitative analysis based on the highest neuritic plaque count of the middle frontal gyrus, superior temporal gyrus, inferior parietal lobule, hippocampus and entorhinal cortex, and midbrain, the subject had frequent neuritic plaques and met CERAD diagnostic criteria C for definite AD (Mirra et al., 1991). Plaque count was done with the modified Bielschowsky silver stain. In addition, the subject had to have had a Braak neurofibrillary stage of 5 or 6 (Braak and Braak, 1996) and therefore fulfill NIA-Reagan criteria for high probability AD (NIA, 1997). We excluded any subjects with Lewy bodies including amygdala only Lewy bodies (Uchikado et al., 2006).
Dementia with Lewy Bodies
Subjects were classified as DLB if there was evidence of widespread α-synuclein positive Lewy bodies in limbic or neocortex that met published criteria for neocortical or limbic variant of dementia with Lewy bodies (McKeith et al., 2005). In addition, to be included in this category, on semiquantitative analysis of the neuritic plaque count as describe above, the subject must have no histological evidence of probable or definite Alzheimer's disease based on the CERAD criteria (Mirra et al., 1991), a Braak score of 1–3 (Braak and Braak, 1996), and low NIA-Reagan (NIA, 1997).
Mixed AD/DLB
Subjects were considered to have mixed AD/DLB if there were evidence of mixed pathologies. That is, if there were findings of neuritic plaque deposition density that met CERAD criteria C for Alzheimer's disease, had a Braak stage 5 or 6, and a high probability of AD based on NIA-Reagan criteria as described above, plus findings of widespread neocortical or limbic Lewy bodies that met published criteria for neocortical or limbic variant of Lewy body disease (McKeith et al., 2005). We excluded subjects with amygdala only Lewy bodies (Uchikado et al., 2006).
FTLD-U
Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes was diagnosed if there were inclusions that stained positive for ubiquitin, yet stained negative for tau, neurofilament, and α-synuclein, in frontal or temporal cortex, and the hippocampus dentate granular cells without any evidence of motor neuron degeneration as previously described (FTLD-U) (Josephs et al., 2006b), and meeting established research criteria (McKhann et al., 2001). Cases with pathological evidence of motor neuron disease were excluded.
Progressive supranuclear palsy
Subjects were diagnosed as PSP if there were neuronal loss and gliosis, as well as characteristic tau-positive lesions including tufted astrocytes, coiled bodies and globose neurofibrillary tangles in cardinal nuclei (subthalamic nucleus, thalamic and brainstem nuclei, etc.) that met diagnostic criteria for PSP (Hauw et al., 1994).
Corticobasal degeneration
Subjects were given a pathological diagnosis of CBD if there were cortical neuronal loss and gliosis with balloon neurons and tau-positive lesions including astrocytic plaques, corticobasal bodies, and abundant neuropil threads that were located in cardinal regions that met diagnostic criteria for CBD (Dickson et al., 2002).
Exclusion criteria
With the exception of the mixed AD/DLB subjects, only subjects that were found to have a primary pathological diagnosis were included. Therefore subjects were excluded if in addition to the primary pathological diagnosis described above for each category, there were any additional findings of large cortical infarcts, diagnosis of another neurodegenerative disease, or brain tumors.
Image analysis
All MRI studies were performed at 1.5T with a standardized imaging protocol that included a coronal T1-weighted 3-dimensional volumetric spoiled gradient echo (SPGR) sequence with 124 contiguous partitions and 1.6mm slice thickness (22×16.5cm FOV, 25° flip angle).
Rates of change of the whole brain and ventricles were made using a software algorithm that has been developed in our lab and described in detail elsewhere (Gunter et al., 2003). Inputs for the algorithm included the baseline and repeat MR scans plus preprocessed brain and ventricular volumes. Whole brain masks were created for all baseline scans using MIDAS image analysis software (Freeborough et al., 1997). This is a semi-automated brain segmentation algorithm that performs an intensity thresholding step, and a series of erosions and dilations to remove non-brain structures. Manual editing was then performed in order to remove any “non-brain” structures from the segmentation. Ventricular segmentations were performed on all repeat scans for each subject using the autotrace subprogram in Analyze/AVW plus manual editing of anatomic outlines. The ventricular region included the lateral ventricles, the temporal horn of the lateral ventricles, and the third ventricle. Serial scan pairs were then registered, i.e. spatially matched, using a 9 degrees of freedom (dof) rigid body registration. This algorithm combines a 6dof rigid registration (three translations, three rotations), which matches the position and orientation of the two brain volumes, with an additional three scaling parameters to correct for drifts in gradient calibration over time. The algorithm also incorporates a correction for intensity inhomogeneity (Sled et al., 1998). Change in brain and ventricle volume was calculated automatically from each registered scan pair using the boundary shift integral (BSI) (Freeborough and Fox, 1997; Gunter et al., 2003). All BSI results were annualized by adjusting for scan interval and atrophy rates were calculated as a percentage change from the baseline volume.
A further scan quality assessment was performed after the scans had been pre-processed. All registered scan pairs were viewed side-by-side to check for consistency of acquisition over time. These assessments were performed without reference to the BSI results. Five scan pairs were thought to have inconsistencies in scan contrast, signal-to-noise, or general scan quality across the two scans. Three cases were diagnosed with mixed AD and DLB, and two had pure DLB. The whole brain BSI results for these five subjects were removed from the final analysis. The ventricular BSI measurements were thought to be more robust to slight quality changes over time due to the well-defined brain-CSF boundary in the ventricles. Therefore, all ventricular BSI data was analyzed.
Statistical analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc, Cary, NC) with statistical significance set at p < 0.05. Gender ratios were compared across groups using a Chi-squared test and Fisher's Exact test for cells with small numbers. Kruskal-Wallis test was used to compare age at scan, education, disease duration (defined as time from disease onset to the time of first MRI scan), and time from second MRI scan to death, Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR) sum of boxes, Braak score (Braak and Braak, 1996), CERAD, NIA Reagan (NIA, 1997) and the BSI variables, including scan interval, whole brain and ventricular BSI, across groups. NIA Reagan and CERAD data were converted to a ordinal scale for analysis (i.e. NIA low = 1, NIA intermediate = 2, NIA high = 3, and CERAD O=0, A=1, B=2, C=3). Because the control group was by definition cognitively normal, we excluded controls from tests of differences in cognitive scores. For analyses which were significant, post hoc testing was performed using the Mann-Whitney U test. This two-stage strategy controls for type 1 errors, even without correcting the p-values for the pair-wise tests through the same mechanism that is employed to control type 1 error in Fisher's Protected Least Significant Differences approach. This mechanism means that pair-wise comparisons are not deemed significant in the absence of a significant global test (Fisher, 1935; Rencher, 2002). Because the global tests were significant, type 1 error on the pair-wise comparisons has been controlled without further corrections for multiple comparisons. Spearman rank correlations were used to assess the relationship between rates of atrophy and disease duration, age at scan, and change over time in the MMSE and CDR. A Linear regression model was used to adjust for any variables predicting the BSI.
RESULTS
Demographic information for all subjects is shown in Table 1. There was no difference across the groups in age at baseline scan, years of education, and gender ratios. Within the disease groups there was also no difference in the time from disease onset to baseline scan. However, there was a difference in the time from baseline scan to death. The mixed group was the furthest from death, while the DLB group was the closest to death. There was no difference in MMSE or CDR across the disease groups, although the PSP group had the largest median MMSE score and lowest CDR, while the CBD group had the smallest median MMSE score. By design, there were significant differences in median CERAD score across the disease groups, with the AD and mixed AD/DLB group showing significantly higher scores than the DLB, FTLD-U, CBD and PSP groups. Braak score was also significantly higher in the AD and mixed groups. The NIA Reagan was low in the FTLD-U, CBD, PSP, and DLB groups, but high in the AD and mixed AD/DLB groups.
Table 1.
Subject demographics
| Controls (n=25) | DLB (n=9) | Mixed (n=13) | AD (n=12) | FTLD-U (n=12) | PSP (n=5) | CBD (n=5) | P values | |
|---|---|---|---|---|---|---|---|---|
| Gender (Male: Female) | 13:12 | 6:3 | 6:7 | 4:8 | 4:8 | 3:2 | 1:4 | NS |
| Age at baseline (yrs) | 76 (51,87) | 72 (59,82) | 76 (52,86) | 77 (52,90) | 72 (55,87) | 71 (61,78) | 69 (43,71) | NS |
| Education (yrs) | 14 (8,20) | 16 (12,20) | 12 (8,20) | 14 (8,18) | 15 (8,18) | 16 (12,18) | 12 (8,16) | NS |
| Time from onset to baseline scan (yrs) | NA | 4 (0,5) | 4 (0,10) | 4 (1,9) | 3 (1,6) | 4 (2,5) | 3 (1,3) | NS† |
| Time from baseline scan to death (yrs) | NA | 3 (1,6) | 7 (3,9) | 5 (3,6) | 6 (1,8) | 4 (2,6) | 4 (2,4) | 0.0065† |
| MMSE at baseline | 29 (24,30) | 24 (19,29) | 23 (13,28) | 24 (11,29) | 25 (19,29) | 28 (23, 30) | 21 (15, 23) | NS† |
| CDR at baseline | 0 (0,0) | 5 (2,11) | 6 (1,10) | 4 (1,17) | 4 (1,8) | 1 (0.5,5) | 6 (2,10) | NS† |
| CERAD | NA | 0 (0,1) | 3 (3,3) | 3 (3,3) | 0 (0,1) | 0 (0,1) | 0 (0,1) | <0.001† |
| Braak score | NA | 2 (1,3) | 6 (5,6) | 6 (5,6) | 1 (0,2) | NA | NA | <0.001† |
| NIA Reagan | NA | L (L, L) | H (H, H) | H (H, H) | L (L, L) | L (L, L) | L (L,L) | <0.001† |
Kruskall-Wallis test was performed across DLB, mixed, AD, FTLD-U, CBD and PSP groups Data is shown as median (range). MMSE = Mini-Mental Status Examination; CDR = Clinical Dementia Rating; NIA = National Institute of Aging; NA = not applicable; NS = not significant; L = Low, I = Intermediate, H = High
All 12 patients with a pathological diagnosis of AD also had a clinical diagnosis of probable AD (McKhann et al., 1984) by inclusion criteria. Similarly all nine cases of DLB had a clinical diagnosis of probable DLB (McKeith et al., 2005); none met criteria for Parkinson's disease dementia (McKeith et al., 2005). Nine of the 13 cases with mixed pathological features of AD/DLB had a clinical diagnosis of AD, two had a clinical diagnosis of DLB, and in two cases the diagnosis was considered to be either AD or DLB. Ten of the 12 FTLD-U cases met criteria for behavioral variant frontotemporal dementia, while the other two met criteria for language variant frontotemporal dementia (Neary et al., 1998). Of the pathologically confirmed PSP cases two were given a clinical diagnosis of apraxia of speech (Josephs et al., 2006a), and one each behavioral variant frontotemporal dementia, corticobasal syndrome, and probable PSP (Litvan et al., 1996). The clinical diagnoses in the five cases of pathologically confirmed CBD were apraxia of speech, corticobasal syndrome, behavioral variant frontotemporal dementia, degenerative dementia, and probable PSP.
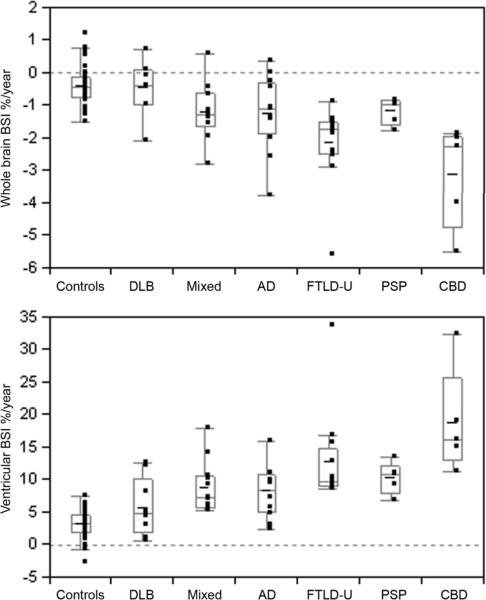
The median interval between the two MRI scans ranged from 1.2 to 2.0 years across the groups, with no significant inter-group differences observed (Table 2). The smallest interval was 6 months, while the largest was 3 years 6 months. There were significant differences in both whole-brain and ventricular atrophy rates across the groups (Table 2 and Figure 1). The results of post hoc testing assessing differences between all groups are shown in Table 3. Rates of both whole brain and ventricular change were larger in the mixed, AD, FTLD-U, CBD and PSP groups compared to controls. There was no significant difference between the DLB group and the control group in either measure. The largest rates of atrophy were observed in the CBD group which had a rate of whole brain atrophy of 2.3% per year and ventricular expansion of 16% per year. The CBD group had significantly greater rates of whole brain BSI and ventricular BSI than the DLB, mixed AD/DLB, AD and PSP groups. There was also a trend for the rates of atrophy in the CBD group to be larger than those observed in the FTLD-U group. The FTLD-U group showed the next largest rates with a rate of whole brain atrophy of 1.7% per year and ventricular expansion of 10% per year which were significantly greater than the rates observed in the DLB group. The rate of whole brain atrophy in the FTLD-U group was also significantly greater than those observed in the mixed AD/DLB and PSP groups. There was no significant difference in the rates of atrophy between the AD, mixed AD/DLB and PSP groups, which all showed rates of whole brain atrophy of approximately 1% per year and rates of ventricular expansion varying from 7 to 11% per year. No correlations were found between rates of whole brain atrophy or ventricular expansion and disease duration, baseline MMSE or CDR within any of the disease groups or across all disease groups. However age at baseline scan correlated with whole brain atrophy (r=0.3; P <0.05) and ventricular expansion (r=−0.5; P< 0.0001) across all groups. After adjusting for age at scan, pathology remained strongly associated with whole brain atrophy and ventricular expansion (P< 0.0001 for both), and we did not find age at scan to be a confounder or an effect modifier.
Table 2.
Whole brain and ventricular data for all groups
| Controls | DLB | Mixed | AD | FTLD-U | PSP | CBD | P values | |
|---|---|---|---|---|---|---|---|---|
| Scan | 1.4 | 1.3 | 1.2 | 1.4 | 1.2 | 2.0 | 1.0 | NS |
| interval | (1.0, 2.7) | (1.0,2.5) | (0.8, 2.7) | (0.6,2.6) | (0.5,2.2) | (1.4,3.5) | (0.9,1.1) | |
| Whole | −0.3 | −0.4 | −1.3 | −1.1 | −1.7 | −1.0 | −2.3 | <0.0001 |
| brain BSI | (1.2, −0.8) | (0.7, −2.1) | (0.6, −2.8) | (0.4, −3.8) | (−0.9,−5.6) | (−0.8,−1.5) | (−1.9,−5.5) | |
| Ventricular | 3.1 | 4.8 | 7.2 | 8.3 | 9.6 | 10.9 | 16.2 | <0.0001 |
| BSI | (−2.8,7.5) | (0.5, 12.6) | (5.1, 17.8) | (2.4, 16.0) | (8.6,33.7) | (6.9,13.4) | (11.2,32.4) |
Data is shown as median (range). Negative rates represent a decrease in volume over time, whereas positive rates represent an increase in volume. P values are calculated using the Kruskal-Wallis test.
Figure 1.
Box-plots showing rates of whole brain loss and ventricular expansion for all groups. Negative rates represent a decrease in volume over time, whereas positive rates represent an increase in volume. Therefore, a brain volume loss is represented as negative rates, and ventricular expansion is represented as positive rates. The horizontal lines of the boxes represent the 25th, 50th (median), and 75th percentiles of the distributions. The vertical lines extending from the boxes stop at the most extreme data point within 1.5 inter-quartile ranges of the box.
Table 3.
P values for all inter-group comparisons of whole brain and ventricular BSI performed using the Mann-Whitney U test
| Group | BSI | DLB | Mixed | AD | FTLD-U | PSP | CBD |
|---|---|---|---|---|---|---|---|
| Controls | Brain | 0.8 | 0.002 | 0.004 | <0.0001 | 0.006 | 0.0004 |
| Ventricle | 0.07 | <0.0001 | 0.0002 | <0.0001 | 0.0006 | 0.0004 | |
| DLB | Brain | 0.09 | 0.13 | 0.007 | 0.12 | 0.012 | |
| Ventricle | 0.06 | 0.19 | 0.005 | 0.07 | 0.006 | ||
| Mixed | Brain | 0.69 | 0.04 | 0.90 | 0.01 | ||
| Ventricle | 0.72 | 0.06 | 0.22 | 0.006 | |||
| AD | Brain | 0.06 | 1.00 | 0.02 | |||
| Ventricle | 0.09 | 0.34 | 0.007 | ||||
| FTLD-U | Brain | 0.04 | 0.09 | ||||
| Ventricle | 0.92 | 0.05 | |||||
| PSP | Brain | 0.009 | |||||
| Ventricle | 0.02 |
DISCUSSION
We report rates of whole brain atrophy and ventricular expansion in a number of different pathologically confirmed neurodegenerative disorders characterized by the deposition of different proteins. The results demonstrate differences between the groups with the lowest rates of atrophy observed in the patients with DLB and the highest rates in the CBD and FTLD-U groups. The rates were very similar between the AD group, the group of subjects with mixed AD/DLB, and the PSP group. These results therefore show that rates of cerebral atrophy do differ according to the underlying pathology and protein biochemistry.
The median rate of whole brain atrophy in the AD subjects was 1.1% per year, with a ventricular expansion of 8.3% per year. The rates of atrophy were significantly greater than in the control group. These rates are comparable with previous studies that have investigated clinical cohorts of subjects with AD (Fox and Freeborough, 1997; Gunter et al., 2003; Jack et al., 2004). These results support previous clinical studies showing that rates of atrophy are good discriminators of AD from controls, but add extra weight because this is a pathologically confirmed sample.
The DLB subjects showed a much lower rate of atrophy compared to the AD group, with rates of whole brain atrophy of only 0.4% per year and ventricular expansion of only 4.8% per year. These rates were not significantly different from the control group suggesting that very little global brain atrophy occurs over time in this group and perhaps that the deposition of α-synuclein itself does not result in wide-spread neuronal loss. However, the subjects with both AD and DLB pathology had larger rates of atrophy which were similar to those observed in the AD group. Therefore, the presence of the AD-type pathological changes is likely driving the loss of brain volume in these subjects. Similarly, a previous study demonstrated that atrophy of the temporal lobe correlated with the presence of AD-type pathology in pathologically confirmed cases of DLB (Harvey et al., 1999). Although there was a large degree of overlap between the groups, rates of both whole brain and ventricular change were substantially larger in the mixed subjects than those with DLB suggesting that rates of atrophy may help differentiate these groups of subjects which are notoriously difficult to differentiate clinically. For example, a subject presenting with a clinical diagnosis of DLB with a low rate of atrophy is more likely to show pure DLB pathology, whereas a subject presenting with a clinical diagnosis of DLB and a high rate of loss is more likely to show mixed AD/DLB pathology. Interestingly, of the 13 subjects with mixed AD/DLB pathology the majority had a clinical diagnosis of AD rather than DLB. This suggests that the memory deficits characteristic of AD are the most likely presentation in cases of mixed pathology. No previous studies have assessed rates of brain atrophy on MRI in pathologically confirmed cohorts of subjects with DLB pathology, although a number of studies have cross-sectionally assessed subjects with a clinical diagnosis of DLB (Ballmaier et al., 2004; Brenneis et al., 2004; Burton et al., 2002; Hanyu et al., 2005; Whitwell et al., In Press) and looked at gross atrophy patterns using post-mortem brains (Cordato et al., 2000). A number of cross-sectional studies have found patterns of brain loss similar to those found in AD (Ballmaier et al., 2004; Burton et al., 2002), although others have suggested that subjects with DLB show more focused loss in the basal areas of the brain (Brenneis et al., 2004; Hanyu et al., 2005; Whitwell et al., In Press). Rates of whole brain atrophy have also been reported to be similar in subjects clinically diagnosed with DLB and AD (O'Brien et al., 2001). The rate observed in DLB was 1.4% per year which is more similar to the rate we observed in the mixed pathology group rather than the pure DLB group, suggesting that the cohorts of subjects diagnosed with DLB on purely clinical criteria are likely to contain subjects with mixed AD/DLB pathology.
The pathological diagnosis of FTLD-U is the most common pathology to underlie the clinical diagnosis of frontotemporal dementia (Josephs et al., 2004a). The rates of whole brain atrophy were 1.7% per year with rates of ventricular expansion of 9.6% per year in this group. There was a trend for these rates to be greater than those observed in the AD group. One previous study has reported rates of whole brain atrophy of 3% per year in a clinical cohort of subjects with frontotemporal dementia (Chan et al., 2001). While these rates were larger than those reported in our FTLD-U cohort these subjects were not pathologically confirmed and were approximately 10 years younger than those in our study. They did however similarly demonstrate a trend for greater rates of loss in frontotemporal dementia than AD, although in contrast to our study they observed a large overlap in rates between controls and the frontotemporal dementia subjects (Chan et al., 2001).
Corticobasal degeneration and PSP can also underlie frontotemporal dementia (Josephs et al., 2006c; Kertesz et al., 2005). Both CBD and PSP are tau-positive disorders whereas FTLD-U is tau-negative. Previous cross-sectional pathological and imaging studies have suggested that patterns of brain atrophy do not differ between tau-positive and tau-negative FTLD pathologies (Broe et al., 2003; Kril et al., 2005; Mann and South, 1993; Whitwell et al., 2004). However, these studies have tended to lump the different pathological subtypes of tau-positive and tau-negative FTLD. The rates of atrophy observed in this study differed across the FTLD-U, CBD and PSP groups suggesting that it is more informative to separate the specific pathological diagnoses. In fact, previous MRI studies have similarly demonstrated different patterns of cerebral atrophy in pathologically confirmed FTLD-U, CBD, PSP and Pick disease (Josephs et al., 2006e; Whitwell et al., 2006; Whitwell et al., 2005). Unfortunately we had no cases of Pick disease with two volumetric head scans, and therefore we were unable to investigate differences from FTLD-U, CBD and PSP.
The rates of whole brain atrophy and ventricular expansion were significantly greater in the CBD group compared to the PSP group even though both had clinically demented subjects. The rates of whole brain atrophy in the PSP group were 1% per year with a ventricular expansion of 11% per year which were similar to those rates observed in the AD group, and those that we have previously published (Josephs et al., 2006d). Although in earlier reports, dementia has been considered one of the discriminators against pathologic confirmed PSP (Josephs and Dickson, 2003), recent studies have demonstrated that PSP can underlie a dementia presentation (Forman et al., 2006; Josephs et al., 2006a; Kertesz et al., 2005). Similar rates of atrophy have previously been reported in a clinically diagnosed PSP cohort without dementia (Paviour et al., 2006). Interestingly, the rates of loss between our PSP subjects and their PSP subjects were similar despite the fact that our subjects presented with a dementia syndrome as opposed to a more classic extrapyramidal presentation. The rates of atrophy in CBD were larger than those observed in all other groups, with rates of whole brain atrophy of 2.3% per year and ventricular expansion of 16.2 % per year. No previous longitudinal MRI studies have reported rates of atrophy in CBD, although cross-sectional studies have shown cortical atrophy affecting the frontal and parietal lobes in pathologically confirmed CBD (Josephs et al., 2004b; Josephs et al., 2006e), and demonstrated greater atrophy cross-sectionally in CBD compared to PSP on MRI and pathologically (Boxer et al., 2006; Groschel et al., 2004; Josephs et al., 2006e; Schofield et al., 2005). The extent and magnitude of cortical atrophy has also been shown to be larger in subjects with CBD compared to an age, gender and severity matched group of AD subjects (Kitagaki et al., 2000). The increased rates of atrophy in CBD, along with the fact that the CBD patients performed worse on tests of clinical severity than the PSP patients, yet had a similar time from disease onset to scan, suggests that CBD is a more rapidly progressive disease. These results suggest first that rates of atrophy will be useful in the differentiation of subjects with pathological CBD from PSP. This differentiation is particularly important since the clinical syndromes associated with these diseases overlap to a large degree and misdiagnosis is common (Boeve et al., 2003). Up to 50% of subjects that are given a clinical diagnosis of CBD actually have PSP on pathology (Josephs et al., 2006c), and subjects with PSP on pathology are most often misdiagnosed as CBD clinically (Josephs and Dickson, 2003). Secondly, these results suggest that it is not just the biochemistry that determines the rates of brain atrophy, but that the distribution and lesion burden may also be important.
There was a trend for the CBD group to have a greater rate of atrophy compared to the FTLD-U group. This was observed for both whole brain atrophy and ventricular expansion, and may have reached significance if our subject groups were larger. Recently, many clinicians and scientists have recognized that CBD pathology may underlie an FTD presentation (Forman et al., 2006; Hodges et al., 2004; Josephs et al., 2006c; Kertesz et al., 2005) and that FTLD-U pathology can underlie a CBS presentation (Benussi et al., 2006; Grimes et al., 1999; Masellis et al., 2006). It is therefore important to be able to differentiate these two pathologies antemortem. Imaging-pathological studies with a larger number of subjects per group may reveal that rates of atrophy calculations can differentiate CBD from FTLD-U pathology.
While the rates of atrophy appear to differ across different pathologies, the role of the specific protein biochemistry is also important. Although most researchers consider one protein to be the primary protein in each of the main pathologies, in some cases there are mixed protein biochemistries. In AD, β-amyloid is considered by many researchers to be the primary protein (Cummings, 2003; Hardy and Selkoe, 2002; Selkoe, 2002), although tau-positive NFT (NIA, 1997) are also present and as important for the pathological diagnosis (NIA, 1997). In addition, while α-synuclein is considered the primary protein in DLB, the majority of cases also have diffuse β-amyloid-positive senile plaques, albeit not of the neuritic type required for a diagnosis of AD (NIA, 1997). These plaques are considered to be similar to those found in pathological aging (Dickson et al., 1989). The fact that our study has shown very low rates of brain atrophy in the subjects with DLB would suggest that neither α-synuclein nor β-amyloid cause wide-spread neuronal loss, although one study has shown that Lewy body density correlates with frontal atrophy in cases of DLB (Cordato et al., 2000). Yet, while the results from the DLB group suggest that the presence of β-amyloid do not result in large degrees of brain loss, it is unclear whether it is the combination of β-amyloid and tau, or only tau that drive the brain loss in AD subjects. In one study there was a correlation between levels of brain amyloid identified on 11C-PIB positron emission tomography and rates of brain atrophy in AD (Archer et al., 2006). This study may suggest a role for amyloid causing atrophy in AD. However, it is almost certain that the results were affected by the presence of tau-positive pathology, since subjects with a large amount of amyloid will also have a high density of tau-positive lesions as both NFT and the neuritic components of the AD plaques are tau-positive. A number of studies have demonstrated correlations between the presence of temporal lobe atrophy and the presence and severity of neurofibrillary tangles (Jack et al., 2002; Nagy et al., 1996), as well as cerebrospinal fluid levels of tau in AD (Hampel et al., 2005). In fact one study found a better correlation between temporal lobe volume and neurofibrillary tangle density than with the density of amyloid plaques (Nagy et al., 1996). Similarly, correlations have been demonstrated between the density of NFT and regional atrophy in PSP (Cordato et al., 2000). The distribution of pathology in PSP also correlates to the distribution of brain atrophy observed on MRI (Paviour et al., 2004; Yagishita and Oda, 1996).
The question of whether the specific protein inclusions in FTLD-U contribute directly to the pattern and degree of brain atrophy is less certain. It has been suggested that ubiquitin-positive inclusions may not play a critical role in the neurodegenerative process in FTLD-U (Kersaitis et al., 2006), and cases of FTLD-U have been reported with a high density of inclusions that have no brain atrophy at post mortem (Josephs et al., 2006f).
The major strengths of this study are that all cases had undergone a detailed autopsy analysis and the biochemical diagnoses were made with strict criteria and not complicated by other secondary pathologies. The groups were also well matched demographically and the clinical diagnoses were made prospectively using modern clinical criteria. However, a limitation of the study is the fact that the control subjects did not have autopsy confirmation. Although it is possible that some of these subjects may show biochemical abnormalities on pathology it is unlikely given the low rates of atrophy and the fact that most of the subjects had been followed for several years and remained clinically normal. A potential confounder to the comparison of CBD and PSP is the fact that the PSP subjects had a larger average scan interval than the subjects with CBD. A large scan interval reduces the accuracy of a linear approximation of the data, while any errors or noise within the measurements are amplified over shorter intervals. It is however difficult to predict the net affect of these factors on our observed rates. The number of subjects in the CBD and PSP groups was also small. However, both groups are representative, in terms of disease onset, duration and severity, of a larger pathological cohort that we have previously published (Josephs et al., 2006c). In addition to the underlying pathology and biochemistry, age at scan does also seem to be playing a role in the rates of brain atrophy. Further investigation of this finding is warranted. It is possible that regional rates of atrophy may also be useful in differentiating the different pathological groups (Paviour et al., 2006). Future studies employing highly deformable registration algorithms, rather than a rigid-body registration algorithm, would enable an assessment of local changes throughout the entire brain.
In summary, this study has demonstrated for the first time increased rates of whole brain atrophy and ventricular expansion in subjects with pathologically confirmed AD, mixed AD/DLB, FTLD-U, CBD and PSP compared to controls, yet low rates of change in subjects with pure DLB pathology. These rates may help differentiate the mixed cases from the cases with pure DLB, although will not help to differentiate the mixed cases from cases with AD. Large differences were also observed between cases with CBD and PSP pathology, with larger rates in the CBD subjects. Therefore, rates of atrophy differ across different pathological disorders and may prove valuable for future disease modifying treatment trials which will target the protein biochemistry underlying each of these diseases.
Acknowledgements
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786, R01 AG15866, and R01 AG11378 from the National Institute on Aging, Bethesda MD, NIRG-03-4842 from the Alzheimer's Association, and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation, U.S.A. DSK has been a consultant to GE HealthCare, GlaxoSmithKline and Myriad Pharmaceuticals, has served on a Data Safety Monitoring Board for Neurochem Pharmaceuticals, and is an investigator in a clinical trial sponsored by Elan Pharmaceuticals. RCP has been a consultant to GE Healthcare and an investigator in a clinical trial sponsored by Elan Pharmaceuticals. We would like to thank Vernon Shane Pankratz, PhD, and Stephen Weigand, MS, for their statistical help and advice, and acknowledge Professor Nick Fox at the Dementia Research Centre, London, UK, for providing the MIDAS image analysis software.
References
- Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, et al. Amyloid load and cerebral atrophy in Alzheimer's disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–7. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, O'Brien JT, Burton EJ, Thompson PM, Rex DE, Narr KL, et al. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004;23:325–35. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999;52:1153–8. doi: 10.1212/wnl.52.6.1153. [DOI] [PubMed] [Google Scholar]
- Benussi L, Binetti G, Sina E, Gigola L, Bettecken T, Meitinger T, et al. A novel deletion in progranulin gene is associated with FTDP-17 and CBS. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–9. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Wenning GK, Egger KE, Schocke M, Trieb T, Seppi K, et al. Basal forebrain atrophy is a distinctive pattern in dementia with Lewy bodies. Neuroreport. 2004;15:1711–4. doi: 10.1097/01.wnr.0000136736.73895.03. [DOI] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–11. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17:618–30. [PubMed] [Google Scholar]
- Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001;57:1756–63. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, et al. Change in rates of cerebral atrophy over time in early-onset Alzheimer's disease: longitudinal MRI study. Lancet. 2003;362:1121–2. doi: 10.1016/S0140-6736(03)14469-8. [DOI] [PubMed] [Google Scholar]
- Cordato NJ, Halliday GM, Harding AJ, Hely MA, Morris JG. Regional brain atrophy in progressive supranuclear palsy and Lewy body disease. Ann Neurol. 2000;47:718–28. [PubMed] [Google Scholar]
- Cummings JL. Toward a molecular neuropsychiatry of neurodegenerative diseases. Ann Neurol. 2003;54:147–54. doi: 10.1002/ana.10616. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal H, Mattiace LA, Kress Y, Schwagerl A, Ksiezak-Reding H, et al. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol (Berl) 1989;78:572–84. doi: 10.1007/BF00691284. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Design of Experiments. Oliver and Boyde; London: 1935. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of A{beta} immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J Magn Reson Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–9. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC, Kitney RI. Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed. 1997;53:15–25. doi: 10.1016/s0169-2607(97)01803-8. [DOI] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, et al. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol. 1994;51:888–95. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Serpell LC, Berriman J, Smith MJ, Jakes R, et al. From genetics to pathology: tau and alpha-synuclein assemblies in neurodegenerative diseases. Philos Trans R Soc Lond B Biol Sci. 2001;356:213–27. doi: 10.1098/rstb.2000.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, Bergeron CB, Lang AE. Motor neuron disease-inclusion dementia presenting as cortical-basal ganglionic degeneration. Mov Disord. 1999;14:674–80. doi: 10.1002/1531-8257(199907)14:4<674::aid-mds1019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Groschel K, Hauser TK, Luft A, Patronas N, Dichgans J, Litvan I, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21:714–24. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- Gunter JL, Shiung MM, Manduca A, Jack CR., Jr. Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Burger K, Pruessner JC, Zinkowski R, DeBernardis J, Kerkman D, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62:770–3. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Tanaka Y, Shimizu S, Sakurai H, Iwamoto T, Abe K. Differences in MR features of the substantia innominata between dementia with Lewy bodies and Alzheimer's disease. J Neurol. 2005;252:482–4. doi: 10.1007/s00415-005-0611-8. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harvey GT, Hughes J, McKeith IG, Briel R, Ballard C, Gholkar A, et al. Magnetic resonance imaging differences between dementia with Lewy bodies and Alzheimer's disease: a pilot study. Psychol Med. 1999;29:181–7. doi: 10.1017/s0033291798007806. [DOI] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–8. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–26. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006a;129:1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathology & Applied Neurobiology. 2004a;30:369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006b;63:506–12. doi: 10.1001/archneur.63.4.506. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006c;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Tang-Wai DF, Edland SD, Knopman DS, Dickson DW, Parisi JE, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004b;61:1881–4. doi: 10.1001/archneur.61.12.1881. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Boeve BF, Shiung MM, Gunter JL, Parisi JE, et al. Rates of cerebral atrophy in autopsy-confirmed progressive supranuclear palsy. Ann Neurol. 2006d;59:200–3. doi: 10.1002/ana.20707. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2006e doi: 10.1016/j.neurobiolaging.2006.09.019. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Jack CR, Parisi JE, Dickson DW. Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol. 2006f;63:1632–8. doi: 10.1001/archneur.63.11.1632. [DOI] [PubMed] [Google Scholar]
- Kersaitis C, Halliday GM, Xuereb JH, Pamphlett R, Bak TH, Hodges JR, et al. Ubiquitin-positive inclusions and progression of pathology in frontotemporal dementia and motor neurone disease identifies a group with mainly early pathology. Neuropathol Appl Neurobiol. 2006;32:83–91. doi: 10.1111/j.1365-2990.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Hirono N, Ishii K, Mori E. Corticobasal degeneration: evaluation of cortical atrophy by means of hemispheric surface display generated with MR images. Radiology. 2000;216:31–8. doi: 10.1148/radiology.216.1.r00ma0531. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Macdonald V, Patel S, Png F, Halliday GM. Distribution of brain atrophy in behavioral variant frontotemporal dementia. J Neurol Sci. 2005;232:83–90. doi: 10.1016/j.jns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Luis CA, Barker WW, Gajaraj K, Harwood D, Petersen R, Kashuba A, et al. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry. 1999;14:526–33. [PubMed] [Google Scholar]
- Mann DM, South PW. The topographic distribution of brain atrophy in frontal lobe dementia. Acta Neuropathol (Berl) 1993;85:334–40. doi: 10.1007/BF00227731. [DOI] [PubMed] [Google Scholar]
- Masellis M, Momeni P, Meschino W, Heffner R, Jr., Elder J, Sato C, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain. 2006;129:3115–23. doi: 10.1093/brain/awl276. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Ballard CG, Perry RH, Ince PG, O'Brien JT, Neill D, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–8. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, OBrien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–90. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Jobst KA, Esiri MM, Morris JH, King EM, MacDonald B, et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7:76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- NIA Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- O'Brien JT, Paling S, Barber R, Williams ED, Ballard C, McKeith IG, et al. Progressive brain atrophy on serial MRI in dementia with Lewy bodies, AD, and vascular dementia. Neurology. 2001;56:1386–8. doi: 10.1212/wnl.56.10.1386. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain. 2006;129:1040–9. doi: 10.1093/brain/awl021. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Schott JM, Stevens JM, Revesz T, Holton JL, Rossor MN, et al. Pathological substrate for regional distribution of increased atrophy rates in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:1772–5. doi: 10.1136/jnnp.2003.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rencher AC. Methods of Multivariate Analysis. Wiley; New York: 2002. [Google Scholar]
- Schofield EC, Caine D, Kril JJ, Cordato NJ, Halliday GM. Staging disease severity in movement disorder tauopathies: brain atrophy separates progressive supranuclear palsy from corticobasal degeneration. Mov Disord. 2005;20:34–9. doi: 10.1002/mds.20286. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr., Senjem ML, Josephs KA. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology. 2006;66:102–4. doi: 10.1212/01.wnl.0000191395.69438.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Warren JD, Josephs KA, Godbolt A, Revesz T, Fox N, et al. Voxel-based morphometry in tau-positive and tau-negative frontotemporal lobar degenerations. Neurodegenerative diseases. 2004;1:225–230. doi: 10.1159/000080990. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in Dementia with Lewy Bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. doi: 10.1093/brain/awl388. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita A, Oda M. Progressive supranuclear palsy: MRI and pathological findings. Neuroradiology. 1996;38(Suppl 1):S60–6. doi: 10.1007/BF02278121. [DOI] [PubMed] [Google Scholar]