Abstract
Background
Mutations in the progranulin gene (PGRN) have recently been identified as a cause of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) in some families.
Objective
To determine whether there is a difference in the patterns of atrophy in cases with FTLD-U with and without a mutation in PGRN.
Design
Case control study
Setting
Brain bank of a tertiary care medical center
Patients
All subjects that had screened positive for mutations in PGRN and had a volumetric MRI were identified (n=8, PGRN (+)). Subjects were then matched by clinical diagnosis to a group of eight subjects with a pathological diagnosis of FTLD-U that had screened negative for mutations in PGRN (PGRN (−)). All subjects were then age and gender-matched to a control subject.
Main outcome Measures
Voxel-based morphometry was used to assess the patterns of grey matter atrophy in the PGRN (+) and (−) groups compared to controls, and compared to each other.
Results
The PGRN (+) group showed a widespread and severe pattern of grey matter loss predominantly affecting the frontal, temporal and parietal lobes. In comparison, the PGRN (−) group showed a less severe pattern of loss restricted mainly to the temporal and frontal lobes. On direct comparison the PGRN (+) group showed greater loss in the frontal and parietal lobes compared to the PGRN (−) group.
Conclusions
This study suggests that PGRN mutations may be associated with a specific and severe pattern of cerebral atrophy in subjects with FTLD-U.
Keywords: Frontotemporal dementia, Voxel-based morphometry, Ubiquitin, Dentate, Progranulin
INTRODUCTION
There are three well recognized clinical variants of frontotemporal lobar degeneration: behavioral variant frontotemporal dementia (bvFTD); progressive non-fluent aphasia (PNFA); and semantic dementia (SD)1. The bvFTD is characterized by behavioral and personality changes and executive dysfunction, while the other two variants are aphasic syndromes. Pathologically the frontotemporal lobar degenerations can be separated in those with tau-positive inclusions and those with ubiquitin-positive inclusions2. Two recent studies have demonstrated that mutations in the progranulin gene (PGRN) are associated with frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U)3, 4.
The aim of this study was to determine if subjects with FTLD-U and a mutation in PGRN have a signature pattern of grey matter atrophy. We hypothesized that the pattern of atrophy in FTLD-U subjects with a mutation in PGRN would be no different from the pattern of atrophy of those without a mutation.
DESIGN AND METHODS
Subjects
We identified six subjects with a pathological diagnosis of FTLD-U from our autopsy database located at the Mayo Clinic in Rochester that had frozen brain tissue or stored DNA blood which had screened positive for mutations in PGRN (PGRN (+)) and which had a useable volumetric head MRI scan. Two additional cases without pathological confirmation were included: each had screened positive for mutations in PGRN and had pathological confirmation in multiple siblings.
All cases had been prospectively studied in our Alzheimer's Disease Research Center (ADRC) or Alzheimer's Disease Patient Registry (ADPR). The historical records of all cases were reviewed by an expert in neurodegenerative diseases (KAJ) for the abstraction of data, including gender, age at onset, illness duration, and short test of mental status (STMS)5, and the signs and symptoms recorded in the historical records by the treating physician up to the time of MRI scan (Table 1). We also abstracted information on whether or not behavioral features, parkinsonism, or significant language impairment developed prior to death. Behavioral features was defined as any change in the subjects behavior or personality; parkinsonism as the presence of two or more of tremor, bradykinesia, rigidity, or postural instability; and language impairment as any loss of syntax, fluency, comprehension, word-finding difficulties, or anomia. Each of the PGRN (+) subjects was then matched by clinical diagnosis to a subject that had screened negative for mutations in PGRN (Table 1). Six of the PGRN (+) subjects were diagnosed with bvFTD and were therefore matched to PGRN (−) subjects with a clinical diagnosis of bvFTD; one PGRN (+) subject had SD and was matched to a PGRN (−) subject with SD; and one PGRN (+) subject had left-sided corticobasal syndrome (CBS) and was therefore matched to a PGRN (−) subject with left-sided CBS. This matching was important since the different clinical phenotypes of FTLD-U may themselves have different patterns of grey matter atrophy6. The aim of this study was to assess the effects of the PGRN mutation independent of clinical phenotype. Each of the PGRN (+) and (−) subjects were age and gender matched to a healthy normal control.
Table 1.
Clinical features of PGRN (+) and (−) subjects
| Sex | Age at onset | Illness duration (years) | STMS at scan | *Diagnosis at the time of Scan | Prominent symptoms up to the time of MRI scan | Symptoms present over entire disease course (+ = present, 0 = absent) | |||
|---|---|---|---|---|---|---|---|---|---|
| Parkinsonism | Language | Behavior | |||||||
| PGRN (−) | |||||||||
| 1 | F | 64 | 13 | 25 | bvFTD | Personality change, apathy, loss of interest | 0 | 0 | + |
| 2 | F | 62 | 8 | 34 | bvFTD | Anhedonic, lack of initiative, poor judgment | 0 | 0 | + |
| 3 | M | 45 | 13 | 26 | bvFTD | Personality change, withdrawn, lack of initiative | 0 | 0 | + |
| 4 | F | 68 | 3 | NA | CBS | Parkinsonism, L>R limb apraxia | + | 0 | 0 |
| 5 | F | 64 | 6 | 34 | bvFTD | Changes in behavior and personality | + | 0 | + |
| 6 | M | 51 | 1 | 30 | bvFTD | Executive dysfunction, decreased personal hygiene, inappropriate laughter, compulsive behaviors | + | + | + |
| 7 | F | 59 | 7 | 26 | SD | Difficulty with naming of people and objects, and circumlocutions, behavioral changes | 0 | + | + |
| 8 | F | 77 | 6 | 31 | bvFTD | Personality change, executive dysfunction, & paranoia | 0 | 0 | + |
| PGRN (+) | |||||||||
| 9 | M | 69 | 7 | 28 | bvFTD | Executive dysfunction, loss of attention, & memory loss | + | + | + |
| 10 | M | 60 | 8 | 31 | bvFTD | Personality change, executive dysfunction & memory loss | + | + | + |
| 11 | F | 52 | 5 | 23 | bvFTD | Personality change, executive dysfunction, abulia, memory loss, and decrease word output & fluency | + | + | + |
| 12 | M | 49 | 6 | 27 | CBS | Parkinsonism, L>R limb apraxia | + | + | 0 |
| 13 | M | 56 | 7 | 34 | bvFTD | Personality change | + | 0 | + |
| 14χ | F | 56 | 7 | 32 | bvFTD | Personality change, executive dysfunction & memory loss | + | + | + |
| 15χ | F | 61 | NA | NA | SD | Difficulty with naming of people, places, and things and comprehension problems | 0 | + | 0 |
| 16 | F | 56 | NA | 22 | bvFTD | Executive dysfunction | + | + | + |
STMS = Short Test of Mental Status Score
Subjects still alive
Using Fishers Exact test p =0.05 for parkinsonism PGRN (−) v PGRN (+) (30% vs. 90%) and P = 0.02 for language (20% vs. 90%) using a one tail test (assuming that the feature would be more frequent in the PGRN (+) group).
Genetic analysis
The 12 coding exons of PGRN were amplified by PCR using our previously published primers and protocol3. Novel PCR primers flanking the non-coding exon 0 and the 3'untranslated region (UTR) of PGRN were also developed. Standard 25μl PCR reactions were performed using Qiagen PCR products (Qiagen, Valencia, CA) and a final concentration of 0.8μM for each primer. The PGRN exon 0 and 3'UTR fragments were cycled using touchdown protocols of 70–55°C and 58–48°C respectively. PCR products were purified with Multiscreen plates (Millipore, Billerica, MA) and sequenced in both directions on an ABI 3730 with the Big Dye chemistry following manufacturer's protocol (Applied Biosystems, Foster City, CA). Genomic characterization of PGRN was performed as previously described7.
Pathological analysis
All cases had undergone extensive neuropathological examination with routine and immunohistochemical stains as previously described in detail2. A pathological diagnosis of FTLD-U was made on the basis of finding neuronal loss, spongiosis and gliosis in frontotemporal neocortex consistent with frontotemporal lobar degenerative changes, as well as, the presence of ubiquitin-positive intraneuronal inclusions and neurites in the neocortex or the dentate granule cell layer of the hippocampus. Any case with evidence of motor neuron disease was excluded as previously described8.
Image analysis
MR studies were performed at 1.5T with a standardized imaging protocol that included a coronal T1-weighted 3-dimensional volumetric spoiled gradient echo (SPGR) sequence with 124 contiguous partitions and 1.6mm slice thickness (22×16.5cm FOV, 25° flip angle). Voxel-based morphometry (VBM) was used to assess group differences, implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm)9, 10. The image processing steps have previously been described in detail10, 11. Briefly, all images were spatially normalized to a customized template created from all subjects in the study. The spatial normalization of each image was optimized by matching the initial grey matter segmentation with the grey matter prior and applying the parameters to the whole head. Images were then segmented using customized prior probability maps, modulated, and smoothed with a 10mm FWHM smoothing kernel. Grey matter differences were assessed between both the PGRN (+) and (−) groups and the control group at a statistical threshold of p<0.05 after correction for multiple comparisons using the false discovery rate (FDR). Direct comparisons between the PGRN (+) and (−) groups were performed at a less stringent threshold of p<0.01 uncorrected for multiple comparisons.
RESULTS
Subjects
Table 1 details the clinical features of the PGRN (+) and (−) subjects. The majority had a clinical diagnosis of bvFTD, although one subject in each group also had a diagnosis of SD and CBS. The demographic features in each of the groups are compared in Table 2. There was no significant difference in the gender ratio or age at scan across all three subject groups. In addition, there was no significant difference between the PGRN (+) and (−) subjects in age at onset, total illness duration, time from onset to scan, or STMS. All subjects were Caucasian and non-Hispanic in origin. The subjects with a mutation in PGRN all had an autosomal dominant pattern of inheritance while only two of the subjects that tested negative for PGRN mutations had any suggestion of a family history. Additional clinical features developed after the time of the MRI scan (Table 1). The PRGN (+) subjects were more likely to develop language impairment (90% vs.20%; p=0.03) and parkinsonian symptoms (90% vs. 30%; p=0.05) compared to the PGRN (−) subjects prior to death.
Table 2.
Demographics of all subjects
| PGRN positive (n=8) | PGRN negative (n=8) | Controls (n=16) | |
|---|---|---|---|
| Gender ratio M/F | 4/4 | 2/6 | 6/10 |
| Age at disease onset | 57.4 (6.1) | 61.3 (9.9) | NA |
| Total illness duration | 6.7 (1.0)* | 7.5 (4.0) | NA |
| Age at scan | 60.1 (6.6) | 64.5 (9.5) | 62.4 (6.9) |
| Time from disease onset to time of MRI scan | 2.7 (1.0) | 3.2 (1.9) | NA |
| STMS at scan† | 28.1 (4.5) | 29.4 (3.8) | 36.3 (2.2) |
Results are shown as mean (standard deviation). Total illness duration represents time from disease onset to time of death. STMS = Short Test of Mental Status Score. Patient demographics were compared using the Mann Whitney U test with significance set at p<0.05. Gender ratios were compared using Chi-square test.
Total illness duration was only available for the six subjects that have died
STMS data was available at the time of scan for seven PGRN positive and seven PGRN negative subjects
Mutations
Sequencing analyses showed that five different PGRN mutations were present in our cohort: c.154delA (p.Thr52HisfsX2), c.910_911insTG (p.Trp304LeufsX58), c.1395_1396insC (p.Cys466LeufsX46), c.1145delC (p.Thr382SerfsX30), c.138+1G>A (IVS1+1G>A).
VBM analysis
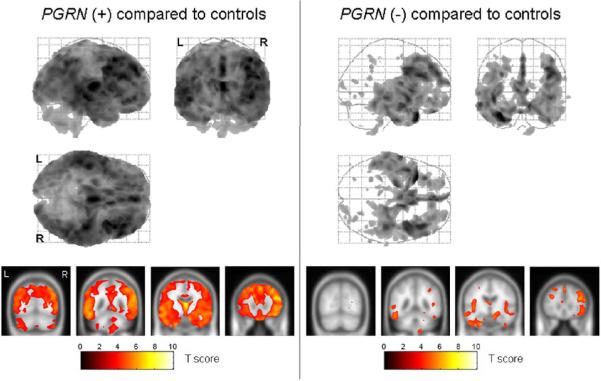
The PGRN (+) group showed a widespread and severe pattern of grey matter loss compared to the control group (Figure 1). The most severe regions of loss were observed in the inferior and middle frontal gyri, the medial frontal lobe, the temporal lobes, and the parietal lobes. The patterns of loss were bilateral, although the frontal and parietal lobe loss appeared to be slightly greater in the right hemisphere. In comparison, the regions of grey matter loss identified in the PGRN (−) group were relatively restricted to the temporal and frontal lobes (Figure 1). It is also noticeable from Figure 1 that the patterns of grey matter loss were less severe than those observed in the PGRN (+) subjects. The temporal lobe loss spread from the anterior temporal pole, back to involve the medial and inferior temporal regions, and the posterior inferior temporal gyri. The frontal lobe loss was observed in the medial frontal cortex, the inferior and middle frontal gyri, and the orbitofrontal cortex. The insula was also involved. As in the PGRN (+) group the frontal lobe loss was slightly greater on the right.
Figure 1.
Grey matter volume differences were assessed between both the PGRN (+) and (−) groups and the control group using VBM at a statistical threshold of p<0.05 after correction for multiple comparisons. The patterns of grey matter loss in the PGRN (+) group compared to controls are shown on the left, while the patterns of grey matter loss in the PGRN (−) group compared to controls are shown on the right. The results of each comparison are shown both on a 3D glass brain render (top) and overlaid on the smoothed customized template at representative slices through the frontal (y=30), temporal (y=−10 and y=−45) and parietal lobes (−70) (bottom). The results show a more widespread and severe pattern of grey matter loss in the PGRN (+) group when compared to controls, than the PGRN (−) group when compared to controls.
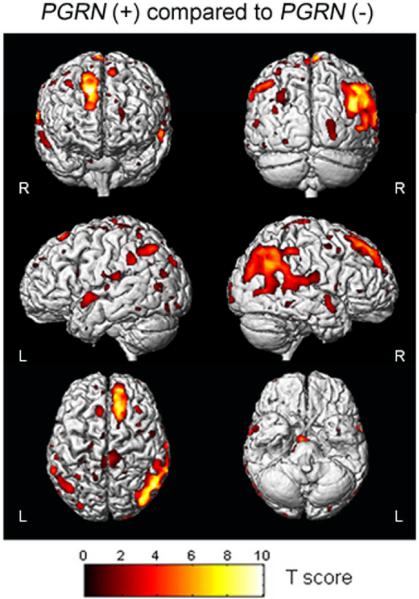
A direct statistical comparison was performed between the two disease groups (Figure 2). The PGRN (+) group showed significantly greater grey matter loss in the superior frontal lobes and the parietal lobes, predominantly on the right, than the PGRN (−) group (uncorrected, p<0.01). No regions showed greater involvement in the PGRN (−) group compared to the PGRN (+) group.
Figure 2.
3D surface renders showing the results of a direct VBM statistical comparison performed between the PGRN (+) and (−) subjects. The highlighted areas show the regions of the brain that have reduced grey matter volume in the PGRN (+) group compared to the PGRN (−) group. No regions of the brain showed reduced grey matter volume in the PGRN (−) group compared to the PGRN (+) group. The results are shown at a statistical threshold of p<0.01, uncorrected for multiple comparisons, and show greater grey matter loss in the frontal and parietal lobes in the PGRN (+) group than the PGRN (−) group.
COMMENT
This study has demonstrated differences in the severity and distribution of grey matter atrophy, as well as clinical differences, in FTLD-U subjects with a mutation in PGRN compared to those without a mutation.
Since both groups had an identical pathological diagnosis of FTLD-U and were matched for clinical diagnosis we had hypothesized that the patterns of atrophy would be similar in those with and without a mutation in PGRN. However, our findings do demonstrate differences between the PGRN (+) and (−) groups. The PGRN (−) subjects showed a pattern of temporal and frontal lobe volume loss as we have previously reported in FTLD-U12, 13. However, the PGRN (+) subjects showed a strikingly more severe and widespread pattern of grey matter loss than the PGRN (−) subjects. The most severe regions of loss were identified in the temporal and frontal lobes, but also in the parietal lobe which was relatively spared in the PGRN (−) group. A direct comparison between the two groups showed that the PGRN (+) subjects had a greater degree of frontal and parietal atrophy than the PGRN (−) subjects. The fact that these results did not survive a correction for multiple comparisons most likely reflects the heterogeneity present in each group. Similarly, our recent pathology study demonstrated significantly lower brain weights at death in PGRN (+) subjects compared to negative subjects (902±160g vs.1030±140g (p<0.01)), and demonstrated greater atrophy of the frontal and parietal lobes in the PGRN (+) subjects (manuscript in submission). Furthermore, another pathology study also found that the frontal lobes were the most severely affected region in PGRN (+) subjects, although the temporal and parietal lobes were also affected14. Single subject MRI scans have also shown frontal, temporal and parietal atrophy in subjects with PGRN mutations14–17. While VBM provides a group-level analysis and should not be generalized to single subjects these results could suggest that a severe pattern of atrophy affecting the temporal and parietal and especially frontal lobes may be suggestive of an underlying mutation in PGRN. It is therefore possible that the mutation results in a more malignant form of FTLD. A recent case study did indeed report a large rate of brain atrophy of over 3.3% in a PGRN (+) subject18. This in turn may reflect differences between familial and sporadic forms of FTLD-U since the majority of the PGRN (−) cases had no family history. Indeed, greater severity of Alzheimer's disease (AD) pathology is found in genetically determined familial AD19, and MRI studies have also shown that younger onset familial cases of AD show a more widespread pattern of atrophy compared to older onset sporadic cases20, 21.
While there were differences between the PGRN (+) and (−) groups it is notable that they both showed involvement of the temporal lobe, particularly the posterior temporal gyri. In two previous VBM studies from separate brain banks we identified a region of severe loss affecting the posterior temporal lobe in FTLD-U and hypothesized that this may be a signature pattern of this pathology12, 13. This study concurs with those results and further suggests that the signature pattern is related to the pathology of FTLD-U and is perhaps independent of the genetic cause of the disease.
The pattern of loss in the PGRN (+) subjects was relatively symmetric, although with slightly greater involvement of the right frontal lobe. However, the lateralization observed in the PGRN (+) subjects may be due to the particular subjects, and particular clinical diagnoses, included in this analysis. Ongoing MRI studies on PGRN at our institution with a larger number of PGRN (+) subjects show greater involvement of the right hemisphere in some, while others show the opposite pattern. Indeed, previous studies reporting on single subject MRI have noted both left-sided17 and right-sided15,18 PGRN (+) cases. Therefore although asymmetry does appear to be a feature of the mutation there appears to be no particular dominant hemisphere.
In our series of PGRN (+) subjects bvFTD was more prevalent than other syndromes, occurring in 75% of the subjects. This is quite similar to the frequency typically reported in FTLD (Miller). These patients presented with personality change, abulia, loss of attention and executive dysfunction1. In addition, bvFTD has been described in other clinical series on PGRN15, 17 and was found to be the most prevalent clinical diagnosis 15. One of our subjects was found to have a diagnosis of CBS22 at the time of scan which has also been described in another family with PGRN mutations16. This subject had been diagnosed with idiopathic Parkinson's disease before presentation at out institution. This is important and suggests that in patients with an initial diagnosis of idiopathic Parkinson's disease, and autosomal dominant family history, a mutation in PGRN should be considered. The one aphasia subject in our cohort had features most in-keeping with the SD syndrome1. This subject had a fluent aphasia with prominent anomia and comprehension problems. This is different from previous PGRN studies in which the aphasia was more in-keeping with the PNFA variant of FTLD14, 17. The PGRN (−) subjects were matched to the PGRN (+) subjects for syndromic diagnosis and therefore 75% were bvFTD, with one each of CBS and SD. As in the bvFTD PGRN (+) subjects, the PGRN (−) subjects had personality changes, apathy, anhedonia and executive dysfunction. The PGRN (−) SD subject had anomia, circumlocutions, and behavioral changes1.
Although our subjects were matched by clinical syndromes at the time of MRI scan, and showed no differences in clinical severity and illness duration, the PGRN (+) subjects were more likely to develop language impairment (90%) and parkinsonism (90%) later in the disease course than the PGRN (−) subjects (20% language, 30% parkinsonism). However, the prevalence of behavioral symptoms was similar between the PGRN (+) (75%) and PGRN (−) (88%) groups. Therefore the later development of significant parkinsonism and language impairment in subjects with an autosomal dominant family history may be a clue to a mutation in the PGRN gene and should prompt the clinician to consider genetic screening. Language impairment and parkinsonism was also found to be more common in the PGRN (+) subjects in our pathological study that included a somewhat different cohort (manuscript submitted). In some of those subjects Lewy Bodies were found which further supports the importance of considering PGRN mutations in autosomal dominant familial Parkinson's disease or in subjects in which parkinsonism is a significant feature. The language impairment was variable with the development of features of both expressive and receptive deficiencies. One feature of note was that the subjects with PGRN (+) mutations frequently became mute. Given the more widespread volume loss seen in the PGRN (+) subjects these developments should be of no surprise. We hypothesize one of two scenarios: Firstly, anatomical structures involved with the development of parkinsonism and aphasia eventually become affected in the PGRN (+) subjects, or they are already affected given the widespread atrophy, but there exists a threshold effect whereby there has to be a certain amount of pathway or anatomic destruction present before the clinical features emerge.
The major strengths of this study were the fact that the PGRN (+) and (−) groups were matched in terms of both clinical and pathological diagnosis, and demographic features. The technique of VBM is also unbiased and allows an assessment of grey matter loss across the whole brain without having to make any a priori assumptions concerning which structures to assess. The limitations include the fact that the number of subjects in each group was relatively small. It is however extremely difficult to find subjects with a mutation in PGRN, artifact free MRI acquired with a standardized protocol, and an autopsy confirmed diagnosis of FTLD-U. Despite the small numbers we still observed well defined patterns of grey matter loss that correlate closely to our previously published findings in larger groups of subjects with FTLD-U12, 13. The results also closely match our recent pathological findings in PGRN (manuscript in submission). However, our cohort represented only five different PGRN mutations and therefore the results may not be generalizable to subjects with other PGRN mutations.
In summary, this study suggests that PGRN mutations may be associated with more severe patterns of cerebral atrophy, particularly involving the frontal and parietal lobes, in subjects with FTLD-U. The subjects with PGRN mutations also developed more parkinsonism and language impairments than the PGRN (−) subjects. It will be important for these results to be validated and correlated to detailed clinical information in a larger group of subjects.
ACKNOWLEDGEMENTS
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078 (KAJ), by grants P50 AG16574, U01 AG06786 and R01 AG11378 from the National Institute on Aging, Bethesda MD, NIRG-03-4842 from the Alzheimer's Association, and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program of the Mayo Foundation, U.S.A. RR is a postdoctoral fellow of the Fund for Scientific Research Flanders (FWO-F).
REFERENCES
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 3.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006 doi: 10.1038/nature05016. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006 doi: 10.1038/nature05017. Epub. [DOI] [PubMed] [Google Scholar]
- 5.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62:281–288. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 6.Whitwell JL, Jack CR, Jr., Duffy JR, et al. MRI correlates of the language and behavioral variants of frontotemporal dementia when both are characterized by ubiquitin-only immunoreactive changes. Alzheimer's & Dementia. 2006;2:S550. [Google Scholar]
- 7.Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 8.Josephs KA, Parisi JE, Knopman DS, et al. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006;63:506–512. doi: 10.1001/archneur.63.4.506. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 10.Senjem ML, Gunter JL, Shiung MM, et al. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitwell JL, Jack CR, Jr., Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.026. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- 13.Whitwell JL, Jack CR, Jr., Senjem ML, Josephs KA. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology. 2006;66:102–104. doi: 10.1212/01.wnl.0000191395.69438.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee O, Pastor P, Cairns NJ, et al. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006;60:374–380. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masellis M, Momeni P, Meschino W, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain. 2006 doi: 10.1093/brain/awl276. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006 doi: 10.1093/brain/awl267. Epub. [DOI] [PubMed] [Google Scholar]
- 18.Boeve BF, Baker M, Dickson DW, et al. Frontotemporal dementia and parkinsonism associated with the IVS1+1G->A mutation in progranulin: a clinicopathologic study. Brain. 2006 doi: 10.1093/brain/awl268. Epub. [DOI] [PubMed] [Google Scholar]
- 19.Lippa CF, Saunders AM, Smith TW, et al. Familial and sporadic Alzheimer's disease: neuropathology cannot exclude a final common pathway. Neurology. 1996;46:406–412. doi: 10.1212/wnl.46.2.406. [DOI] [PubMed] [Google Scholar]
- 20.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer's disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26:333–340. [PMC free article] [PubMed] [Google Scholar]
- 21.Frisoni GB, Testa C, Sabattoli F, et al. Structural correlates of early and late onset Alzheimer's disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2005;76:112–114. doi: 10.1136/jnnp.2003.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]