Summary
Patients with malignant mesothelioma (MM), an aggressive cancer associated with asbestos exposure, usually present clinically with advanced disease and this greatly reduces the likelihood of curative treatment. MM is difficult to diagnose without invasive techniques; the development of non-invasively detectable molecular markers would therefore be highly beneficial. DNA methylation changes in cancer cells provide powerful markers that are potentially detectable non-invasively in DNA shed into bodily fluids. Here we examined the methylation status of 28 loci in 52 MM tumors to investigate their potential as molecular markers for MM. To exclude candidate MM markers that might be positive in biopsies/pleural fluid due to contaminating surrounding non-tumor lung tissue/DNA, we also examined the methylation of these markers in lung samples (age- or environmentally-induced hypermethylation is frequently observed in non-cancerous lung). Statistically significantly increased methylation in MM vs. non-tumor lung samples was found for estrogen receptor 1 (ESR1; p=0.0002), solute carrier family 6 member 20 (SLC6A20; p=0.0022) and spleen tyrosine kinase (SYK; p=0.0003). Examination of associations between methylation levels of the 28 loci and clinical parameters suggest associations of the methylation status of metallothionein genes with gender, histology, asbestos exposure, and lymph node involvement, and the methylation status of leucine zipper tumor suppressor 1 (LZTS1) and SLC6A20 with survival.
Keywords: APC, CpG islands, DNA methylation, ESR1, lung, mesothelioma, metallothionein, SLC6A20, SYK
Introduction
Malignant mesothelioma (MM) is an aggressive cancer of the pleura, peritoneum or pericardium strongly associated with exposure to asbestos [1–3]. MM usually becomes clinically apparent after a 30- to 40-year latency period following asbestos exposure [4]. The use of asbestos in developed countries has continuously declined in the past 30 years and because of this, mesothelioma incidence is expected to peak in many countries. In the United States, the mesothelioma incidence peaked in the 1990’s [5]. However, more than 2000 cases are still diagnosed annually and workers previously active in construction, railroads and shipyards will continue to be at risk for developing MM [4, 6]. Recently, concerns were raised about asbestos exposure of New York residents and public servants such as firefighters, rescue workers, and recovery teams after the collapse of the World Trade Center on September 11, 2001 [7]. In the United Kingdom, the peak of mesothelioma deaths is expected to fall around 2020 [3]. In contrast to the diminished use of asbestos in developed countries, asbestos use in Asian and Latin American nations has become a more common trend [3]. For this reason, it is projected that worldwide mesothelioma incidence will continue to increase in the next 10–20 years.
Because clinical symptoms only appear with advanced disease, the median survival for mesothelioma patients is approximately 9 months [8]. Mesothelioma is usually diagnosed by means of biopsies of pleura or peritoneum. Diagnosis of MM can be a challenge due to (i) the atypical features of mesothelial cells or lack of cells in the pleural or peritoneal fluids [8] and (ii) difficulties in differentiating MM from other afflictions, such as metastatic adenocarcinoma and benign pleural inflammation [4]. To resolve these problems, various approaches have been developed, including immunohistochemical assays [4, 9]. Although immunohistochemical markers can be highly specific and sensitive, a substantial sample of tumor tissue is necessary for accurate diagnosis, requiring invasive surgery [10]. Therefore, the development of MM-specific molecular markers that could allow a diagnosis through the analysis of blood, pleural fluid or other bodily fluids and that does not require the presence of intact cells, would be highly desirable.
The first phase of marker development is the discovery of candidate genes or proteins by identifying molecular changes specific for tumor presence [11]. Exciting progress has been made in developing protein-based as well as expression-based (mRNA-derived) molecular markers for MM [1, 12–18]. While these approaches are valuable, development of DNA-based markers would provide a powerful complementary approach with the advantages that the signal is exponentially amplifiable and relatively stable in bodily fluids. DNA methylation consists of the addition of a methyl group to the 5-position of cytosine in the context of a CpG dinucleotide. Hypermethylation of clusters of CpG dinucleotides (“CpG islands”) in the promoter regions of genes appears to lead to transcriptional silencing, and is thought to be a very common mechanism for the inactivation of tumor suppressor and growth regulatory genes in cancer [19, 20]. Different types of cancer show distinct DNA methylation profiles, suggesting that it should be possible to develop cancer-type specific methylation signatures [21]. The power of DNA methylation as a marker derives not only from its ability to be detected in a wide variety of samples (from fresh specimens to bodily fluids and archival paraffin-embedded specimens) but also from the defined localization of the lesion (in promoter CpG islands of genes), allowing the design of gene-specific, targeted probes [22]. To date, findings regarding the role of DNA methylation in MM are still rather limited [23–35].
The goals of this study were dual: to identify new MM-specific methylation markers that could be used to develop highly specific and sensitive panels to effectively diagnose MM, and to explore possible relationships between methylation profiles and clinicopathological patient characteristics. Associations with patient features could be of importance for treatment or prognostication, and could also provide insights into the molecular mechanism of MM development. We examined the methylation status of 28 CpG islands of genes encoding tumor suppressor proteins and other growth regulatory proteins in 52 MM samples. For comparison, we analyzed 38 non-tumor lung samples from patients with lung carcinomas. The choice of loci was based on previous exploratory studies in our laboratory ([24] and unpublished data) as well as reports in the literature. Our data show that CpG island hypermethylation is common in MM, and that analysis of methylation profiles can provide MM-specific methylation markers, as well as insights into the potential role of epigenetic changes in the development and progression of MM.
Materials and methods
Study subjects and tissue samples
MM samples were obtained from 52 patients treated by one of us (HIP) at the Karamanos Cancer Institute between May 2000 and February 2004. Study subjects included 42 males and 9 females (gender unknown for one sample) ranging from 44–83 years old at time of surgery (median: 64 years old, age unknown for 1 subject). Thirty-nine of the 49 subjects were exposed to asbestos (asbestos exposure data was incomplete for 3 subjects). Histological subtypes were epithelioid (35), mixed (9) and sarcomatoid (4), with incomplete histological data for 4 cases. Lymph node involvement was observed in 38/49 patients (data was missing for 3 subjects). The mean survival of the 33 patients who died during the study was 13 months; surviving patients (n=17) were followed for an average of 19 months. Survival information was missing for 2 cases. Primary histologically verified MM tumors were fresh-frozen 2–3 cm chunks of whitish tissue, clearly different from lung, pleura, diaphragm etc. which, depending upon the amount of tumor stroma in the specimen, consisted of 85–95% tumor tissue. Histologically non-tumor-bearing lung samples were derived from paraffin sections from separate cancer-free blocks of the adjacent lung of lung cancer patients from the Los Angeles County Hospital. These lung cancer patients consisted of 23 male and 15 female subjects and ranged in age from 40 to 82 years old, with a median age of 58. All studies were institutionally approved. Patient identity was not made known to the laboratory investigators.
DNA extraction and methylation analysis
DNA was extracted from MM and non-tumor lung samples via proteinase K digestion [36]. The DNA was bisulfite converted as previously described [37]. DNA methylation was analyzed by MethyLight [37, 38]. The primers and probes used are listed in Table 1. In addition to primers and probe sets designed specifically for the gene of interest, duplicates of an internal reference primer and probe set designed to analyze Alu repeats (Alu) were included in the analysis to normalize for input DNA [39]. Percentage methylated reference (PMR) is a relative measure comparing methylation in the sample to methylation in enzymatically methylated DNA. The PMR is calculated from the GENE:ALU ratio of a sample divided by the GENE:ALU ratio of human DNA methylated by treatment with SssI methylase, multiplied by 100. The average PMR for each locus in each sample was calculated using values for duplicate ALU controls. PMR values over 100 may occasionally be obtained for genes that are very heavily methylated because in spite of extensive SssI treatment, the control DNA may not always be fully methylated. However, this in no way affects the obtained statistical significance, as the same batch of SssI-treated DNA was used throughout the study.
Table 1.
Summary of MethyLight primers for 28 loci studied
| HUGO1 | Forward Primer Sequence | Reverse Primer Sequence | Probe Oligo Sequence |
|---|---|---|---|
| APC | GAACCAAAACGCTCCCCAT | TTATATGTCGGTTACGTGCGTTTATAT | 6FAM-CCCGTCGAAAACCCGCCGATTA-BHQ-1 |
| ATM | ACGGAGAAAAGAAGTCGTGGTC | GCGACGATAACTACAACGCAAAT | 6FAM-CGACTCCTCTCGCCTCCTCCCG-BHQ-1 |
| CDH1 | AATTTTAGGTTAGAGGGTTATCGCGT | TCCCCAAAACGAAACTAACGAC | 6FAM-CGCCCACCCGACCTCGCAT-BHQ-1 |
| CDH13 | AATTTCGTTCGTTTTGTGCGT | CTACCCGTACCGAACGATCC | 6FAM-AACGCAAAACGCGCCCGACA-BHQ-1 |
| CDKN2A | GCGTTCGAGTGGCGGA | CTCCCGAACAACGTCGTACAC | 6FAM-CAATTAAACTCCGCGCCGTAAAACAACAA-BHQ-1 |
| CDKN2B | AGGAAGGAGAGAGTGCGTCG | CGAATAATCCACCGTTAACCG | 6FAM-TTAACGACACTCTTCCCTTCTTTCCCACG-BHQ-1 |
| CDX2 | GGTAATCGTCGTAGTTCGGGTATT | ACTCCGTACGCCACTCTAACG | 6FAM-CAACCTAACGCCGCAAAACTTCGTCA-BHQ-1 |
| CHFR | CGGGAGTTTTTATGGGCGT | AACCGTCCCCAAAACTACGAC | 6FAM-CCTCGAACCGCTCCATCGAAATTCA-BHQ |
| CYP1B1 | GTGCGTTTGGACGGGAGTT | AACGCGACCTAACAAAACGAA | 6FAM-CGCCGCACACCAAACCGCTT-BHQ-1 |
| ESR1 | GGCGTTCGTTTTGGGATTG | GCCGACACGCGAACTCTAA | 6FAM-CGATAAAACCGAACGACCCGACGA-BHQ-1 |
| HMGA1 | CGTGATTTTTTTGCGCGTG | ACCTAAACTACGAACTCGAATCGAC | 6FAM-CACTCCTCGCAATCCCGAACGAA-BHQ-1 |
| HOXA1 | GTTGTTGCGGCGATTGTAAA | CGCGCAAAACGCAACTT | 6FAM-TACTCTTCTTCGCTCCAACACTCCAAATCG-BHQ-1 |
| LZTS1 | GCGGCGTTGTAGGGACG | CGCGCGCTAACTCTTCTACG | 6FAM-ATTACCGCCTTTAAACTCCGAACCCTCCA-BHQ-1 |
| MGMT | GCGTTTCGACGTTCGTAGGT | CACTCTTCCGAAAACGAAACG | 6FAM-CGCAAACGATACGCACCGCGA-BHQ-1 |
| MT1A | CGTGTTTTCGTGTTATTGTGTACG | CTCGCTATCGCCTTACCTATCC | 6FAM-TCCACACCTAAATCCCTCGAACCCACT-BHQ-1 |
| MT2A | GCGTTTTCGTCGTGTGTATAGTTT | TTCCCAAATCCCGCTTTCA | 6FAM-CGCGCGCTAACGACTCAAATTCG-BHQ-1 |
| OPCML2 | CGTTTCGAGGCGGTATCG | CGAACCGCCGAAATTATCAT | 6FAM-AACAACTCCATCCCTAACCGCCACTTTCT-BHQ-1 |
| PGR | GGCGGTGACGGTCGTATTC | ACAAACCGTCCCGCGAA | 6FAM-AACAACCGCTCGCGCCCGA-BHQ-1 |
| PTEN | GTTTCGCGTTGTTGTAAAAGTCG | CAATATAACTACCTAAAACTTACTCGAACCG | 6FAM-TTCCCAACCGCCAACCTACAACTACACTTA-BHQ-1 |
| RASSF1 | ATTGAGTTGCGGGAGTTGGT | ACACGCTCCAACCGAATACG | 6FAM-CCCTTCCCAACGCGCCCA-BHQ-1 |
| SFRP1 | GAATTCGTTCGCGAGGGA | AAACGAACCGCACTCGTTACC | 6FAM-CCGTCACCGACGCGAAAACCAAT-BHQ-1 |
| SFRP4 | GTTGTTCGGGCGGGTTC | GCGAAACTCCGCCGTCTA | 6FAM-AAACACGAACAACGCCAACTCTCAACCT-BHQ-1 |
| SFRP5 | GCGTTTGTAGTTTATCGTGTGGTAGA | GAACCGCTACACGACCGCT | 6FAM-CGCCGCAATACCTTAACATCCCTACCG-BHQ-1 |
| SLC6A20 | AGGCGAATACGAATTGTAGCG | TAAAACGACGCGCCTAACG | 6FAM-CCGCGCACTAAAACTACCGTACCGAA-BHQ-1 |
| SOCS4 | TATATTGCGTTTGCGTCGATTC | ACGCCGCAACTAACGCC | 6FAM-CCCGACGACCTCTATCGAAACCTACGCTA-BHQ-1 |
| SYK | GGGCGCGATATTGGGAG | GCGACTCTTCCTCATTTTAAACAAC | 6FAM-CCTTAACGCGCCCGAACAAACG-BHQ-1 |
| TWIST1 | GTAGCGCGGCGAACGT | AAACGCAACGAATCATAACCAAC | 6FAM-CCAACGCACCCAATCGCTAAACGA-BHQ-1 |
| VHL | CGGGAGCGCGTACGTAGTT | CTCCGAAACATTCCCTCCG | 6FAM-CGAACCGAACGCCGCGAAA-BHQ-1 |
Human Genome Organization nomenclature. Acronyms are defined in the text except for the following loci: CHFR (checkpoint with forkhead and ring finger domains), HMGA1 (high mobility group AT-hook 1), MGMT (O-6-methylguanine-DNA methyltransferase), PTEN (phosphate and tensin homolog) and VHL (von Hippel Lindau tumor suppressor).
This primer/probe set shares homology with the CpG island of the adjacent HNT gene, which appears to have arisen via gene duplication.
Statistical analysis
For each gene, PMR values in MM versus non-tumor lung were compared by means of the Mann-Whitney U test. To minimize the risk of finding random associations that are not truly significant, we applied a multiple comparisons threshold [40] to the analysis of the 24 loci that were studied here for the first time (Table 2). The relationship between the top markers was examined by two-dimensional hierarchical clustering using log-transformed PMR values. Ward’s hierarchical clustering method was applied using JMP version 6.0 software (SAS Institute, Inc., Cary, NC).
Table 2.
Frequency and median PMR values of mesothelioma and non-tumor lung tissues for 28 loci
| Locus1 | MM2 (n=52) | NTL5(n=38) | p-value6 | MC | |||
|---|---|---|---|---|---|---|---|
| Frequency(%)3 | Median PMR4 | Frequency3(%) | Median PMR4 | Locus1 | MM vs NTL | Threshold7 | |
| APC | 58 | 0.36 | 94 | 4.84 | APC | <.0001 | n.a. |
| ATM | 37 | 0.01 | 59 | 0.16 | ESR18 | 0.0002 | n.a. |
| CDH1 | 98 | 4.33 | 82 | 12.7 | CDH1 | 0.0143 | n.a. |
| CDH13 | 65 | 2.91 | 37 | 5.45 | PGR | 0.0848 | n.a. |
| CDKN2AEX2 | 100 | 43.8 | 96 | 43.3 | CDKN2B | <.0001 | 0.0021 |
| CDKN2B | 98 | 5.43 | 96 | 9.66 | ATM | 0.0001 | 0.0023 |
| CDX28 | 86 | 7.11 | 66 | 3.40 | SYK | 0.0003 | 0.0025 |
| CHFR | 13 | 0.04 | 4 | 1.65 | SLC6A20 | 0.0022 | 0.0027 |
| CYP1B1 | 27 | 1.66 | 24 | 0.44 | HOXA1 | 0.0033 | 0.0030 |
| ESR18 | 71 | 3.29 | 32 | 1.29 | CDX28 | 0.0091 | 0.0033 |
| HMGA18 | 6 | 0.00 | 18 | 0.02 | LZTS1 | 0.0098 | 0.0037 |
| HOXA1 | 63 | 0.43 | 63 | 3.82 | MT2A | 0.0685 | 0.0042 |
| LZTS1 | 100 | 133 | 100 | 183 | CDH13 | 0.1006 | 0.0047 |
| MGMT | 13 | 1.75 | 14 | 9.22 | RASSF18 | 0.1421 | 0.0053 |
| MT1A | 100 | 134 | 100 | 110 | SFRP58 | 0.1971 | 0.0061 |
| MT2A | 100 | 11.0 | 71 | 12.2 | SFRP18 | 0.1985 | 0.0071 |
| OPCML | 94 | 6.19 | 69 | 9.20 | PTEN | 0.2562 | 0.0083 |
| PGR | 27 | 4.28 | 4 | 6.95 | MT1A | 0.2701 | 0.0099 |
| PTEN | 15 | 0.05 | 20 | 0.85 | HMGA18 | 0.2736 | 0.0120 |
| RASSF18 | 57 | 32.4 | 58 | 0.86 | CDKN2AEX2 | 0.3776 | 0.0148 |
| SFRP18 | 88 | 4.39 | 87 | 10.01 | OPCML | 0.5029 | 0.0188 |
| SFRP48 | 37 | 0.65 | 42 | 1.03 | CHFR | 0.5455 | 0.0245 |
| SFRP58 | 98 | 4.08 | 92 | 5.78 | MGMT | 0.5482 | 0.0333 |
| SLC6A20 | 46 | 6.60 | 10 | 0.41 | VHL | 0.7562 | 0.0480 |
| SOCS48 | 12 | 1.60 | 13 | 1.70 | SOCS48 | 0.9174 | 0.0500 |
| SYK | 67 | 0.22 | 22 | 0.43 | CYP1B1 | 0.8831 | 0.0500 |
| TWIST1 | 75 | 3.25 | 53 | 7.45 | SFRP48 | 0.5671 | 0.0500 |
| VHL | 4 | 0.90 | 0 | 0.00 | TWIST | 0.6444 | 0.0500 |
HUGO nomenclature (on right loci are listed alphabetically by p-value, with previously identified loci at the top);
MM: malignant mesothelioma;
percentage of samples with positive methylation values;
median percent methylated reference calculated from positive methylation values;
NTL: non-tumor lung;
p-value calculated using Mann-Whitney U test;
MC threshold: multiple comparison p-value threshold according to Benjamini et al.[40]. This threshold was not applied to the four loci for which previous research suggested significant differences between MM and non-tumor lung;
MM n=51
Spearman correlation coefficients were calculated to investigate associations between age at the time of surgery and methylation, and associations between the individual methylation markers MT1A, MT2A, SFRP4 and SOCS4. P-values to test H0: r=0 were also calculated, using a permutation test, because the large number of zeros for some loci violates the standard assumptions of the Spearman correlation. To investigate the association between age at time of surgery and other clinicopathological variables including sex, histology type, asbestos exposure and lymph node involvement, the Mann-Whitney U test was performed. Associations among gender, histology type, asbestos exposure and lymph node involvement were investigated using Fisher’s exact test. The log-rank test was performed to test the association of methylation with survival by dichotomizing the PMR. Dichotomization was done using the median, or if the distribution of PMR values indicated two distinct groups of roughly similar size, at the PMR value separating both groups. Alternatively, if a substantial fraction of subjects showed no methylation, dichotomization was done by dividing the subjects into those with and without methylation. In order to adjust for the effect of age (calculated from the date of surgery) on survival, the stratified log-rank test was also performed using age at surgery as the stratifying variable. Age at surgery was dichotomized at the median age of 64. Data from those who did not die were censored at the date of last follow up. The Pike estimates of relative hazard ratio were calculated with the use of observed and expected numbers of events as calculated in the log-rank test. The survival probabilities were estimated using the product limit method. All statistical tests were two-sided.
Results
Hypermethylation is common in MM
Our first goal was to examine the 28 loci for their utility as MM-specific DNA methylation markers. Because methylation markers are under development for many different kinds of cancer, establishing methylation marker panels for different types of cancer will be possible in the future. However, it is also important that potential markers not be hypermethylated in non-tumor tissues near the cancer. We and others have observed substantial hypermethylation in normal lung samples ([24, 41] and our unpublished observations). Through cellular contamination or shedding of DNA, such hypermethylation could potentially contaminate biopsies or pleural fluids, compromising marker specificity. In order to ensure cancer-specificity of methylation markers for MM, it would be important to determine that the chosen markers would not be as highly methylated in non-cancer lung. Because basal levels of methylation in the lung may increase with environmental exposures and/or age [42, 43], we chose to make the most rigorous comparison; we used lung samples that would be most likely to show high levels of background methylation: non-cancer lung samples from lung cancer patients. Lung cancer tends to occur in older subjects with substantial exposure to environmental agents such as tobacco smoke. Utilizing histologically verified cancer-free lung from these patients would thus set stringent requirements for possible MM methylation markers; the ideal MM methylation marker should show higher methylation levels than any kind of non-tumor lung samples, even from heavy smokers.
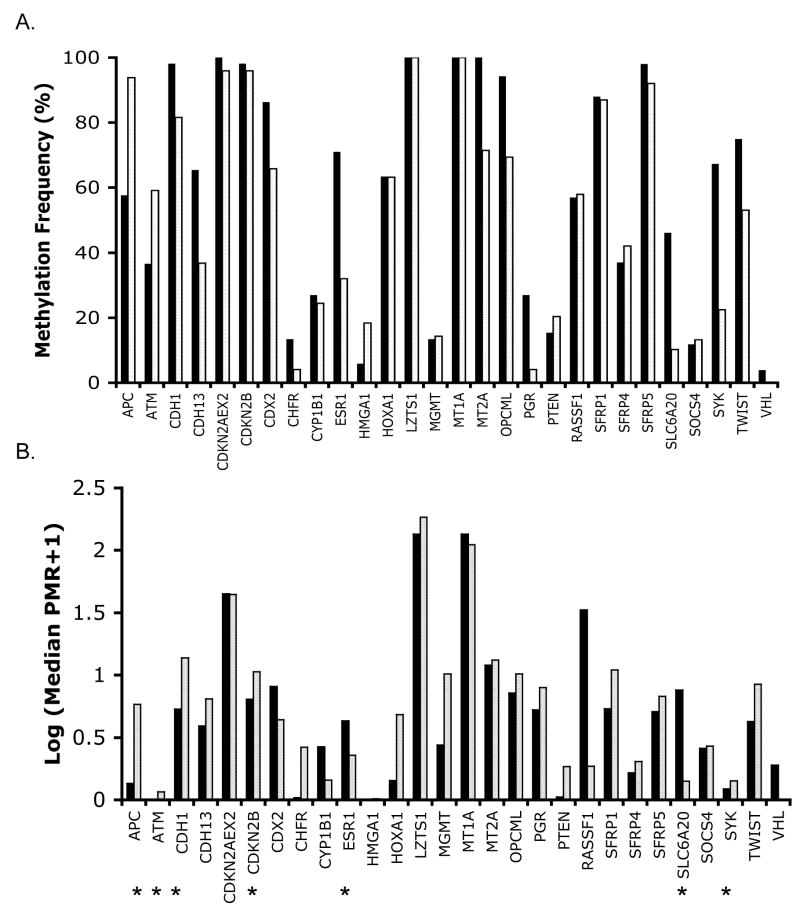
Examination of methylation in the MM and non-tumor lung samples showed that the frequency of methylation in the sample collection and the median PMR values varied substantially for loci studied (Fig. 1, Table 2). CpG islands for adenomatosis polyposis coli (APC), epithelial and heart cadherins (CDH1 and CDH13), cyclin-dependent kinase inhibitors 2A and 2B (CDKN2A EX2/p16 and CDKN2B), caudal type homeobox transcription factor 2 (CDX2), ESR1, homeobox A1 (HOXA1), LZTS1, MT1A, MT2A, opioid binding protein/cell adhesion molecule-like (OPCML), ras association domain family 1 (RASSF1), secreted frizzled-related proteins 1 and 5 (SFRP1 and SFRP5), SLC6A20, SYK and twist homolog 1 (TWIST1) were methylated in 46–100% of the MM cases. However, many of these loci showed a similar frequency of methylation in non-tumor lung.
Figure 1.
Graphical representation of methylation of 28 loci in MM cases. Percentage of MM (black) or non-tumor lung (light gray) samples showing methylation is indicated in (A). Median PMR value of the samples showing methylation in MM (black) or non-tumor lung (white) is indicated in (B). Because the spread in PMR values was large, log-transformed values were plotted in (B). Stars indicate statistically significant differences in methylation between MM and non-tumor lung samples, using PMR as a continuous variable and taking into account a multiple comparison correction threshold (see Table 2 for values).
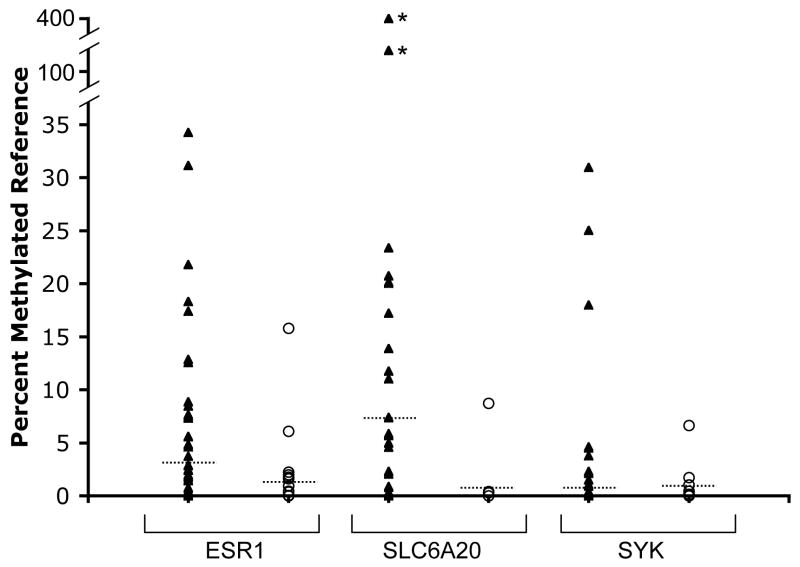
We examined the statistical significance of methylation frequency and levels (expressed as percentage methylated reference or PMR, see methods) in MM vs. non-tumor lung samples by comparing the PMR values of all samples for each locus using the Mann-Whitney U test. Because examination of multiple markers can lead to spurious associations, we incorporated a multiple comparisons threshold for those markers for which no demonstration of significant differences in methylation in MM vs. normal lung had been previously shown (Table 2). Seven loci showed statistically significant differences in methylation between MM and non-tumor lung: APC, ataxia telangiectasia (ATM), CDKN2B, CDH1, ESR1, SLC6A20 and SYK. Of these, APC, ATM and CDKN2B showed highly significantly elevated methylation in non-tumor lung (p≤0.0001, Table 1). This methylation in non-tumor lung could be related to aging [42]. However, we found no evidence for age-related increases of methylation of these genes in MM or lung tissue (see below). More likely, the methylation in non-tumor lung is related to environmental exposures, which have been shown to increase methylation of various loci in the lung, possibly creating a “field defect” (reviewed in [43, 44]). Three loci, ESR1, SLC6A20 and SYK showed a substantially elevated frequency and/or level of methylation in MM compared to non-tumor lung (p=0.0002, 0.0022 and 0.0003, respectively, Table 2 and Fig. 1 and 2). Examination of the ability of these three loci to distinguish MM from non-tumor lung samples indicated that neither marker on its own was very strong (Receiver Operating Characteristic curves for the three markers based on the tissue collection used showed areas under the curve ranging from 0.70–0.73, data not shown). Combination of SLC6A20 and SYK methylation as positive markers for MM yielded a sensitivity of 81% and specificity of 73% based on evaluation of the current collection of tissues. Adding ESR1 methylation as a third positive marker increased sensitivity to 94% but reduced specificity to 55%, since ESR1 methylation is relatively frequent in non-tumor lung. Because many samples with ESR1 methylation were also methylated in SYK or SLC6A20, we explored using APC methylation as a negative marker instead. The APC/SYK/SLC6A20 combination resulted in a sensitivity of 92% and a specificity of 73%. A two-dimensional clustering analysis using these three markers shows separation of most samples into the correct categories (Fig. 3). The diagram illustrates the utility of combining markers with complementary methylation patterns (SYK and SLC6A20) to maximize sensitivity. One main branch of the dendrogram consists of non-tumor lung, while the second branch consists of MM interspersed with seven non-tumor lung samples, pointing to the need to identify additional markers to bolster specificity.
Figure 2.
Comparison of distribution of PMR values for ESR1, SLC6A20 and SYK in MM (triangles) and non-tumor lung (open circles). The median PMR of methylation positive samples is indicated by the dashed line. Two MM samples (indicated with an asterisk) showed very high PMR values for SLC6A20 as indicated by the discontinuous scale.
Figure 3.
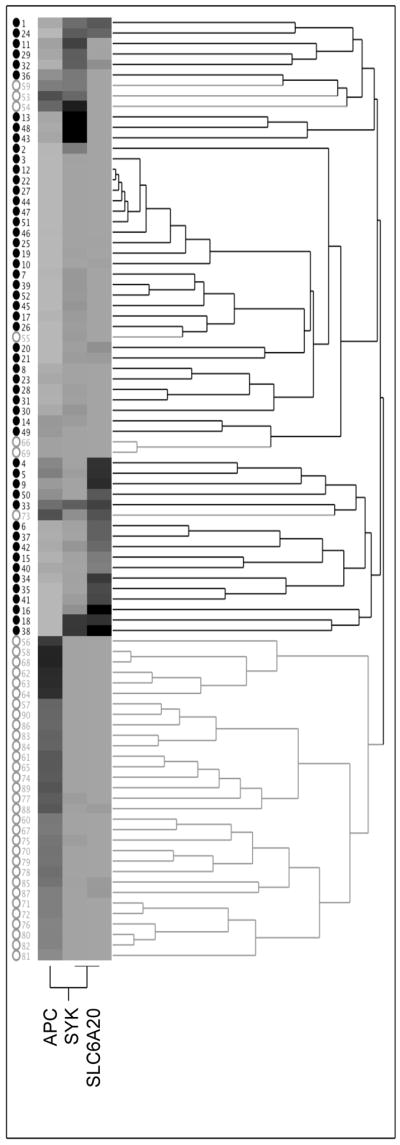
Two-dimensional hierarchical clustering analysis of methylation data for APC, SYK and SLC6A20. MM and non-tumor lung samples are indicated by closed black and open gray circles, respectively. Methylation levels range from undetectable (light gray) to most elevated (black).
Clinicopathological associations with methylation of loci in MM
Besides their potential role as molecular markers for early cancer detection, DNA methylation of loci in MM could also provide information about the nature of the disease in each patient. Because methylation of certain genes can increase with age [42], we first compared the age of the patients at time of surgery vs. methylation. We found that TWIST1, cytochrome P450, family member 1B1 (CYP1B1), RASSF1, and OPCML methylation increased with age (p=0.008, 0.013, 0.018, and 0.038, respectively). No clear associations with age were observed for other genes (p>0.05), so that the statistically significant differences in methylation between MM and non-tumor lung (Table 2) do not appear to be due to the slightly higher mean age of the MM patients. None of the clinicopathological variables (gender, histological subtype, asbestos exposure, lymph node involvement) was significantly associated with age.
We next examined possible association between the clinicopathological variables and methylation. Toyooka and colleagues had reported a significant difference in the methylation frequency of RASSF1A in epithelioid vs. sarcomatoid/mixed patterns [45], but we did not find such a correlation (p=0.2). However, we did note associations between gender, histology, asbestos exposure and lymph node involvement vs. methylation levels for two members of the metallothionein family, MT1A and MT2A, and the SFRP4 and SOCS4 loci (Table 3). As expected, age adjustment did not significantly affect the results (not shown). Because many associations were examined (methylation levels at 28 loci vs. four clinical variables), application of a multiple comparisons threshold would be appropriate, and under those conditions the associations would not be deemed significant. Thus, we cautiously interpret this data as providing suggestions for further study. However, in the case of MT1A and MT2A, we observe associations with three variables (Table 3), which might support the significance of methylation at these two loci. A higher median PMR was observed for MT1A in men, in patients exposed to asbestos, and in patients showing lymph node involvement. A higher median PMR for MT2A was associated with female gender, epithelioid histology, and lack of exposure to asbestos. The opposite trends in the methylation levels of the two loci were supported by the observation of a negative correlation between MT1A and MT2A methylation levels (p=0.019). Because of the similarity in methylation trends of each of these two loci for multiple clinical characteristics, we reasoned that association with one trend might drive association with the others. Therefore, we examined associations among gender, histological type, asbestos exposure, and lymph node involvement. Asbestos exposed patients were more likely to be male (p=0.016) and to show lymph node involvement (p=0.008). All other associations showed p>0.05. Thus it is possible that at least in the case of MT1A, multiple associations are driven by one variable.
Table 3.
Summary of associations between clinicopathological characteristics and methylation for which p≤0.05
| Characteristics | Locus1 | Median PMR2 | p-value3 | |
|---|---|---|---|---|
| Gender |
Male (n=42; 41)4 |
Female (n=9; 6)4 |
||
| MT1A | 153 | 91 | 0.048 | |
| MT2A | 10 | 25 | 0.006 | |
| SFRP4 | 1 | 3 | 0.022 | |
| Histology |
Mixed/Sarcomatoid (n=13) |
Epithelioid (n=35) |
||
| MT2A | 7 | 13 | 0.017 | |
| Asbestos Exposure |
Exposed (n=39) |
Not Exposed (n=10) |
||
| MT1A | 151 | 93 | 0.050 | |
| MT2A | 10 | 21 | 0.018 | |
| Lymph Node Involvement |
Involved (n=38; 37)4 |
Not Involved (n=11; 10)4 |
||
| MT1A | 162 | 106 | 0.047 | |
| SOCS4 | 3 | 1 | 0.005 | |
HUGO nomenclature;
For SFRP4 and SOCS4 value is median of all positive samples;
Mann-Whiteny U test;
SFRP4 methylation was available for 41 males and 6 females; SOCS4 methylation data was available for 47 cases.
The two other loci for which associations with clinicopathological variables were suggested were SOCS4, associated with lymph node involvement (but methylated only in 6 cases), and SFRP4, which trends toward association with female gender (methylated in 37% of MM). SFRPs encode a family of modulators of wingless-type mouse mammary tumor virus integration site (WNT) signaling that function through direct binding to the WNT protein. Lee and colleagues reported a much higher methylation frequency for SFRP4 (89%, or 17/19 MM samples), but to our knowledge, an association with gender was not examined [31]. The same investigators reported frequent (>80%) methylation of SFRP1 and 5, similar to our results [31]. Interestingly, the SFRPs are not the only WNT antagonists that are methylated in MM; the Wnt inhibitory factor 1 gene (WIF1) was recently shown to be methylated in 11/12 MMs [35]. Thus, it appears that methylation may be a common mechanism of inactivation of genes in the Wnt pathway.
Methylation and survival
The observed trends linking methylation of certain genes to gender, histology, asbestos exposure and lymph node involvement prompted an investigation of methylation vs. survival for all loci examined. It should be noted that the sample size is relatively small, which makes observing survival differences challenging. Indeed, we did not detect a significant survival difference when we compared gender, lymph node involvement, histology and asbestos exposure (data not shown), even though a better survival has been reported in the literature for women, epithelioid subtype, and in some studies, patients lacking involved nodes [10, 46].
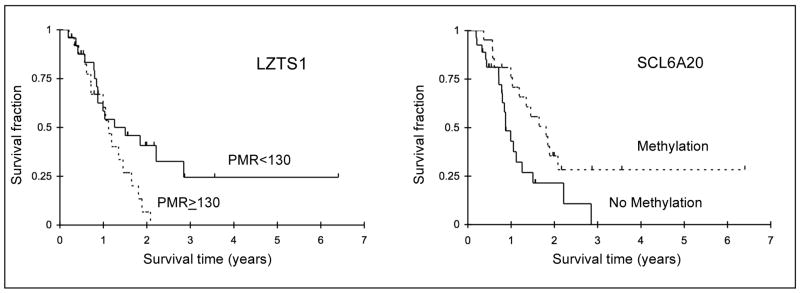
Associations between survival and methylation were examined by dichotomizing PMR values into low or high methylation groups or into methylated and unmethylated cases when a substantial fraction of subjects showed no methylation (see Materials and Methods). Like other researchers, we found no association of survival of MM patients with the methylation of the individual loci APC, CDH1, CDH13, CDKN2A, MGMT and RASSF1 [25, 34]. Two marginally significant associations were observed (Fig. 4): a better survival of the group with the lower LZTS1 PMR values (p=0.046; age-adjusted p=0.065), and a better survival of the group showing SLC6A20 methylation (p=0.022; age-adjusted p=0.023). Neither of these associations would remain statistically significant if a multiple comparisons correction were applied, and thus their potential role should be further evaluated.
Figure 4.
Kaplan Meier analyses of survival vs. methylation for LZTS1 (left) and SLC6A20 (right) loci. LZTS1 PMR values were dichotomized at 130. P=0.046 without and 0.065 with age adjustment respectively, and the relative hazard ratio for PMR≥130 was 1.93 (0.94–3.97). SLC6A20 subjects were divided into with and without methylation; p=0.022 without and 0.023 with age adjustment respectively, and the relative hazard ratio for absence of methylation was 2.14 (1.05–4.37).
Discussion
To our knowledge, the present study of 28 methylation markers in MM is the largest such analysis to date. We generally find a higher methylation frequency compared to other investigators (e.g. [25, 30]), which is likely due to our use of the quantitative and highly sensitive MethyLight technique. We have identified three loci (ESR1, SLC6A20 and SYK) that are significantly more methylated in MM than in non-tumor lung from lung cancer patients and several negative markers, of which APC is the strongest. Combination of SLC6A20, SYK, and APC yields a sensitivity of 92% and specificity of 73% based on evaluation of the current collection of tissues. Adding ESR1 methylation as a third positive marker increases sensitivity but reduces specificity, illustrating that the best results are obtained with a minimum number of complementary markers. Identification of further markers is required to increase specificity, and the utility of the current markers for the analysis of bodily fluids (effusions, plasma or serum) remains to be determined.
Some of the loci we examined were also studied by Toyooka and colleagues, in a comparison of methylation levels in 66 MM and 40 lung adenocarcinomas [25]. All loci that were found by Toyooka and coworkers to show statistically significant differences between the two cancer types (APC, CDH13, CDKN2A, O-6-methylguanine-DNA methyltransferase (MGMT) and retinoic acid receptor beta (RARB)) showed elevated methylation in adenocarcinoma, and thus would be potential negative markers for MM. Similar to our findings, APC showed the strongest difference in methylation between adenocarcinoma and MM.
One interesting aspect of our results is the frequent methylation of the genes encoding heavy metal binding proteins MT1A and MT2A, and the potential link to gender, asbestos exposure, histology and nodal status. The MT genes are located in the metallothionein family gene cluster, a 75-kb region of chromosome 16q [47]. Tissue-specific methylation has been reported for MT1A and MT1J; while both genes showed methylation in breast tumors, only the latter gene showed methylation in normal breast tissue [48]. This differential methylation is surprising, given that the two CpG islands lie less than 2 kilobases apart [48]. It indicates that CpG islands within the MT cluster are not necessarily coordinately regulated, an observation supported by our analysis of MT1A and MT2A methylation. Epigenetic regulation has been implicated in the control of various MT genes [49]. Hypermethylation of the MT1G promoter has been reported to be associated with higher tumor stage in prostate cancer [50], and MT3 and MT1G expression are downregulated by methylation in oesophageal cancer and thyroid carcinoma respectively [51, 52]. The biological role of silencing of these metal-binding proteins in cancer remains to be further investigated. It has been suggested that MT expression might retard growth of cancer cells, or that lack of metallothioneins could increase availability of cellular zinc for cell growth [53].
Our observation of potential associations of LZTS1 and SLC6A20 with survival is intriguing but needs to be borne out by further studies. How methylation of the SLC6A20 transporter gene might be associated with increased survival is not clear at this time, but it is conceivable that methylation-associated silencing might affect resistance of cells to chemotherapy. Given its potential utility as part of a marker panel, SCL6A20 methylation might be of both diagnostic and prognostic value, and merits further investigation.
Conclusion
The presence of highly methylated genes in MM demonstrates that hypermethylation occurs in this type of cancer. A three-marker methylation panel can distinguish between our MM and non-tumor lung tissues with considerable sensitivity but specificity needs to be strengthened through the identification of additional loci. The modest number of informative genes identified to date suggests that other types of loci might need to be examined [30]. An epigenomic approach, in which methylation of thousands of CpG islands can be quickly prescreened, followed by a detailed evaluation of selected markers by MethyLight, might prove more fruitful in rapidly identifying strong candidate genes that are highly MM-specific. Such studies are in progress in our laboratory. Aside from its relevance for marker development, analysis of methylation profiles in MM can provide clues to the epigenetic hits that may play a role in MM development and progression. The studies presented here hint at a role for metallothioneins, among others, and underscore the utility of methylation research in uncovering new avenues of exploration in MM. Ongoing efforts in many laboratories to identify loci hypermethylated in MM promise to provide important insights that will facilitate early diagnosis and may one day lead to new therapeutic applications.
Acknowledgments
The authors would like to thank the members of the Laird lab for their technical advice, in particular Daniel Weisenberger, Mihaela Campan, and Tiffany Long, and members of the Laird-Offringa lab for critically reviewing this manuscript. This research was supported by a grant from the Mesothelioma Applied Research Foundation (to I.A.L.-O) and by NCI P30 CA 14089 which supports W.Y. and S.G. in the Norris Comprehensive Cancer Center Biostatistics Core. The funding sources did not play a role in the study or in the decision to publish, or manner and content of publication. We thank MARF and all those who support it, including all the courageous and inspiring mesothelioma patients and their family members. We dedicate this manuscript in memory of Alan Reinstein, who passed away from mesothelioma in May 2006, and who was co-founder of the Asbestos Disease Awareness Organization.
Footnotes
Conflict of interest statement
I.A.L.-O. and P.W.L. are shareholders of Epigenomics AG, which has a commercial interest in the development of DNA markers for disease detection and diagnosis. None of the work performed in the laboratories of any of the authors is or has been supported by Epigenomics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramos-Nino ME, Testa JR, Altomare DA, Pass HI, Carbone M, Bocchetta M, Mossman BT. Cellular and molecular parameters of mesothelioma. J Cell Biochem. 2006;98:723–34. doi: 10.1002/jcb.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy SS, Testa JR. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J Cell Physiol. 1999;180:150–157. doi: 10.1002/(SICI)1097-4652(199908)180:2<150::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Kazan-Allen L. Asbestos and mesothelioma: worldwide trends. Lung Cancer. 2005;49 Suppl 1:S3–8. doi: 10.1016/j.lungcan.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Boylan AM. Mesothelioma: new concepts in diagnosis and management. Curr Opin Pulm Med. 2000;6:157–163. doi: 10.1097/00063198-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Weill H, Hughes JM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–41. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg M. Changing trends in US mesothelioma incidence. Occup Environ Med. 2005;62:134. doi: 10.1136/oem.2004.018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landrigan PJ, Lioy PJ, Thurston G, Berkowitz G, Chen LC, Chillrud SN, Gavett SH, Georgopoulos PG, Geyh AS, Levin S, Perera F, Rappaport SM, Small C. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112:731–9. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer M, Byrne M, Clarke S. Alimta: A new option for malignant mesothelioma. Rev Clin Oncol. 2003;1:4–8. [Google Scholar]
- 9.Ordonez NG. Mesothelioma with clear cell features: an ultrastructural and immunohistochemical study of 20 cases. Hum Pathol. 2005;36:465–73. doi: 10.1016/j.humpath.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Baas P. Predictive and prognostic factors in malignant pleural mesothelioma. Curr Opin Oncol. 2003;15:127–30. doi: 10.1097/00001622-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan Pepe M, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 12.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–83. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 13.Singhal S, Wiewrodt R, Malden LD, Amin KM, Matzie K, Friedberg J, Kucharczuk JC, Litzky LA, Johnson SW, Kaiser LR, Albelda SM. Gene expression profiling of malignant mesothelioma. Clin Cancer Res. 2003;9:3080–97. [PubMed] [Google Scholar]
- 14.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, Winzell P, Hellstrom KE, Hellstrom I. Soluble mesothelin-related protein--a blood test for mesothelioma. Lung Cancer. 2005;49 Suppl 1:S109–11. doi: 10.1016/j.lungcan.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–73. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 16.Pass HI, Liu Z, Wali A, Bueno R, Land S, Lott D, Siddiq F, Lonardo F, Carbone M, Draghici S. Gene expression profiles predict survival and progression of pleural mesothelioma. Clin Cancer Res. 2004;10:849–59. doi: 10.1158/1078-0432.ccr-0607-3. [DOI] [PubMed] [Google Scholar]
- 17.Onda M, Nagata S, Ho M, Bera TK, Hassan R, Alexander RH, Pastan I. Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res. 2006;12:4225–31. doi: 10.1158/1078-0432.CCR-06-0472. [DOI] [PubMed] [Google Scholar]
- 18.Gordon GJ, Jensen RV, Hsiao LL, Gullans SR, Blumenstock JE, Ramaswamy S, Richards WG, Sugarbaker DJ, Bueno R. Translation of microarray data into clinically relevant cancer diagnostic tests using gene expression ratios in lung cancer and mesothelioma. Cancer Res. 2002;62:4963–4967. [PubMed] [Google Scholar]
- 19.Laird PW. Cancer epigenetics. Hum Mol Genet. 2005;14 Spec No 1:R65–76. doi: 10.1093/hmg/ddi113. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–56. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 21.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 22.Laird PW. The power and the promise of DNA methylation markers. Nature Reviews Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 23.Wong L, Zhou J, Anderson D, Kratzke R. Inactivation of p16(INK4a) expression in malignant mesothelioma by methylation. Lung Cancer. 2002;38:131–136. doi: 10.1016/s0169-5002(02)00178-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsou JA, Shen LYC, Siegmund KD, Long TI, Laird PW, Seneviratne C, Koss MN, Pass HI, Hagen JA, Laird-Offringa IA. Distinct DNA methylation patterns in malignant mesothelioma, lung adenocarcinoma, and non-tumor lung. Lung Cancer. 2005;47:193–204. doi: 10.1016/j.lungcan.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M, Minna JD, Gazdar AF. Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung cancer. Cancer Res. 2001;61:4556–4560. [PubMed] [Google Scholar]
- 26.Suzuki M, Toyooka S, Shivapurkar N, Shigematsu H, Miyajima K, Takahashi T, Stastny V, Zern AL, Fujisawa T, Pass HI, Carbone M, Gazdar AF. Aberrant methylation profile of human malignant mesotheliomas and its relationship to SV40 infection. Oncogene. 2005;24:1302–8. doi: 10.1038/sj.onc.1208263. [DOI] [PubMed] [Google Scholar]
- 27.Shivapurkar N, Toyooka S, Toyooka KO, Reddy J, Miyajima K, Suzuki M, Shigematsu H, Takahashi T, Parikh G, Pass HI, Chaudhary PM, Gazdar AF. Aberrant methylation of trail decoy receptor genes is frequent in multiple tumor types. Int J Cancer. 2004;109:786–792. doi: 10.1002/ijc.20041. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu H, Suzuki M, Takahashi T, Miyajima K, Toyooka S, Shivapurkar N, Tomlinson GE, Mastrangelo D, Pass HI, Brambilla E, Sathyanarayana UG, Czerniak B, Fujisawa T, Shimizu N, Gazdar AF. Aberrant methylation of HIN-1 (high in normal-1) is a frequent event in many human malignancies. Int J Cancer. 2005;113:600–604. doi: 10.1002/ijc.20622. [DOI] [PubMed] [Google Scholar]
- 29.Ohta Y, Shridhar V, Kalemkerian GP, Bright RK, Watanabe Y, Pass HI. Thrombospondin-1 expression and clinical implications in malignant pleural mesothelioma. Cancer. 1999;85:2570–2576. [PubMed] [Google Scholar]
- 30.Marsit CJ, Houseman EA, Christensen BC, Eddy K, Bueno R, Sugarbaker DJ, Nelson HH, Karagas MR, Kelsey KT. Examination of a CpG island methylator phenotype and implications of methylation profiles in solid tumors. Cancer Res. 2006;66:10621–10629. doi: 10.1158/0008-5472.CAN-06-1687. [DOI] [PubMed] [Google Scholar]
- 31.Lee AY, He B, You L, Dadfarmay S, Xu Z, Mazieres J, Mikami I, McCormick F, Jablons DM. Expression of the secreted frizzled-related protein gene family is downregulated in human mesothelioma. Oncogene. 2004;23:6672–6676. doi: 10.1038/sj.onc.1207881. [DOI] [PubMed] [Google Scholar]
- 32.Kobatake T, Yano M, Toyooka S, Tsukuda K, Dote H, Kikuchi T, Toyota M, Ouchida M, Aoe M, Date H, Pass HI, Doihara H, Shimizu N. Aberrant methylation of p57KIP2 gene in lung and breast cancers and malignant mesotheliomas. Oncol Rep. 2004;12:1087–92. [PubMed] [Google Scholar]
- 33.Hirao T, Bueno R, Chen CJ, Gordon GJ, Heilig E, Kelsey KT. Alterations of the p16(INK4) locus in human malignant mesothelial tumors. Carcinogenesis. 2002;23:1127–1130. doi: 10.1093/carcin/23.7.1127. [DOI] [PubMed] [Google Scholar]
- 34.Fischer JR, Ohnmacht U, Rieger N, Zemaitis M, Stoffregen C, Kostrzewa M, Buchholz EC, Manegold C, Lahm H. Promoter methylation of RASSF1A, RARbeta and DAPK predict poor prognosis of patients with malignant mesothelioma. Lung Cancer. 2006;54:109–16. doi: 10.1016/j.lungcan.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Batra S, Shi Y, Kuchenbecker KM, He B, Reguart N, Mikami I, You L, Xu Z, Lin YC, Clement G, Jablons DM. Wnt inhibitory factor-1, a Wnt antagonist, is silenced by promoter hypermethylation in malignant pleural mesothelioma. Biochem Biophys Res Commun. 2006;342:1228–32. doi: 10.1016/j.bbrc.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 36.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001;25:456–462. doi: 10.1006/meth.2001.1268. [DOI] [PubMed] [Google Scholar]
- 39.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, Ehrlich M, Laird PW. Analysis of Repetitive Element DNA Methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–36. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 41.Waki T, Tamura G, Sato M, Motoyama T. Age-related methylation of tumor suppressor and tumor-related genes: an analysis of autopsy samples. Oncogene. 2003;22:4128–4133. doi: 10.1038/sj.onc.1206651. [DOI] [PubMed] [Google Scholar]
- 42.Issa JP. CpG-island methylation in aging and cancer. Curr Top Microbiol Immunol. 2000;249:101–118. doi: 10.1007/978-3-642-59696-4_7. [DOI] [PubMed] [Google Scholar]
- 43.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nature Reviews Cancer. 2004;4:1–11. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 44.Kerr KM, Galler JS, Hagen JA, Laird PW, Laird-Offringa IA. The role of DNA methylation in the development and progression of lung adenocarcinoma. Dis Markers. 2007;23:5–30. doi: 10.1155/2007/985474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toyooka S, Pass HI, Shivapurkar N, Fukuyama Y, Maruyama R, Toyooka KO, Gilcrease M, Farinas A, Minna JD, Gazdar AF. Aberrant methylation and Simina Virus 40 Tag sequences in malignant mesothelioma. Cancer Res. 2001;61:5727–5730. [PubMed] [Google Scholar]
- 46.Sugarbaker DJ, Strauss GM, Lynch TJ, Richards W, Mentzer SJ, Lee TH, Corson JM, Antman KH. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol. 1993;11:1172–8. doi: 10.1200/JCO.1993.11.6.1172. [DOI] [PubMed] [Google Scholar]
- 47.Gius D, Cui H, Bradbury CM, Cook J, Smart DK, Zhao S, Young L, Brandenburg SA, Hu Y, Bisht KS, Ho AS, Mattson D, Sun L, Munson PJ, Chuang EY, Mitchell JB, Feinberg AP. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell. 2004;6:361–71. doi: 10.1016/j.ccr.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Piotrowski A, Benetkiewicz M, Menzel U, de Stahl TD, Mantripragada K, Grigelionis G, Buckley PG, Jankowski M, Hoffman J, Bala D, Srutek E, Laskowski R, Zegarski W, Dumanski JP. Microarray-based survey of CpG islands identifies concurrent hyper- and hypomethylation patterns in tissues derived from patients with breast cancer. Genes Chromosomes Cancer. 2006;45:656–67. doi: 10.1002/gcc.20331. [DOI] [PubMed] [Google Scholar]
- 49.Majumder S, Kutay H, Datta J, Summers D, Jacob ST, Ghoshal K. Epigenetic regulation of metallothionein-i gene expression: differential regulation of methylated and unmethylated promoters by DNA methyltransferases and methyl CpG binding proteins. J Cell Biochem. 2006;97:1300–16. doi: 10.1002/jcb.20738. [DOI] [PubMed] [Google Scholar]
- 50.Henrique R, Jeronimo C, Hoque MO, Nomoto S, Carvalho AL, Costa VL, Oliveira J, Teixeira MR, Lopes C, Sidransky D. MT1G hypermethylation is associated with higher tumor stage in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1274–8. doi: 10.1158/1055-9965.EPI-04-0659. [DOI] [PubMed] [Google Scholar]
- 51.Smith E, Drew PA, Tian ZQ, De Young NJ, Liu JF, Mayne GC, Ruszkiewicz AR, Watson DI, Jamieson GG. Metallothionien 3 expression is frequently down-regulated in oesophageal squamous cell carcinoma by DNA methylation. Mol Cancer. 2005;4:42. doi: 10.1186/1476-4598-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, de la Chapelle A, Pellegata NS. Hypermethylation, but not LOH, is associated with the low expression of MT1G and CRABP1 in papillary thyroid carcinoma. Int J Cancer. 2003;104:735–44. doi: 10.1002/ijc.11006. [DOI] [PubMed] [Google Scholar]
- 53.Jacob ST, Majumder S, Ghoshal K. Suppression of metallothionein-I/II expression and its probable molecular mechanisms. Environ Health Perspect. 2002;110 Suppl 5:827–30. doi: 10.1289/ehp.02110s5827. [DOI] [PMC free article] [PubMed] [Google Scholar]