Abstract
Purpose
To use a histologic approach to obtain dimensional and morphologic information on the cornea in three commonly used strains of mice.
Methods
Adult mice (three each of 129/SVJ, C57BL/6, and BALB/c) were euthanatized, and the eyes were enucleated, immersed in 2% glutaraldehyde fixative, and prepared for light and transmission electron microscopy. The full corneal, epithelial, stromal, and posterior limiting lamina (PLL) with endothelium thicknesses were measured at the same location centrally and peripherally.
Results
All three strains showed a statistically significant (P0.001) decrease in overall thickness in the peripheral compared with the central cornea. The decrease was due to a reduced thickness of both the epithelium and the stroma. The stroma and epithelium contributed to approximately two thirds and one third of the total corneal thickness, respectively. The epithelium had the classic stratified layout and consisted of 13.00 ± 1.41 layers centrally versus 10.33 ± 1.37 peripherally. Some adaptation of stromal tissue was found immediately adjacent to the epithelial basement membrane, but a clearly defined anterior limiting lamina did not exist. The stroma was organized into lamellae but lacked the anterior branching and interweaving reported in humans and had unmyelinated nerve fibers within micrometers of the endothelium. The PLL was 2.17 ± 0.3 μm thick and was divided into pre- and postnatal layers, with striated bodies in the postnatal portion.
Conclusions
This study demonstrated that in the three strains of mice examined, the cornea becomes significantly thinner toward the periphery. Dimensionally, proportionally, and anatomically the three strains used appeared to be similar. However, morphologic differences were observed compared with other mammals, and awareness of these differences is important when using the mouse as an animal model applicable to the human.
For many years, the rabbit was generally the preferred animal model for ophthalmic research, but recently the mouse has become widely used in corneal research and studies involving other ocular regions, such as the retina and the crystalline lens.1,2 The main reason for the increased utilization of the mouse is that its genome has been sequenced, and there are many different transgenic and knockout strains available for different types of research. Additional advantages of using the mouse are that it is easy to breed, it grows rapidly, and it is cost effective.3–5
An obvious disadvantage with the mouse as a model is that its corneal diameter is much smaller than that of the human. However, the mouse cornea contains five layers (epithelium, anterior limiting lamina [ALL], stroma, posterior limiting lamina [PLL], and endothelium) as described by Smith et al.,4 although the presence of an anterior limiting lamina has been disputed.6,7 The literature also contains conflicting data on the dimensions of the mouse cornea. For instance, there are large discrepancies between different reports on the overall central corneal thickness and central stromal thickness that may be related to variations in the measuring techniques and strains of mice used. Zhang et al.3 reported a central corneal thickness of 170 μm in the BALB/c mouse, detected by confocal microscope (Helmut Hund, Wetzlar, Germany). They also reported a central epithelial thickness of 51 μm and a central stromal thickness of 119 μm. These measurements were obtained from cryostat sections photographed by microscope (Photomicro-scope OM-2; Olympus, Tokyo, Japan).3 In contrast Schulz et al.8 based their thickness assessment on optical low coherence reflectometry and reported the central corneal thickness to be 106 ± 3.45 μm in the same strain of mice, the BALB/c. Jester et al.9 used yet another technique, in vivo confocal microscopy by a tandem scanning confocal microscope (Tandem Scanning Corp., Reston, VA) to determine corneal thickness and reported a central corneal thickness of 112.9 ± 7.0 μm in wild-type littermates of PEPCK-TGFβ1 transgenic mice. Song el al.10 used the same in vivo confocal microscope to measure the central epithelial and stromal thickness in the wild-type CD1 mouse and reported values of 49.3 ± 5.7 and 81.5 ± 10.7 μm, respectively.
In addition to these discrepancies, available literature provides only central corneal measurements, and no information is available about the mouse peripheral cornea. An understanding of the normal corneal structure together with normative data is desirable for future comparisons when using the mouse model for anterior segment research.
The purpose of this study was to use a histologic approach to provide needed normative morphometric data on the dimensions of the overall cornea and its components, both centrally and peripherally, while assessing the mouse corneal morphology in three commonly used strains of mice, the BALB/c, C57BL/6, and 129/SVJ.
Materials and Methods
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Nine adult mice, 6 to 8 weeks of age (three 129/SVJ, three C57BL/6 and three BALB/c) were euthanatized, and a few drops of 2% glutaralde-hyde in 80 mM sodium cacodylate buffer, 330 mOsm/kg fixative11 were immediately applied to the cornea after resection of the eye lids. The whole eyes were carefully enucleated and immersed in fixative for 4 to 6 hours, to ensure proper cross-linking and preservation of the tissue.
After fixation, corneal diameter measurements were obtained on one eye per animal under a dissecting microscope (SZ60; Olympus) for increased magnification. A pair of digital calipers with sensitivity down to 0.1 mm was used to obtain measurements from all eyes. The corneal diameter was measured from limbus to limbus by using the easily visualized (at 25× magnification) corneoscleral junction to define the limit of the cornea. The corneal limbus-to-sclera transitional zone is very narrow, at approximately 0.1 mm and was for this reason not considered separately. The mouse cornea was found to be circular, not oval like the human cornea, and, therefore, the measurements do not differ in the horizontal and vertical meridians. All three measurements on the same cornea were obtained along the same meridian, and the corneal diameter measurements were performed on fixed eyes for consistency with the other measurements. The tissue-processing protocol used in the present study has shown that a fixative maintaining near physiological osmolality produces minimal tissue shrinkage, with the result that the undistorted, natural contour of the tissue is preserved.11 The average corneal radius (diameter/2) was used to identify the location where the central corneal measurements were made.
The fixed corneas were bisected, and small pieces (1 × 1.5 mm) were cut from the central region. The corneal pieces were washed three times in sodium cacodylate buffer (pH 7.4) at room temperature and left for 10 minutes in each wash. Subsequently, the samples were immersed in a freshly prepared 1% solution of osmium tetroxide in 100 mM sodium cacodylate buffer for 1 hour under dim light. The samples were once again washed several times in sodium cacodylate buffer and left 10 minutes in each wash. A tissue processor (EM TP; Leica; Wetzlar, Germany) was used for the following steps: dehydration, transition, infiltration, and embedding. First, the tissue samples were dehydrated through a graded alcohol series (30%–100% in six steps) at room temperature. Next the tissue samples were infiltrated with propylene oxide. Embedding with agitation was achieved through an initial mixture of propylene oxide and Araldite resin 2:1 for 3 hours, followed by overnight immersion in a 1:1 mixture of propylene oxide and Araldite resin. Thereafter, the tissue samples were immersed in propylene oxide and Araldite resin 1:3 for 4 to 8 hours before final transfer to 100% Araldite resin overnight. The tissue samples were then oriented in embedding molds and left 12 hours for polymerization in an oven at 60°C.
An ultramicrotome (MT-7000; Research Manufacturing Co. Inc., Tucson, AZ) was used to cut thick transverse sections (0.5–1 μm). These sections were stained with 1% toluidine blue for examination with a light microscope (BX51; Olympus). For morphologic analysis, ultrathin sections were obtained and mounted on parallel bar copper grids (200MP, cat no. G200P; Electron Microscopy Sciences, Fort Washington, PA). The sections were double stained, first, in 3.5% uranyl acetate for 20 minutes at 60°C, followed by Reynold's lead citrate for 10 minutes at room temperature. The grids were examined in a transmission electron microscope (Tecnai G2 Bio Twin Spirit; FEI Co., Eindhoven, The Netherlands) and the images captured digitally.
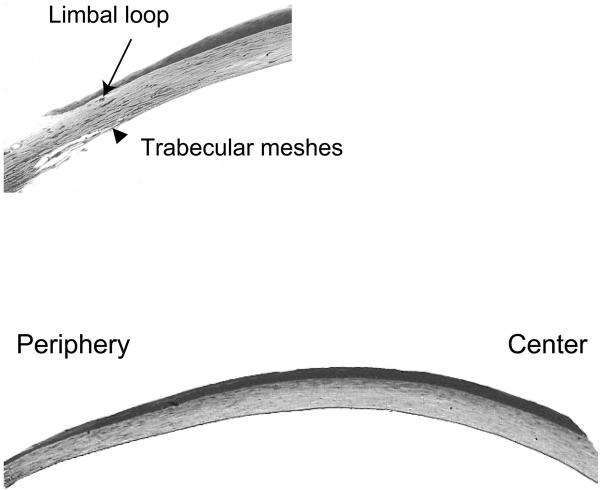
Digital images were captured at 40× and 200× of two thick toluidine-stained corneal sections, cut from two levels of the same block separated by approximately 200 μm. Peripheral measurements were taken at the extremity of the cornea, defined histologically as immediately central (anterior) to limbal capillaries and central to the anterior edge of the trabecular meshes (Fig. 1A).
Figure 1.
(A) The peripheral extremity of the cornea. Peripheral measurements were taken immediately central (anterior) to limbal loops and central to the anterior edge of the trabecular meshes. (B) Thick section stained with toluidine blue showing the thickness variation between the central and peripheral mouse cornea in a C57BL/6 mouse. Magnification: (A) ×100; (B) ×40.
Measurements for the central cornea were taken at a distance, half the corneal diameter ±14% from the peripheral measurements where the cornea had its natural contour, had taken up stain in a uniform manner, and was free of artifacts. All measurements were made with NIH Image Software (Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) in trip-licate, at the same location centrally and peripherally.
The layers of cells forming the epithelium were counted in electron micrographs taken from both the central and peripheral regions. The cells were counted in a straight line from the basement membrane to the corneal surface and did not have to display a nucleus in the plane of the section.
An unpaired Student's t-test was used to compare the two corneal sections cut from two levels of the same block but separated by approximately 200 μm, to ensure no difference throughout the tissue sample. An unpaired Student's t-test was also used to compare the average central with the average peripheral corneal measurements in three mice within the same strain. The statistical significance was set at P < 0.05.
Results
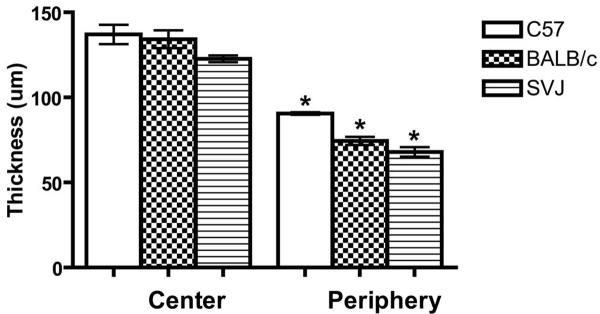
The averages of three measurements per mouse for the three strains (129/SVJ, BALB/c, and C57BL/6) are presented in Table 1. The corneal diameter for the 129/SVJ mice measured 2.3 mm and was slightly smaller than the diameter for the BALB/c and the C57BL/6 mice, which both measured 2.6 mm. All three strains of mice showed a statistically significant difference (P < 0.001) between the total central corneal thickness compared with the periphery, with the periphery being thinner compared to the center (Table 1, Figs. 1B, 2).
Table 1.
Thickness of Corneal Layers
| 129/SVJ | C57BL/6 | BALB/c | |
|---|---|---|---|
| Corneal diameter (mm) | 2.3 ± 0.1 (n = 5) | 2.6 ± 0.2 (n = 4) | 2.6 ± 0.1 (n = 4) |
| Full corneal thickness (μm) (n = 3) | C: 122.68 ± 4.8 | C: 137.02 ± 14.0 | C: 134.16 ± 12.9 |
| P: 68.00 ± 6.8 | P: 90.55 ± 1.9 | P: 74.48 ± 5.8 | |
| Stromal thickness (μm) (n = 3) | C: 80.53 ± 6.7 | C: 90.88 ± 14.8 | C: 81.84 ± 8.1 |
| P: 45.42 ± 2.9 | P: 64.11 ± 2.5 | P: 51.70 ± 4.2 | |
| Epithelial thickness (μm) (n = 3) | C: 37.12 ± 2.3 | C: 40.59 ± 5.8 | C: 46.88 ± 4.9 |
| P: 17.86 ± 6.6 | P: 22.39 ± 2.5 | P: 18.81 ± 4.2 |
The average and standard deviation of the measurements obtained from the three strains of mice. C, measurements from the central cornea; P, measurements from the peripheral cornea.
Figure 2.
Overall corneal thickness. Average full central and peripheral corneal thickness in the BALB/c, C57BL/6, and 129/SVJ mice. Central and peripheral corneal thickness within each strain was statistically compared by Student's t-test. *P < 0.001. Error bars, SD.
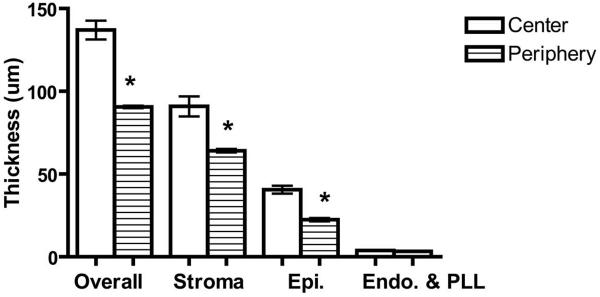
All three strains of mice showed a similar dimensional relationship between the stroma and the epithelium, with the center being thicker than the periphery (Table 1, Fig. 3). In none of the strains did the endothelium with PLL measurement vary between the central and peripheral cornea. The stroma contributed to approximately two thirds of the total corneal thickness, with the epithelium accounting for approximately one third.
Figure 3.
Central and peripheral overall, stromal, epithelial, and endothelial with PLL corneal thickness in the C57BL/6 mice. Central and peripheral corneal thicknesses of each of the corneal layers individually were compared by Student's t-test. *P < 0.001. Error bars, SD.
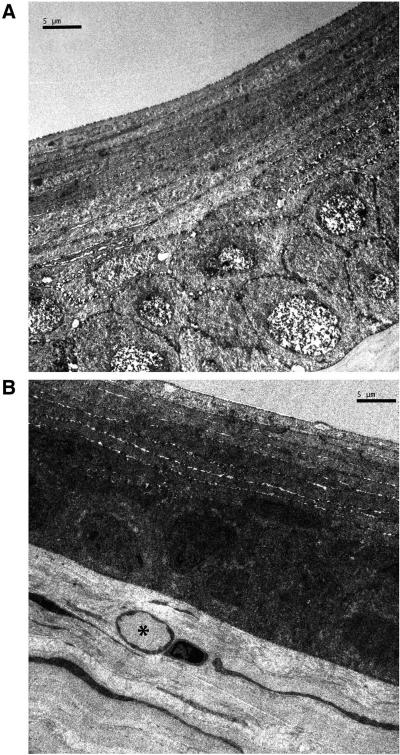
Morphologically the epithelium had the classic stratified layout. Centrally, it consisted of 13 layers (Fig. 4A), whereas peripherally it was reduced by approximately 3 layers (Fig. 4B). The increased number of layers compared with the primate was attributable to the addition of multiple layers of squamous cells. The epithelial cells were tightly packed and adhered to its neighbors through numerous desmosomes and to its basement membrane through hemidesmosomes.
Figure 4.
(A) Central epithelium. The stratified epithelium is formed by 12 layers of cells and most numerous are the squamous cells. The cells are tightly packed with no obvious spaces between them. (B) Peripheral cornea. The stratified epithelium is noticeably thinner than the central cornea (nine layers in the one in the image). Subepithelially a limbal capillary (*) is in view. The stromal organization remains similar to the central cornea.
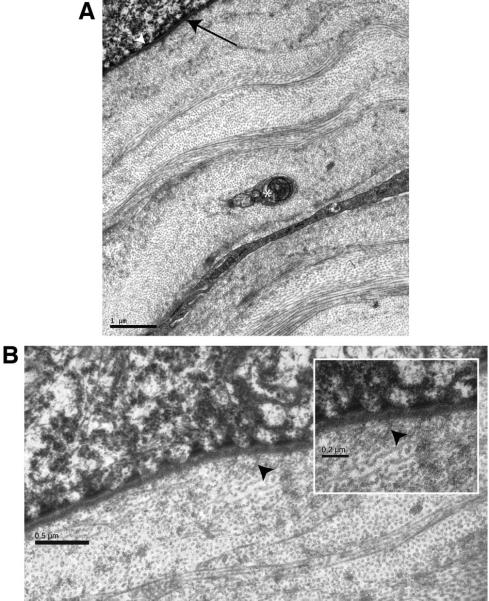
There was scant evidence of an ALL. However, fibers oriented and appearing like type VII collagen were in place at the epithelial basement membrane, and at intervals there were individual or a few collagen fibrils that departed from the characteristics of the stromal lamellar organization (Fig. 5A).
Figure 5.
(A) Epithelial-stromal interface. The internal aspect of the epithelium is outlined by hemidesmosomes (white arrowhead) and a linear basement membrane (black arrow). Immediately internal to the basement membrane, the collagen fibers are organized as stromal lamellae. An unmyelinated nerve fiber bundle (*) at preterminal level is present in the field of view. (B) Epithelial-stromal interface. Except for apparent collagen type Vll anchoring fibers (black arrowhead) all collagen fibers in this view are essentially parallel to the corneal surface. Inset: higher magnification shows collagen type Vll fibers (black arrowhead) inserting into the basement membrane.
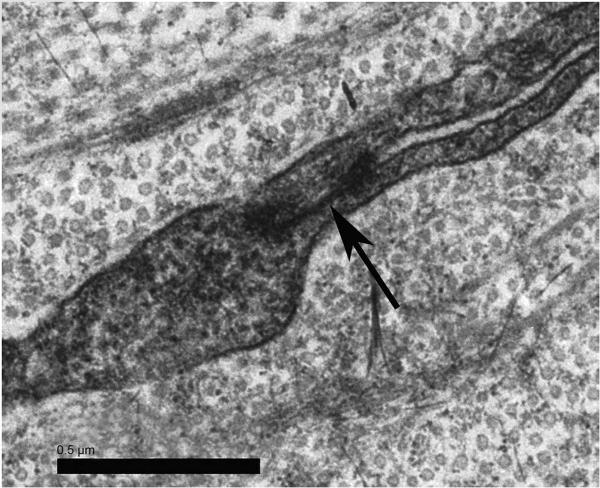
The substantia propria or the stroma was essentially organized into lamellae, which anteriorly did not show the extensive and complex interweaving seen in the human cornea. No electron-dense formations suggesting midcorneal lamellar terminations were recorded (Fig. 5B). Posteriorly, the lamellae followed the humanlike layered arrangement. Scattered across the cornea were keratocytes in a density that approximates that of the primate. Gap junctions between adjacent keratocyte processes were noted (Fig. 6).
Figure 6.
Keratocytes. Processes from two separate keratocytes form a gap junction (arrow) in the midstroma.
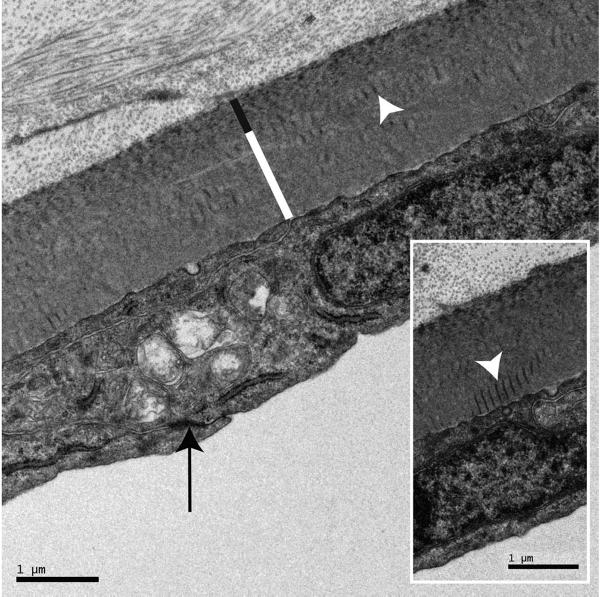
Measurements and morphologic analysis of the PLL and endothelium individually were made from electron micro-graphs. The animals examined were all young adults and the PLL measured on average 2.17 ± 0.3 μm in cross section. The mouse PLL did not possess the distinct banded and the non-banded division seen in the primate cornea but had a thin anterior layer facing the stroma that appeared to correlate to the banded fetal portion in humans. It had a texture that was different, with a tendency toward banding compared with the underlying and presumably postnatal basement membrane. Scattered within the posterior portion of the basement membrane were numerous bodies with collagen-like cross striations. The endothelium, measuring on average 2.15 ± 0.4 μm in thickness, provided full corneal coverage all the way to the trabeculae, and had a smooth anterior and posterior outline. The lateral sides showed intimate interdigitation with its neighbors and a lateral flap extending from one cell to bridge the narrow gap between two cells. This junction between two cells contained a zonula occludens (Fig. 7).
Figure 7.
Endothelium and PLL. Neighboring endothelial cells are linked by a zonula occludens (black arrow), have essentially parallel apical and basal sides, and are richly provided with organelles. The PLL has a thin banded portion (black line) and a thicker, nonbanded portion (white line). Collagen-like striated bodies (white arrowhead) are scattered along the nonbanded portion at all levels. Inset: high magnification of PLL showing the banded portion and striated bodies (white arrowhead) in greater detail.
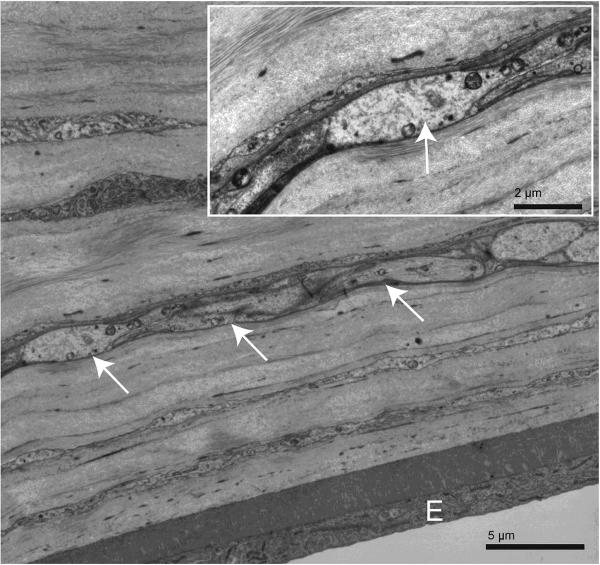
An interesting departure from the primate pattern was the distribution of corneal nerves. Stromal nerve fibers were observed throughout the full corneal thickness. Unmyelinated nerve fiber bundles were regularly seen within a few micrometers of the PLL and the endothelium, where they often exhibited axon varicosities (Fig. 8).
Figure 8.
Stromal unmyelinated nerve fiber bundle within 10 μmof the endothelium. The nerve fiber (white arrows), sectioned obliquely to longitudinally, runs parallel to the endothelial layer (E) and is at a preterminal-to-terminal stage. Inset: higher magnification of this posterior stromal nerve fiber. One of its axons (white arrow) is forming a varicosity and at this point is devoid of its Schwann cell wrapping.
Discussion
The present study qualitatively and morphometrically assessed the corneal anatomy of three strains of mice. With minor variations, all types of mice showed the same general structural arrangement and dimensions, while also possessing features distinct to this species.
Little information is available concerning the dimensions of BALB/c and 129/SVJ and C57BL/6 mouse corneas. As noted in the introduction, the existing data show a large discrepancy between central corneal thickness measurements (106.00 ± 3.45 μm versus 170 μm).3,8 This difference is likely to be due to the various methodologies used to obtain the measurements. The protocol followed in the present study eliminated shrinkage and tissue distortion, which, together with the presence of histologically well defined reference points, allowed the observer to conduct accurate intracorneal measurements.11
Schultz et al.8 used optical low coherence reflectometry, which is a type of noncontact pachymeter to obtain the thickness measurements. To use this technique, one must know the accurate refractive index of the cornea. The refractive index used in Schultz et al.8 was based on a rat schematic eye reported by Massof and Chang,12 which may be similar but does not correspond exactly to the refractive index of the mouse cornea. Another drawback with using optical low coherence reflectometry is that accurate calibration of this type of instrument is known to be difficult.13
Zhang et al.,3 on the other hand, used a confocal microscope (Helmut Hund) in one animal to arrive at a 170-μm central corneal thickness for the mouse. In addition to a small sample (n = 1), this confocal instrument, of nondescript technical specification, may not have had the precision to make such an assessment. The corneal diameter ranged from 2.3 to 2.6 mm in the three strains assessed in the present study. Zhang et al.3 made a measurement on one BALB/c mouse eye and found a corneal diameter of 3.5 mm, which is substantially larger than the same measurements on our BALB/c mice or the other strains used in this study. The reason for this discrepancy may be the age of the animals (6 to 8 weeks versus 6 months) and also the methodology used for this measurement. Higher magnification and resolution through the use of a high-quality dissection microscope, in conjunction with a precision caliper allowed our measurements to be very precise. We also found a good agreement between the different strains. Zhang et al.3 also determined the stromal and the epithelial proportions toward the total corneal thickness in cryosections, which have known disadvantages such as poor morphologic preservation and limited resolution.14 Both Jester et al.9 and Song et al.10 used in vivo confocal microscopy to obtain their measurements, but the drawback with this method is its reproducibility due to the difficulty in locating the appropriate area for the assessment.
Another issue to consider relates to how the central cornea was defined in the aforementioned studies. Schultz et al.8 defined the central cornea by the visual corneal apex, whereas Zhang et al.3 did not state any criteria for locating the central cornea. In the present study, both the central and the peripheral measurements were taken from clearly defined corneal zones. Therefore, we argue that our measurements are likely to be accurate for each of the three strains.
The data showed that there was a significant decrease (P < 0.001) in thickness between the central and peripheral cornea in the 129/SVJ, C57BL/6, and BALB/c mice. The cornea thinned between 55% to 66% going from center to periphery depending on the mouse strain. The decreased thickness of the mouse cornea toward the periphery appeared mainly to be due to tissue thinning of the stroma and the epithelium but some compacting of the tissue may also occur toward the periphery.
It is also interesting to note the difference in shape between the human and the mouse cornea. The average human cornea measures 535 ± 20 μm in the center compared with 657 ± 71 μm in the periphery.15 Thus, the human cornea is thinner in the center than in the periphery, making it a negative-meniscus lens. In contrast, the mouse cornea is thicker in the center and thinner in the periphery and could therefore be described as having the shape of a positive-meniscus lens. For comparison, the rabbit cornea maintains a uniform thickness across its width and is therefore, similar to a plano lens.16 The thinning of the mouse cornea toward the periphery is a new discovery, and the structural cause to the thinning has yet to be explained. A possible explanation for this difference in thickness is that the peripheral cornea is formed by fewer stromal lamellae. Alternatively, the cornea may have the same number of lamellae centrally and peripherally but with the peripheral lamellae being thinner. Further ultrastructural histologic studies are needed and are under way in our laboratory to uncover the structural reason for this corneal thickness variation, which may also explain the nonuniform thickness of the human cornea.
It was also concluded in the present study that the epithelium contributes approximately 30% percent and the stroma approximately 70% to the total central and peripheral corneal thickness in the mouse. In contrast, the corneal epithelium represents approximately only 10% and the stroma 90% of the total corneal thickness in humans.17 In an experimental model, it is important to be aware of the greater relative contribution of the epithelium toward the overall corneal thickness in the mouse compared with the human. Such differences are likely to have an impact when relating corneal wound-healing studies on the mouse to those in the human. For the mouse epithelium to heal and return to full thickness may take longer than in the primate, since the mouse epithelium is formed by more layers of cells. Ultraviolet radiation experiments on the cornea are another example in which where a species variation make interspecies comparisons more uncertain, since the epithelium and the stroma have different absorption characteristics.18
The stratified layout of the murine corneal epithelium is consistent with the description of this epithelium in the mammalian cornea, such as rat, rabbit, cat, and human.1,19–22 Compared with the human epithelium, that of the mouse consists of approximately twice the layers of cells, and the increased number of cells is due to a comparative elevation in the number of squamous cells.23,24 The average of 13 cell layers in the epithelium was a consistent finding in our specimens, as was the thinning of the epithelium by approximately three layers in the peripheral cornea. Other epithelial features, such as desmosomal junctions, hemidesmosomes, and basement membrane were present in number and form similar to that described for the primate.24
The presence or absence of an ALL in the cornea of a particular species varies according to how this layer is defined. If the criteria for the presence of an ALL include a layer of primate-like thickness of 8 to 10 μm, clearly the mouse does not possess one. However, if some type VII collagen fibers and one or a few type I fibers not strictly following the lamellar pattern of stroma proper is all that is required, then it may be argued that the mouse has an ALL. However, we propose that the ALL in the primate is a distinct and separate layer sandwiched between the epithelium and the stroma. In the mouse there is no such layer, but there is some adaptation of stromal tissue immediately adjacent to the epithelial basement membrane. This is what others 4,25 may have elected to call an ALL but to do so will only cause confusion, because there is no resemblance between the primate ALL and the same region in the mouse. In this respect we are in agreement with Reh-binder,6 who also concluded that the mouse does not possess an ALL.
The mouse corneal stroma was organized into lamellae crossing each other at various angles while remaining parallel to the ocular surface, as is also the case in the human cornea.21,23 However, the anterior interweaving and branching lamellae reported in humans24,26,27 were not present in mice, and the electron-dense formations recently described in humans were also absent.27 Thus, there are significant differences between the anterior stroma of the human and the mouse. In the mouse, the anterior stromal layout consisted essentially of lamellae parallel to the corneal surface and laid down on top of each other at different angles. The architecture of the mouse anterior stroma was similar to the human mid- and posterior stroma.
Keratocytes were present primarily between the lamellae and had a morphology similar to that reported in humans and rabbits.22,28 They seemed to be there in a sufficient number and possessed gap junctions to permit the intracorneal network suggested by Watsky.29
The PLL consists of a fetal and a postnatal portion,4,23,24 which in the case of the human cornea divides the membrane into a distinctly banded and a more uniformly textured portion. Our observations suggest that the mouse PLL has a similar but less distinct organization into pre- and postnatal layers, perhaps because of the relatively shorter gestation period in mice. The presence of striated bodies in the PLL postnatal portion was also noted by Smith et al.4 and pictured but not commented on by Rehbinder.6 This feature may be peculiar to mice. Of note, similar features were observed in the human PLL in a patient with Fuchs' endothelial dystrophy, but in this case the bodies were located in the posterior collagenous layer, which is formed by the abnormal basement membrane material pathologically produced by the endothelium in this disease.30 The function and exact composition of these collagen-like bodies are not yet known. The termination of the PLL at the trabecula resembled the primate and the rabbit.
The structure of the mouse corneal endothelium closely resembles that of both the human23,24 and the rabbit.1,11 However, the mouse endothelium at 2.1 μm in transverse section is thinner than the rabbit (2.6 μm) and human (5 μm) endothelia.11,31 The general shape and outline, the extensive interdigitation of the lateral sides, and the position of junctions show striking similarity between the two species and presumably also provide the same functions.
The distribution of the mouse corneal nerve fibers is at variance with the primate. In the primate cornea, nerve fibers are not found deeper than 50 μm from the epithelium, especially in the central cornea, where most stromal fibers are within 25 μm of the epithelium.24,32 However, the mouse cornea contains unmyelinated nerves in the deeper layers of the cornea within micrometers of the endothelium. Some of these fibers harbor terminals and therefore must perform their functions at this posterior location. The mouse cornea, being a relatively thin one, may permit these terminals to respond to stimuli from an anterior direction. Alternatively, the posterior corneal nerve fibers may have other functions than those associated with the primate corneal innervation.
Dimensionally, proportionally, and anatomically, all three strains of mice in our study appeared to be similar, and this may be true of other strains of mice as well. However, there are significant dimensional differences between mice and other mammals including humans. In addition, some noticeable anatomical variations were demonstrated ultrastructurally. Overall, the mouse is a useful model for corneal research; however, awareness of the morphologic features peculiar to the mouse is important when using the mouse as an animal model applicable to the human.
Acknowledgments
The authors thank Alan Burns for providing the C57BL/6 and Richard Bond for providing the BALB/c specimens used for this experiment, and Margaret Gondo for excellent technical advice and support.
Supported by National Eye Institute Core Grant P30 EY007551 to the University of Houston College of Optometry. JTH received a graduate student stipend from Optikbranschen (Stockholm, Sweden).
Footnotes
Disclosure: J.T. Henriksson, None; A.M. McDermott, None; J.P.G. Bergmanson, None
References
- 1.Prince JH. The Rabbit in Eye Research. Thomas; Springfield, IL: 1964. pp. 86–134. [Google Scholar]
- 2.Smith RS, Krob D, John SWM. A goniolens for monitoring of the mouse iridocorneal angle and the optic nerve. Mol Vis. 2002;8:26–31. [PubMed] [Google Scholar]
- 3.Zhang E, Schründer S, Hoffmann F. Orthotopic corneal transplantation in the mouse: a new surgical technique with minimal endothelial cell loss. Graefes Arch Clin Exp Ophthalmol. 1996;243:714–719. doi: 10.1007/BF00292359. [DOI] [PubMed] [Google Scholar]
- 4.Smith RS, John SWM, Nishina PM, Sundberg JP. The anterior segment and ocular adnexae. In: Smith RS, editor. Systematic Evaluation of the Mouse Eye, Anatomy, Pathology, and Biomethods. CRC Press; Boca Raton, FL: 2002. pp. 3–24. [Google Scholar]
- 5.Garcia de la Cera E, Rodrigues G, Liorenta L, Schaffel F, Marcos S. Optical aberrations in the mouse eye. Vision Res. 2006;46:2546–2553. doi: 10.1016/j.visres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Rehbinder C. Fine structure of the mouse cornea. Z Versuchstierk. 1978;20:28–34. [PubMed] [Google Scholar]
- 7.Hazlett LD. Corneal and ocular surface histochemistry. Prog Histochem Cytochem. 1993;25(1):2. doi: 10.1016/s0079-6336(11)80031-8. [DOI] [PubMed] [Google Scholar]
- 8.Schulz D, Iliev ME, Ftueh BE, Goldblum D. In vivo pachymetry in normal eyes of rats, mice and rabbits with the optical low coherence reflectometer. Vision Res. 2003;43:723–728. doi: 10.1016/s0042-6989(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 9.Jester JV, Lee YG, Li J, et al. Measurements of corneal sublayer thickness and transparency in transgenic mice with altered corneal clarity using in vivo confocal microscopy. Vision Res. 2001;41:1283–1290. doi: 10.1016/s0042-6989(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 10.Song J, Lee Y-G, Houston J, et al. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest Ophthalmol Vis Sci. 2003;44(2):548–557. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doughty MJ, Bergmanson JPG, Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolarity glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tissue Cell. 1997;29(5):533–547. doi: 10.1016/s0040-8166(97)80054-7. [DOI] [PubMed] [Google Scholar]
- 12.Massof RW, Chang FW. A revision of the rat schematic eye. Vision Res. 1972;12:793–796. doi: 10.1016/0042-6989(72)90005-3. [DOI] [PubMed] [Google Scholar]
- 13.Reader AL, Salz JJ. Differences among ultrasonic pachymeters in measuring corneal thickness. J Refract Surg. 1987;3(1):7–11. [Google Scholar]
- 14.Ward TS, Rosen GD, von Bartheld CS. Optical dissector counting in cryosections and vibratome sections underestimates particle numbers: effects of tissue quality. Microsc Res Tech. 2008;71:60–68. doi: 10.1002/jemt.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367–407. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 16.Doughty MJ. Physiological state of the rabbit cornea following 4°C moist chamber storage. Exp Eye Res. 1989;49:807–827. doi: 10.1016/s0014-4835(89)80041-7. [DOI] [PubMed] [Google Scholar]
- 17.Li HF, Petroll WM, Pedersen TM, Mauer JK, Cavanagh HD, Jester JV. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CNTF) Curr Eye Res. 1997;16:214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 18.Walsh JE, Bergmanson JPG, Koehler LV, Doughty MJ, Fleming D, Harmey JH. Fibre optic spectrophotometry for the in vitro evaluation of ultraviolet radiation (UVR) spectral transmittance of rabbit corneas. Physiol Meas. 2008;29:1–14. doi: 10.1088/0967-3334/29/3/008. [DOI] [PubMed] [Google Scholar]
- 19.Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26(suppl 1):S75–S81. doi: 10.1097/ICO.0b013e31812f6d8e. [DOI] [PubMed] [Google Scholar]
- 20.Madigan M, Holden B. Reduced epithelial adhesion after extended contact lens wear correlates with reduced hemidesmosome density in cat cornea. Invest Ophthalmol Vis Sci. 1992;33(2):314–323. [PubMed] [Google Scholar]
- 21.DukeElder S, Wybar KC. System of Ophthalmology. Vol 2. The Anatomy of the Visual System. Vol. 92. Henry Kimpton; St. Louis: 1961. The eye; 131 pp. [Google Scholar]
- 22.Clareus F. Thesis. Uppsala University Medical School, E. Westrell; Stockholm: 1857. Hornhinnans histologi. [Google Scholar]
- 23.Bron AJ, Tripathi RC, Tripathi BJ. Wolff's Anatomy of the Eye and the Orbit. 8th ed Chapman & Hall Medical; London: 1997. The cornea and sclera; pp. 233–267. [Google Scholar]
- 24.Bergmanson JPG. Clinical Ocular Anatomy and Physiology. 15th ed Texas Eye Research and Technology Center; Houston: 2008. pp. 67–94. [Google Scholar]
- 25.Hayashi S, Osawa T, Tohyama K. Comparative observations on corneas, with special reference to Bowman's layer and Descemet's membrane in mammals and amphibians. J Morphol. 2002;254:247–258. doi: 10.1002/jmor.10030. [DOI] [PubMed] [Google Scholar]
- 26.Bergmanson JPG, Horne J, Doughty MJ, Garcia M, Gondo M. Assessment of the number of lamellae in the central region of the normal human corneal stroma at the resolution of the transmission electron microscope. Eye Contact Lens. 2005;31(6):281–287. doi: 10.1097/01.icl.0000165280.94927.0d. [DOI] [PubMed] [Google Scholar]
- 27.Mathew JH, Bergmanson JPG, Doughty MJ. Fine structure of the interface between the anterior limiting lamina and the anterior stromal fibrils of the human cornea. Invest Ophthalmol Vis Sci. 2008;49(9):3914–3918. doi: 10.1167/iovs.07-0707. [DOI] [PubMed] [Google Scholar]
- 28.Doughty MJ, Seabert W, Bergmanson JPG, Blocker Y. A descriptive and quantitative study of the keratocytes of the corneal stroma of albino rabbits using transmission electron microscopy. Tissue Cell. 2001;33(4):408–422. doi: 10.1054/tice.2001.0195. [DOI] [PubMed] [Google Scholar]
- 29.Watsky MA. Keratocyte gap junctional communication in normal and wounded rabbit corneas. Invest Ophthalmol Vis Sci. 1995;36(13):2568–2576. [PubMed] [Google Scholar]
- 30.Bergmanson JPG, Sheldon TM, Goosey JD. Fuchs' endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol Opt. 1999;19(3):210–222. doi: 10.1046/j.1475-1313.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara T. Current concepts in anatomy and histology of the cornea. Contact Intraocul Lens Med J. 1978;4:101–132. [Google Scholar]
- 32.Bergmanson JPG, Doughty MJ. Anatomy, morphology and electron microscopy of the cornea and conjunctiva. In: Benett E, Weissman BA, editors. Clin Contact Lens Pract. Lippincott Williams & Wilkins; 2005. pp. 11–39. [Google Scholar]