Abstract
Glia have emerged as key contributors to pathological and chronic pain mechanisms. On activation, both astrocytes and microglia respond to and release a number of signalling molecules, which have protective and/or pathological functions. Here we review the current understanding of the contribution of glia to pathological pain and neuroprotection, and how the protective, anti-inflammatory actions of glia are being harnessed to develop new drug targets for neuropathic pain control. Given the prevalence of chronic pain and the partial efficacy of current drugs, which exclusively target neuronal mechanisms, new strategies to manipulate neuron–glia interactions in pain processing hold considerable promise.
Under normal conditions, in otherwise healthy subjects, acute-pain processing consists of a chain of events that begins with stimuli that activate specialized receptive endings on peripheral sensory nerve fibres. Following such activation, excitatory impulses carried by the sensory afferent axons induce excitatory postsynaptic potentials in pain-transmission neurons located in the dorsal horn of the spinal cord, with the final synaptic relay occurring in the cortex. However, pain processing is not simply a relay of signals from the body to the brain, it is a redundant, multi-pathway and dynamic system in which profound pain suppression or enhancement can occur at any level of synaptic communication. When dysfunctional pain signalling (for example, pain signalling in the absence of tissue damage) occurs, pathological pain ensues. Lesions of the nervous system can produce a form of pathological pain, neuropathic pain, which is characterized by unexplainable widespread pain, a sensory deficit, a burning sensation, pain caused by light touch (allodynia), or acute pain in the absence of a noxious stimulus1. Furthermore, chronic neuropathic pain can persist for months, without the underlying cause being treatable or identifiable.
Our understanding of pathological pain has primarily revolved around neuronal mechanisms. However, neighbouring astrocytes and microglia have recently been recognized as powerful modulators of pain and are emerging as a new target for drug development. Although glia are well known for having a number of housekeeping functions that are necessary for healthy neuronal communication, on strong activation they act as immunoresponsive cells or exert a neuroprotective effect. Glia can contribute to neuropathic-pain processing by releasing a number of glial and neuronal signalling molecules. These include, among others, the classic immune signals: cytokines and chemokines. There is substantial evidence that both astrocyte and microglia activation lead to pro-inflammatory responses with pathological effects, such as neuronal hyperexcitability, neurotoxicity and chronic inflammation. However, the notion that activation of glia in the CNS may also lead to beneficial outcomes has recently been gaining attention. Evidence that both astrocytes and microglia respond to and contribute to a local immune environment that is beneficial for nearby healthy glia and neurons is emerging. These beneficial roles of glia include the release and maintenance of anti-inflammatory factors, and processes that serve to restore normal pain signalling and protect against neurotoxicity. This Review first examines the evidence for glial roles in the regulation of pain, and then discusses how glia activation could be exploited to provide new research avenues for therapeutic pain control. Taking into account both the protective and the pathological effects of activated glia will be key to the development of effective therapeutics.
Pain processing
Pain is a sensory system that under normal conditions is protective and adaptive. It serves as a warning signal for the body (of tissue inflammation and damage) and induces behavioural changes that facilitate wound healing and recuperation. Acute pain signalling is produced when stimuli that signal potential or actual tissue damage are detected in the body by the peripheral nerve terminals of nociceptive neurons (FIG. 1a). Specific stimuli (for example, high heat, pressure or chemicals) that evoke a response activate specialized areas on nociceptive nerve terminals and induce electrical impulses that are then conducted from the nociceptive fibre terminal along the fibre’s axon to the spinal cord dorsal horn2. Acute pain is transient and lasts several weeks at most, whereas persistent pain associated with clearly identifiable causes, such as infection or inflammation, can last for up to 6 months3. Both acute and persistent pain arise from tissue damage and/ or inflammation, and so the nociceptive transmission in these cases is not necessarily dysfunctional. These pain conditions are mediated by well-known, multisynaptic pathways, which are triggered in the periphery and are processed at multiple sites in the CNS. The peripheral component of the pain pathway is referred to as the nociceptor and consists of a specialized ending at the peripheral nerve terminal (containing transient receptor potential (TRP) channels, tetrodotoxin channels and ion channels) that transduces energy from noxious (potential or actual tissue-damaging stimuli), temperature and mechanical signals, or information from chemical signals, into action potentials; an axon that conducts action potentials; a sensory nociceptive neuron located in the dorsal root ganglia (DRG) (FIG. 1 inset); and a central nerve terminal, which is the presynaptic portion of the synaptic relay to pain-projection neurons in the dorsal horn of the spinal cord4.
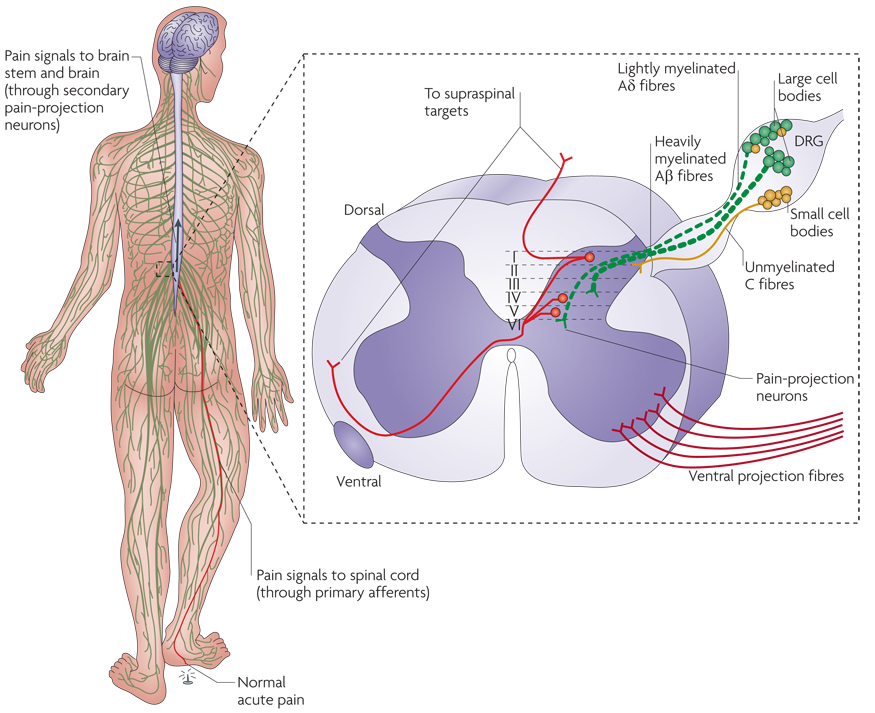
Figure 1. Nociception.
Normal pain signalling in the body is transmitted to the spinal cord dorsal horn through nociceptors. Nociceptive pain, such as a pin prick to the foot, leads to the release of pain transmitters from primary afferent terminals that project onto pain-projection neurons primarily to laminae I, IV and V in the spinal cord dorsal horns. It is notable that Aβ, Aδ and C fibres additionally project to laminae II–VI to a much lesser extent, but can alter pain-projection neuron activity. However, when tissue injury and skin inflammation ensue, chronic inflammatory signals are released from surrounding cells at the peripheral nerve terminal and lead to the sensitization of the nociceptors. Such factors include, but are not limited to, adenosine and its related mono- or polyphosphorylated compounds (AMP, ADP and ATP), bradykinin, glutamate, histamine, interleukin 1, interleukin 6, nerve growth factor, platelet-activating factor, prostaglandin E2, protons, serotonin, substance P and tumour-necrosis factor-α. Nociceptive signalling from the dorsal root ganglia (DRG) is then relayed to the dorsal spinal cord, brain stem and brain, where the experience of pain occurs. The inset shows a cross section of spinal cord including the spinal cord dorsal horn and the DRG. The DRG contain pseudounipolar sensory neurons — so called because they give rise to a single axon that bifurcates, with one part projecting to the periphery and the other projecting to the dorsal horn of the spinal cord. The cell bodies of nociceptive neurons in the DRG are broadly classified into large and small types. Immunohistochemical staining studies have shown that slowly conducting C and Aδ fibres have small cell bodies, whereas faster-conducting Aβ fibres tend to have larger cell bodies.
Primary sensory neurons convey information about non-noxious and noxious stimuli, and their axons are classified into four major groups on the basis of their conduction velocity. Large, myelinated fibres (Aα and Aβ fibres) conduct the fastest; A δ fibres, which are lightly myelinated, and unmyelinated C fibres conduct more slowly (FIG. 1 inset). Whereas mechanical, chemical and thermal ‘threats’ to tissue integrity induce high-threshold nociceptors to respond, low-threshold sensory receptors at the nerve terminals of Aβ fibres respond to non-noxious stimuli, such as vibration or light touch. Information conveyed by Aβ fibres can be greatly altered during disease conditions or after tissue damage has resolved, leading to abnormal pain signalling (as the Aβ fibres begin to signal pain in response to non-noxious stimuli (see below)).
The central projections of Aδ and C fibres synapse onto nociceptive interneurons and pain-responsive second-order pain-projection neurons in the dorsal horn of the spinal cord (FIG. 1 inset) or in the hindbrain in the case of trigeminal ganglia. The axons of these second-order neurons decussate (cross to the contralateral side) at the spinal cord level, where they join ascending fibres of the anterolateral system and project to supra-spinal pain-processing centres (FIG. 1a). These higher-order areas include the brainstem and thalamic nuclei, including the ventral posterior nucleus, the intralaminar nucleus and the parafascicular nucleus. In turn, axons from the thalamus project to and synapse on to several cortical areas, such as the primary and secondary somatosensory cortex and the association cortex of the posterior parietal lobe (see REF. 7 for a review). All these components of the pain pathway have to work in concert to produce a protective, adaptive response in the host organism.
Several well-characterized chemical signals trigger pain transmission in response to incoming noxious stimuli (FIG. 2a). For example, presynaptic afferent neurons release substance P (also known as tachykinin, precursor 1), which binds to the neurokinin 1 receptor (also known as tachykinin receptor 1) located on post-synaptic pain-projection neurons5. The co-expression of receptors for the excitatory amino acid glutamate, AmPA (α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid) and NMDA (N-methyl-d-aspartate) receptors, helps to regulate neurotransmitter release and the release of substance P. Although glutamate binds to both AMPA and NMDA receptors on the pre- and postsynaptic neurons, glutamate binding to NMDA receptors under normal, non-pathological conditions does not lead to changes in membrane potential and subsequent pain-projection neuron excitation, as the NMDA receptor-associated ion channel is plugged by Mg2+. The highest concentration of the neurokinin 1 receptor is located on postsynaptic terminals in lamina I of the dorsal spinal cord. Many neurokinin 1 receptor-expressing neurons internalize the receptor following activation by substance P6. Other neuromodulators, such as calcitonin gene-related peptide (CGRP), galanin, vasoactive intestinal polypeptide and somatostatin, also have crucial roles at the first synapse in the dorsal spinal cord and have been thoroughly reviewed elsewhere7. The activity of these pain-projection neurons is also influenced by local inhibitory interneurons in the spinal cord and by supra-spinal and brainstem-to-spinal-cord mechanisms (for reviews see REFS 8,9).
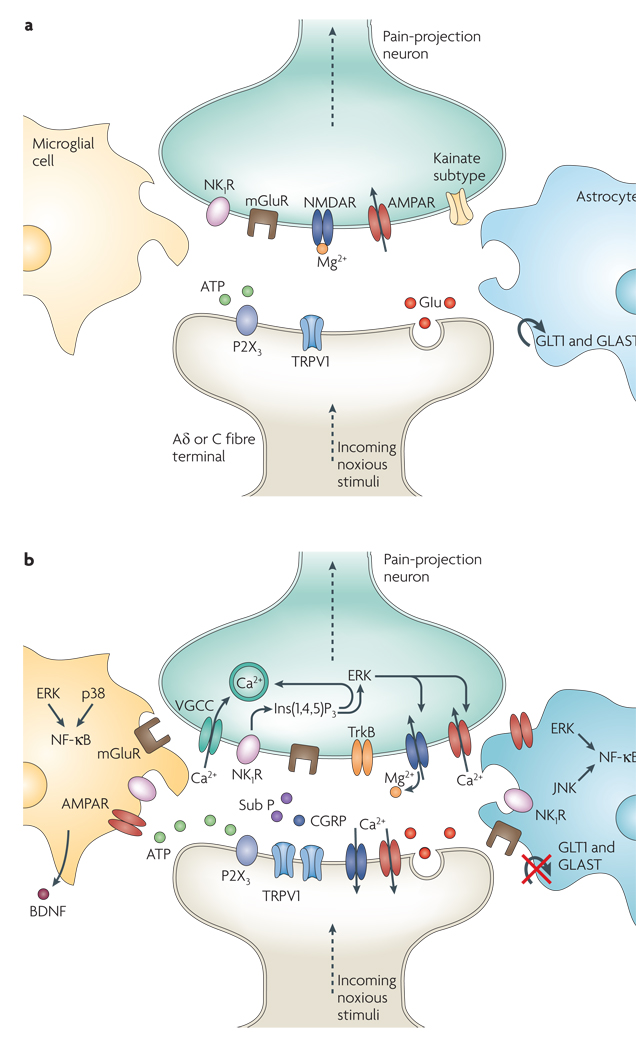
Figure 2. Molecules involved in pain processing.
a | Under healthy circumstances, low-frequency activation of Aδ and C fibre nociceptors by mild noxious stimuli leads to glutamate (Glu) release from the central presynaptic afferent nerve terminals in the spinal cord dorsal horn. Short-term activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid) and kainate subtypes of ionotropic glutamate receptors ensues. Although also present, the NMDA (N-methy-l-d-aspartate) ionotropic glutamate receptor subtype (NMDAR) remains silent because it is plugged by Mg2+. This signalling to dorsal horn pain-projection neurons provides information about the time of onset, duration and intensity of noxious stimuli from the periphery. Both astrocytes and microglia remain unchanged by these synaptic events. b | After repetitive synaptic communication, which can occur after a short barrage of nociceptive afferent input, there is an increase in the responsiveness of dorsal horn pain-projection neurons to subsequent stimuli (known as central sensitization) (see BOX 1). A co-release of glutamate and neurotransmitters such as substance P (sub P) and calcitonin gene-related peptide (CGRP) mediates NMDAR activation, leading to voltage-gated Ca2+ currents (VGCCs). In addition, inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) signalling and mitogen-activated protein kinases, such as extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK), are activated. In neurons, ERK can further sensitize excited AMPA receptors (AMPARs) and NMDARs. Activation of purinoreceptors (P2X3) by ATP, activation of sub P receptors (the neurokinin 1 receptor (NK1R)), activation of metabotropic glutamate receptors (mGluR), and release of brain-derived neurotrophic factor (BDNF) all contribute to enhanced nociceptive transmission. Astrocytes and microglia express various neurotransmitter receptors and are activated by glutamate, ATP and sub P. At synapses the glutamate transporters, glutamate transporter 1 (GLT1) and glutamate–aspartate transporter (GLAST), which are crucial for clearing synaptic glutamate, become dysregulated after prolonged exposure to high levels of synaptic glutamate. Ongoing excitation can induce ERK, p38 and JNK activation in microglia and astrocytes. Each of these kinases can activate the transcription factor nuclear factor-κB (NF-κB), which induces the synthesis of inflammatory factors. Upregulation of the V1 transient receptor potential channel (TRPV1) after inflammation further contributes to the sensitization to noxious signals. During this time, normally non-nociceptive Aβ fibres can also activate pain-projection neurons.
Pathological pain processing
Although acute pain from an identifiable trauma is considered a symptom of tissue injury or disease, chronic and recurrent pain that persists for more than 3 months is itself a disease condition10. Normally the pain pathway is activated to induce an adaptive and protective response that facilitates recuperation and helps to prevent further tissue damage. Treating clearly identifiable causes of pain normally leads to a resolution of such pain. When injury or inflammation is prolonged, ongoing excitation of primary nociceptive neurons causes chronic pain. Injured or inflamed tissue in the CNS can also sensitize neurons in the spinal cord, leading to chronic pain7,11. These chemical and neural changes occur at the site of tissue injury in the body, either at the nerve endings of pain fibres or along their axons, and at first-order synapses, both pre- and postsynaptically in the dorsal horn of the spinal cord and/or in supra-spinal pain-processing areas. These well-characterized changes in neuronal and biochemical processing, from normal conditions to a pain-facilitatory state in the dorsal horn of the spinal cord, are collectively known as ‘central sensitization’ (REF. 12) (BOX 1). Altered activity of spinal and/or brain neurons that is relevant to the pain pathway, as well as of primary sensory nociceptors, leads to chronic neuropathic pain1,13. Neuropathic pain is often associated with direct trauma and/or inflammation of peripheral nerves14. There are various peripheral neuropathies, in which damage to the PNS leads to allodynia and to increased sensitivity to noxious stimulation (hyperalgesia).
Box 1 central sensitization
Several mechanisms that bring about central sensitization have been described7,11,88,91,118,119. The best characterized involves a change in the function of neuronal NMDA (N-methyl-d-aspartate) receptors in the spinal cord dorsal horn88. Activation of sensory neurons by painful stimuli leads to the release of transmitters (for example, substance P and excitatory amino acids) that bind to and activate pain-projection neurons in the spinal cord. During conditions that produce strong and/ or persistent nociceptive stimulation, sufficient amounts of substance P and glutamate are released to sustain the depolarization of the spinal cord neurons. When this happens, Mg2+ that is normally present in the NMDA channel is removed, allowing Ca2+ to flow into the cell and facilitating signal transmission. The influx of Ca2+ causes the production and release of nitric oxide by Ca2+-activated neuronal nitric oxide synthase and of prostaglandins by cyclooxygenase enzymes120. These molecules both enhance the excitability of spinal cord neurons in response to incoming pain signals and cause an exaggerated release of neurotransmitters from sensory neuron presynaptic terminals to the spinal cord. Together, these downstream effects of NMDA activation result in the amplification of pain messages being relayed to higher brain centres88.
The first pain-relevant chemical signals to be identified at first-order synapses included glutamate and substance P (FIG. 2). Many additional factors in the periphery are now known to create an ‘inflammatory soup’ that sensitizes sensory nerve endings (for a review see REF. 15) (FIG. 2b).
Role of glia in pathological pain processing
It is now thought that solely considering neuronal activity provides an incomplete understanding of the creation and maintenance of chronic neuropathic pain16. Spinal cord glial activation seems to be a common underlying mechanism that leads to pathological pain in a number of pain syndromes with widely different aetiologies (for example, diabetic neuropathy, chemotherapy-induced neuropathy, peripheral nerve inflammation and trauma, and spinal cord inflammation)17. Astrocytes and microglia (collectively referred to as glia) have well-documented roles in pain facilitation: they can modulate neuronal synaptic function and neuronal excitability by various mechanisms18,19. Although astrocytes and microglia in the CNS each have unique roles in the modulation of neuronal function20,21, they do have some overlapping actions. Both cell types are key mediators of the CNS innate immune response, which resembles many aspects of the classical immune response22.
Collectively, glia greatly outnumber neurons in the CNS. There are various cellular subtypes, such as astrocytes, resident microglia, perivascular microglia and oligodendrocytes, that have distinct functions in the regulation of pain processing. Oligodendrocytes, best known for their role in myelinating axons in the CNS, are now emerging as modulators of neuronal function23,24. The modulating functions of astrocytes, resident microglia and perivascular microglia are well established; their role in pain processing will be the focus of this Review.
Astrocytes, developmentally derived from the neuroectoderm, are the most abundant glial cell type in the CNS. In addition to their neuron-supportive functions, astrocytes also directly alter neuronal communication because they completely encapsulate synapses and are in close contact with neuronal somas20. The close astrocyte–neuron contact allows for astrocyte activation by neurotransmission as astrocytes express various functional neurotransmitter receptors25. These include ionotropic non-NMDA and NMDA receptors as well as metabotropic glutamate (mGluR3 and mGluR5), purinergic and substance P receptors. On astrocyte activation, the extracellular signal-regulated kinase (ERK; also known as mitogen-activated protein kinase 1 (MAPK1)) and c-Jun N-terminal kinase (JNK; also known as MAPK8) signalling pathways (among others) are activated (for a review see REF. 26). These lead to an increase in the synthesis of inflammatory factors (for example, interleukin 1β (IL-1 β), IL-6, tumour-necrosis factor-α (TNF α), prostaglandin E2 (PGE2) and nitric oxide (NO)), which ultimately alter glial glutamate transporter function27,28 and gap-junction proteins, which are known to facilitate astrocyte–astrocyte activation through Ca2+ cascades27. Although similar pathways are activated in microglia after nerve injury29, the temporal patterns of enzyme activation and pro-inflammatory cytokine release are distinct for microglia and astrocytes. For example, distinct releases of matrix metalloproteinases (MMPs), which facilitate pro-inflammatory IL-1β activity, contribute to the development and maintenance of pathological pain after nerve injury. Furthermore, chronic astrocyte activation after nerve injury has been shown to involve ERK activation and subsequent down-regulation of excitatory amino acid transporters (glutamate transporter 1 (GLT1; also known as SLC1A2) and glutamate–aspartate transporter (GLAST; also known as SLC1A3))30; discussed in more detail below), leading to a decrease in glutamate uptake and an increase in excitatory synaptic transmission31. During chronic neuropathic conditions, astrocytes remain activated in response to the initial microglia-derived inflammatory factors31,32; this is likely to account for their ongoing responses during these conditions.
Resident microglia, which are classically known as the macrophages of the CNS, are bone marrow-derived haematopoietic cells that invade the CNS during embryonic development and are never replenished. Conversely, perivascular microglia are continuously replenished in adulthood by bone marrow-derived haematopoietic precursors33, and this replenishment increases during CNS inflammation34 and after peripheral nerve damage35. The exact role of each subpopulation of microglia in the healthy and the injured CNS remains unknown, but perivascular microglia have been shown to alter the blood–brain barrier and exert an anti-inflammatory effect in response to inflammatory conditions. Resident microglia are known to survey the CNS and to rapidly proliferate on activation, and exert both inflammatory and anti-inflammatory effects36. Generally, microglia act as a first line of defence against pathogen invasion, by recognizing, sequestering and processing antigens. However, microglia (both the resident and the perivascular subtypes) express many of the neurotransmitter receptors that are found in astrocytes and neurons19. Once microglia become activated, they produce and release many substances that activate nearby astrocytes, microglia and neurons.
Glia as CNS immunocompetent cells
Factors that are released as a result of tissue injury and that induce glial activation include reactive oxygen species, NO, aggregated or misfolded proteins, and nuclear factors that are often detected in apoptotic cells (for a review see REF. 37). In addition, glia, in their role as immunocompetent cells, are activated on pathogen invasion. Similar to the classical innate immune cells, including macrophages and dendritic cells, both astrocytes and microglia become activated by pathogens by recognizing specific molecular patterns on the pathogenic proteins and toxic cell debris during infection or tissue injury22,38. Both astrocytes and microglia express a wide range of pattern-recognition receptors, including toll-like receptors (TLRs)39,40 (FIG. 3a). Upon activation, TLRs initiate immune-like processes, such as the release of pro-inflammatory cytokines and phagocytosis41,42. To date, 13 TLRs have been identified40,43. Some, like TLR2 and TLR4, are predominantly expressed by microglia but can under some circumstances be expressed by astrocytes. The activation of TLR2 and TLR4 induces the release of IL-1β, TNFα and IL-6 (REFS 44–46). TLR activation in the CNS modulates glial–neuronal communication, creating an excitatory positive-feedback loop in the pain pathway. Congruent with typical macrophage and dendritic cell activity, astrocytes and microglia can not only phagocytose47,48, but also clear both pathogens and tissue debris49. These processes are known to further stimulate IL-1β, TNFα and IL-6 release from glia46, thereby maintaining glial activation (FIG. 3a).
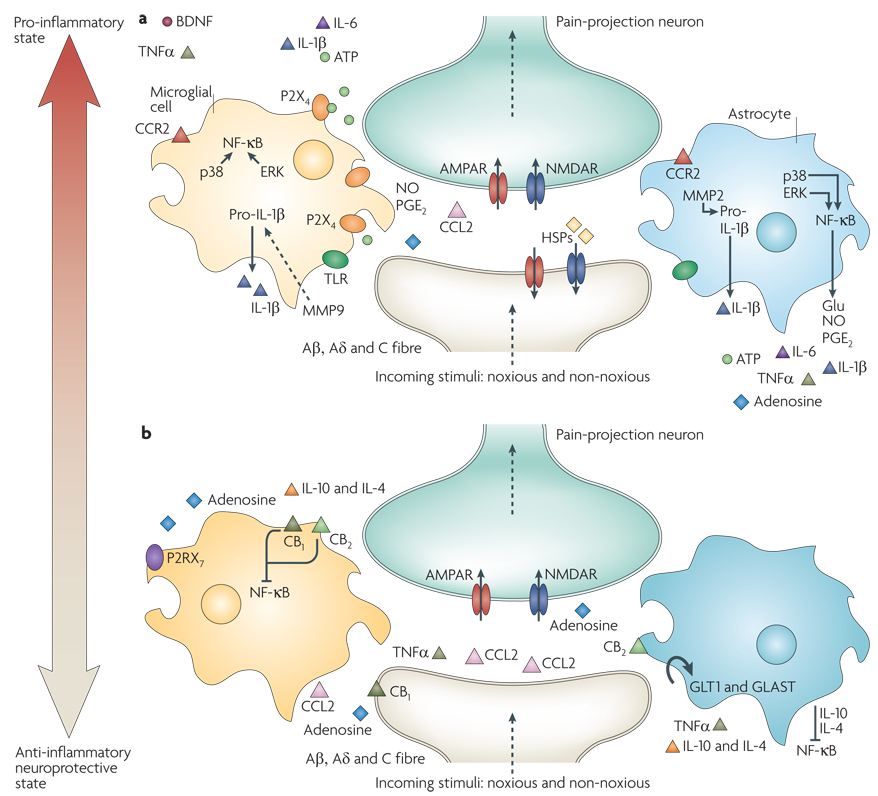
Figure 3. Glia activation: from pro-inflammatory to anti-inflammatory states.
a |Pro-inflammatory roles for glia. If a noxious input persists, such as during chronic inflammation or nerve damage, sustained central sensitization leads to transcriptional changes in dorsal horn neurons that alter these neurons’ function for prolonged periods. Astrocytes respond to this ongoing synaptic activity by mobilizing internal Ca2+, leading to the release of glutamate (Glu), ATP that binds to P2X4, tumour-necrosis factor-α (TNFα), interleukin 1β (IL-1 β), IL-6, nitric oxide (NO) and prostaglandin E2 (PGE2). Activated microglia are also a source of all of these pro-inflammatory factors. Matrix metalloproteinase 9 (MMP9) induces pro-IL-1β cleavage and microglial activation, whereas MMP2 induces pro-IL-1β cleavage and maintains astrocyte activation. The activation of p38 mitogen-activated protein kinase (p38 MAPK) is induced in both microglia and astrocytes on IL-1β signalling. Astrocytes and microglia express the chemokine receptors CX3CR1 (not shown) and CCR2 and become activated when the respective chemokines bind. After nerve damage, heat shock proteins (HSPs) are released and can bind to Toll-like receptors (TLRs) expressed on both astrocytes and microglia, leading to the further activation of these cell types. b | Activated glia can also be neuroprotective, as they release anti-inflammatory cytokines such as IL-10 and IL-4 and express cannabinoid receptors (CB1 and CB2) that have been shown to exert anti-inflammatory functions and to inhibit microglial toxicity by suppressing chemotactic responses and MAPK signal transduction, with consequent pro-inflammatory cytokine inhibition134,135. The glutamate transporters, glutamate transporter 1 (GLT1) and glutamate– aspartate transporter (GLAST), can resume normal glutamate clearance. Activation of microglial P2RX7 purinoreceptors by ATP leads to TNFα release that protects neurons from glutamate-induced toxicity. Activated astrocytes reduce the spread of tissue degeneration after direct injury through the controlled removal of dying neurons and tissue debris, another neuroprotective effect. In quiescent cells (not shown), nuclear factor-κB (NF-κκB) is sequestered in the cytosol by inhibitor of κb (IκB), which binds to specific regions on NF-κB and thereby prevents exposure of the nuclear-localization signal. On stimulation with pro-inflammatory cytokines, IκB proteins are phosphorylated, leading to their ubiquitin-dependent degradation. As a consequence, NF-κB translocates to the nucleus and binds to elements in the promoters of target genes, leading to activation of pro-inflammatory cytokine genes26
Glia in neuropathic pain
Neuropathic pain is typically associated with either peripheral nerve or CNS damage caused by compression, transection, inflammation and/or altered metabolism11. Once central sensitization begins, pain signalling is no longer the adaptive, protective mechanism that normal pain is, and no longer serves for recuperation and wound healing.
Glia activation
Several reports now demonstrate that TLRs have crucial roles in chronic neuropathic pain after peripheral nerve injury. Studies show that TLRs can be activated not only by well-known ‘non-self ’ molecular signals but also by endogenous signals (IL-1β, TNFα, IL-6 and NO) produced during chronic neuropathic pain states. TLR2 activation in astrocytes and microglia and TLR3 activation in microglia mediate neuropathic pain processing50–52. TLR4 is also key for microglial activation and the development of pathological pain after peripheral nerve trauma53. Fibronectin, an endogenous TLR4 ligand that is produced in response to tissue injury, leads to an upregulation of the purinoceptor P2X4, which is expressed exclusively on microglia54, and TLR4 antagonism in the spinal cord has been reported to reverse neuropathic pain resulting from sciatic nerve damage55,56. Opioids have recently been shown to activate glia through a receptor that is distinct from the classical opioid receptor on neurons55. This opioid-induced activation of glia induces them to release neuroexcitatory pro-inflammatory cytokines, suppressing or counter-regulating opioid analgesia. Repeated exposure to opioids leads to enhanced pro-inflammatory cytokine release from glia57. Blocking such opioid-induced glial activation enhances acute opioid analgesia and suppresses the development of opioid tolerance55,57. Furthermore, suppressing glial activation suppresses the development of opioid dependence and withdrawal, suppresses opioid reward linked to drug abuse and suppresses other negative side effects, such as respiratory depression56,58,59. Thus, preventing glial activation in response to opioids represents a promising way to enhance the clinical efficacy of opioid drugs.
The glial receptor that recognizes opioids is TLR4 (REFS 55,56), the very same receptor that is activated under conditions of neuropathic pain. These studies show, using endotoxin-free reagents to avoid contamination by the classical TLR4 agonist lipopolysaccharide (LPS), that morphine activates TLR4 signalling in human embryonic kidney cells stably transfected to express TLR4 and its associated signalling components (HEK-TLR4 cells). This morphine-induced TLR4 receptor signalling was blocked by TLR4 competitive antagonists (forms of LPS that bind to TLR4 but fail to activate its signalling), which is suggestive of opioid interactions at or near the TLR4 receptor complex55. Intriguingly, endotoxin-free opioid agonists non-stereoselectively activate TLR4, and endotoxin-free naloxone (an opioid antagonist) non-stereoselectively inhibits TLR4 signalling by either LPS or opioids55,56. Indeed, intrathecally injected naloxone, mutant LPS and LPS-R/S (two forms of LPS that are competitive TLR4 antagonists) each potentiate spinal morphine analgesia and suppress neuropathic pain, supporting a role for TLR4 in activating spinal cord glia and neuropathic pain.
In addition, heat shock proteins (HSPs) have been implicated in glia activation and neuropathic pain60. In vitro studies revealed that HSPs can activate TLRs in glia61,62 (FIG. 3a). HSPs are constitutively expressed intracellular chaperones that bind to proteins and facilitate their folding into their native and active conformations. In response to cellular stress and inflammation, HSPs are upregulated and protect cells from protein misfolding and aggregation63. When cells are damaged, dying or dead, HSPs are released and activate nearby glia directly, by binding to their TLRs or by promoting the release of glial activators from stressed glia or neurons63. HSP60, HSP70 and HSP96 can induce TNFα release from activated glia and innate immune cells through TLR2 and TLR4 signalling63, and a striking upregulation of HSP27 has been reported in damaged sensory fibres in the spinal cord dorsal horn and surrounding white matter months after peripheral nerve injury60. Although HSP27 has not yet been shown to bind directly to TLRs, these findings support the suggestion that HSPs trigger, through TLRs, glial activation and pro-inflammatory processes that contribute to neuropathic pain64 (FIG. 3a).
Finally, astrocytes and microglia express various functional neurotransmitter receptors and are activated by classic neurotransmitters and neuromodulators; thus, they can receive and respond to signals during synaptic transmission. Astrocytes are best known for their active, integrative role during synaptic signalling, and both microglia and astrocytes are ideally situated in close physical contact with neurons, as well as with other astrocytes and microglia, to carry out modulatory functions19,20. When neurons release ATP, glutamate and substance P, they are acting as sources of stimuli for astrocytes and microglia. In turn, astrocytes and microglia release glutamate and/or ATP, leading to further neuronal and nearby glial activation (FIG. 3a).
Neuron-to-glia signals in neuropathic pain
As described above, neuromodulators and neurotransmitters released upon peripheral nerve injuries activate astrocytes and microglia65. Under such conditions, subsequent glial responses to classic neurotransmitters and glial–neuronal interactions are altered. During neuropathic conditions, activated glia release the same neuroexcitatory signals that are released under immunogenic conditions. For instance, the initiation and maintenance of neuropathic pain in animal models involves neuro excitatory signals including IL-1β, TNFα and IL-6 (REFS 66,67). In addition, all of the neuroexcitatory or neuromodulatory signals that glia release (such as substance P, excitatory amino acids, NO and ATP) and/or for which glia express receptors are key players in neuropathic pain states15. The pioneering work of Coull and colleagues68 showed that ATP-stimulated microglia signal to pain-projection neurons in the dorsal horn of the spinal cord. It is now understood that neuron-derived ATP activates purinergic ionotropic receptors (P2X4) on microglia, which in turn leads to the release of microglial ATP and brain-derived neurotrophic factor (BDNF), causing a depolarization shift that inverts the polarity of currents activated by the inhibitory neurotransmitter GABA (γ-aminobutyric acid) in spinal lamina neurons68.
Other studies have shown that neuropathic pain signalling also requires the activation of microglial p38 MAPK (also known as MAPK14). p38 MAPK is involved in Ca2+-sensitive intracellular signalling cascades that lead to the production of pro-inflammatory cytokines. It has been speculated that activation of P2X4 increases intracellular Ca2+ and activates p38 MAPK65 (FIG. 3a). It has also been shown that TNFα and MMPs trigger p38 MAPK activation in microglia in the spinal cord dorsal horn during peripheral neuropathic pain69. MMP9-induced pro-IL-1β cleavage leads to p38 MAPK activation in microglia during the onset and early stages of neuropathic pain, whereas MMP2-induced pro- IL-1β cleavage leads to astrocyte activation during the ongoing and later stages of neuropathic pain70. These studies indicate that, rather than glial activation being limited to pathological pain produced from purely inflammatory conditions, glia can be activated by neuron-to-glia signalling initiated by neurotransmitters and neuromodulators.
A role for chemokines as neuron-to-glia signals during ongoing neuropathic pain states is also emerging. Chemokines were initially recognized as having a role in the maturation and trafficking of leukocytes during inflammatory diseases71,72. Chemokines and their receptors are expressed not only in peripheral immune cells (leukocytes) and diverse populations of glia, but also in neurons73. Several reports have shown that the chemokine CCL2 (also known as monocyte chemoattractant protein 1 (MCP1)) and its receptor, CCR2, are not normally expressed in healthy conditions but are dramatically upregulated in DRG and spinal cord neurons after peripheral nerve injury35,74,75. CCL2 and CCR2 expression are upregulated in TRPv1-expressing sensory neurons after sciatic nerve injury, and CCL2 has been shown to be secreted from neurons upon capsaicin- or K+-dependent depolarization in a Ca2+-dependent and an activity-dependent manner76,77. CCR2 activation enhanced the responses of both TRPv1- and TRPA1- expressing neurons to capsaicin treatment77. Spinal cord expression of CCR2 and downstream signalling, identified in both bone marrow-derived microglia and resident microglia, results in the infiltration of macrophages into the spinal meninges that differentiate into microglia on further parenchyma penetration35. Expression of CCL2 in the superficial lamina of the spinal cord dorsal horns is thought to trigger spinal astrocyte and microglia activation75,78. Whereas the activation of microglia surrounding CCL2-expressing spinal cord neurons peaked by day 14 after peripheral nerve injury, astrocytes surrounding CCL2-expressing spinal cord neurons remained robustly activated throughout the entire testing period of 150 days after nerve injury75. This sustained astrocyte activation may be important during the persistent phase of chronic neuropathic pain.
The chemokine fractalkine (also known as CX3CL1) has also been documented to mediate pathological pain79–83. Fractalkine binds to only one receptor, CX3CR1 (which binds only fractalkine)84, and is expressed by spinal cord neurons. CX3CR1 is expressed mostly on microglia, and exogenous fractalkine has been shown to produce IL-1β- and IL-6-mediated pain responses through the activation of microglia81,82 (FIG. 3a). Peripheral nerve injury or neuronal excitation in the spinal cord triggers MMP-induced cleavage of fractalkine and an increase in the expression of CX3CR1 in microglia in pain-relevant areas85. Activated microglia in the dorsal horn also express the lysosomal cysteine protease cathepsin S, an enzyme that may induce fractalkine cleavage during neuropathic pain states79. Fractalkine and CX3CR1 expression in astrocytes have also been found to increase during ongoing pathological pain, suggesting that CX3CR1 activation might be important for the chronic stages of neuropathic pain, as astrocyte activation is strongly implicated in ongoing pain changes. Other chemokines, such as RANTES (also known as CCL5), IP-10 (also known as CXCL10) and SDF1 (also known as CXCL12) are also implicated in neuropathic pain conditions84, supporting the suggestion that chemokines are important neuron-to-glia signals that lead to pro-inflammatory processes and pathological pain.
Activated glia produce pathological pain
One way in which pro-inflammatory cytokines released from glia lead to pathological pain is by acting on their receptors expressed on neurons in the pain-responsive regions of the spinal cord. For example, TNFα and IL-1β from astrocytes increase neuronal excitability and synaptic strength by increasing the conductivity of AMPA and NMDA receptors, as well as by increasing the number of these receptors on the surface of neurons86,87. Other neuroexcitatory substances that are released by activated glia include d-serine, which potently enhances NMDA channel function88. Intriguingly, IL-1β has been shown to increase the inward Ca2+ conductance of NMDA receptors in pain-responsive neurons in the spinal cord89. A recent report demonstrated that IL-1β induced the phosphorylation of a specific NMDA receptor subunit, leading to its activation90. Furthermore, IL-1β, TNFα and IL-6 mediate DRG and spinal cord dorsal horn central sensitization and exaggerated pain, either independently or in combination91,92. NMDA receptor channel opening leads to the influx of Ca2+ (REF. 11) and to increased production of NO and PGE2, which are involved in amplifying the excitability of pain-projection neurons93. The excitability of pain-responsive neurons is rapidly increased on exposure to these glial products, which suggests that they exert a direct effect on neurons. As discussed above, one of the initial events that occurs following peripheral nerve injury is elevation of intracellular Ca2+, leading to p38 MAPK and ERK activation in microglia. These MAPKs can also be activated by IL-1 β and TNFα, leading to the activation of various transcription factors (including nuclear factor κB (NF-κB)) and to further secretion of IL-1β, TNFα, IL-6, PGE2 and BDNF. All of these factors contribute to infiltration and phagocytosis and thereby to the pathological effects of glia in neuropathic pain states. Although the mechanism(s) by which glial pro-inflammatory products enhance neuronal excitability is still under investigation, these data support the suggestion that traditional immune signals such as IL-1β, TNFα and IL-6 mediate glial–neuronal communication resulting in pathological pain16 (BOX 2).
Box 2 Techniques to identify glia-derived pain neuromodulators
Neither astrocytes nor microglia can synaptically relay information about pain from the spinal cord to the brain, because they do not have axons. Rather, glia exert their influence on pain processing through the substances they release, which amplify neuronal excitation and pain signalling111. Several methods have been used to examine glial neuromodulators, including electrophysiological studies87,121,122, pharmacological blockade of glial activation65,67 and mRNA-based techniques coupled with immunohistochemical expression of neuropeptides, neurotransmitter receptors and pro-inflammatory cytokines in microglia and astrocytes19, 46,91,123–125. For example, in pharmacological glial blockade studies, a broad array of compounds has been used to disrupt glial function that, in turn, disrupts hyperalgesia and allodynia in animal models of neuropathic pain. The best-characterized compounds in neuropathic animal models include fluorocitrate, a glial metabolic inhibitor of the citric acid cycle126; minocycline, a tetracycline derivative that specifically inhibits microglia123,127; and propentophyline, a methylxanthine derivative30. Electrophysiological recordings of neurons treated with minocycline revealed the specific role of microglia in the early and chronic phases of neuropathic pain in rodent models121, verifying prior work using protein analysis, mRNA analysis and immunohistochemical techniques that demonstrated a strong role for activated microglia in neuropathic pain processing123. More recently, phosphorylated p38 mitogen-activated protein kinase (p38 MAPK) in the spinal cord dorsal horn was found to be robustly increased after the onset of peripheral neuropathic pain32. Pharmacological blockade of p38 MAPK activation reversed ongoing allodynia and blocked increases in the levels of dorsal spinal cord interleukin 1β (IL-1β). IL-1β, IL-6 and tumour-necrosis factor-α (which are released by glia) enhanced neuroexcitatory synaptic transmission and potentiated AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid)- and NMDA (N-methyl-d-aspartate)-induced currents in spinal slices70. These examples illustrate that the identification of glial-derived factors and their actions on the spinal cord and DRG neurons that contribute to the development and maintenance of neuropathic pain in animal models requires a combination of techniques, ranging from behavioural assessment to sensitivity to noxious and non-noxious stimuli, pharmacological manipulations, immunohistological procedures, protein and mRNA analysis and electrophysiological recordings.
Protective roles of activated glia
A growing number of studies aimed at understanding the mechanisms involved in neurodegenerative disease provide evidence that microglia and astrocytes can also be neuroprotective22,94 through some of the same factors that have been demonstrated to exert sensitizing and/or toxic effects in the CNS (FIG. 3b).
Signals produced by peripheral nerve injury or spinal cord injury that initiate astrocyte and microglia activation are likely to be different from the signals that are induced during other circumstances, such as by pathogen invasion or neurodegenerative diseases. Thus, the resulting downstream signalling cascades may also be different. However, all of these events involve the activation of the local innate immune response, which is mediated by microglia and astrocytes.
Both of these cell types can recognize ‘danger signals’, which include cellular debris produced from apoptotic or necrotic cells and signals from pathogens38, and can clear the pathogen or cellular debris by phagocytosis. The ongoing, daily processes that are involved in phagocytosis occur during healthy conditions and often go unnoticed. The therapeutic potential of manipulating these functions has led to intense research of neuroimmune regulatory proteins (for a review see REF. 37). Neuroimmune regulatory proteins include various cell-surface proteins referred to as ‘self-associated molecular patterns’, which are expressed on viable cells and are thought to function as ‘self ’ signals to prevent phagocytosis of healthy cells37, and ‘altered self molecular patterns’ that trigger an immune response37. Astrocytes and microglia express pattern-recognition receptors that recognize these surface proteins, resulting in the phagocytosis of ‘altered’ cells. This process is accompanied by a downregulation of pro-inflammatory cytokines to limit damage to nearby healthy tissue95,96. In neurodegenerative disease states, in which damaged neurons release HSPs that bind TLRs, these immune-regulatory processes often become dysfunctional. Thus, it seems that without appropriate clearance of dying cells and cellular debris by activated phagocytic glia, toxicity and cellular damage readily spread in the CNS97.
‘Self ’ versus ‘non-self ’ recognition
Recognition and phagocytosis of apoptotic cells, such as those that are observed after spinal cord injury, are thought to result in responses that are different from those that are elicited after microbial ligands or misfolded proteins are recognized97, as often occurs during neurodegenerative diseases. Astrocytes and microglia recognize pathogen-associated molecular patterns (PAMPs), resulting in the activation of intracellular signalling cascades that are distinct from those that are elicited during the clearance of apoptotic ‘self ’ cells96,98. For example, on pathogen stimulation with LPS, MAPK cascades, such as those involving ERK, p38 MAPK and jNK, become activated in glia, leading to the activation of the NF-κB family of transcription factors26. Activation of the NF-κB pathway in both astrocytes and microglia leads to the activation of a wide array of inflammatory genes (including those that encode TNFα, IL-1β and IL-6) and chemokines (such as CCL2)99, in addition to NO and PGE2. Furthermore, enzymes that cleave complement C3 are assembled and membrane attack complexes are formed. However, apoptotic cells, which are readily phagocytosed in the absence of TLR activation, induce the expression of a family of cytokine regulatory proteins, suppressors of cytokine signalling (SOCS)96. Consequently, apoptotic cells exert suppressive effects on pro-inflammatory signal transduction that result in continued tissue homeostasis. Given that pathogens lack self-associated molecular pattern surface proteins, which can act as complement inhibitors, full activation of the complement cascade can proceed, resulting in cell lysis and phagocytosis37.
The expression of self-associated molecular pattern proteins in the spinal cord after peripheral nerve injury may be crucial for the activation of astrocytes and microglia and the activation of the innate immune response. This response leads to neuropathic pain, but can also have a neuroprotective effect. Proteins that signal ‘self ’ to astrocytes and microglia in the spinal cord may dampen and control local immune events that result from signals released by damaged afferent nerve terminals. For example, chemokines (such as CX3CL1 and CCL2) are released from sensory nerve terminals in the spinal cord after peripheral nerve damage and activate both astrocytes and microglia, with consequential neuropathic pain changes. Interestingly, cell-surface CX3CL1 has been proposed to act as a ‘self’ signal to glia37. On its enzymatic cleavage by TNFα cleaving enzyme (TACE; also known as ADAM17), CX3CL1 binds to its receptor (CX3CR1), which is expressed on astrocytes and microglia. Could the cleavage of CX3CL1 signal ‘altered self ’ and trigger the clearance of excitatory signals like chemokines by glia? It is noteworthy that phagocytosis of apoptotic cells occurs with a downregulation of pro-inflammatory cytokines37,96, suggesting that phagocytosis and clearance are protective mechanisms that control inflammatory processes and promote survival. Thus, one can speculate that the fine balance between pro- and anti-inflammatory events in the spinal cord after peripheral nerve damage could be controlled by varying the expression of ‘self-associated molecular pattern’ and ‘altered self’ proteins in astrocytes and microglia.
Although astrocytes are similar to microglia in some aspects of local immune signalling, the functions of the two cell types probably differ with respect to the local immune response when peripheral nerve injuries progress from acute to more chronic stages. For example, although intracellular MAPK cascades followed by NF-κB activation are observed in both cell types, only activated microglia respond to MMP9. By contrast, prolonged astrocyte activation leads to pro-IL-1β cleavage and activation in an MMP2-dependent manner, and also causes p38 MAPK activation. In addition, GLT1 and GLAST are disrupted in astrocytes on neuronal damage, whereas microglia do not express these glutamate transporters (FIG. 2b). Thus, microglia seem to respond to injury-induced factors that are released by damaged neurons by promoting the initial production and release of pro-inflammatory cytokines and other pain-relevant substances, whereas astrocytes become activated later (and for longer), when alterations in normal glutamate transporter activity occur27, reflecting the early-onset and long-duration neuropathic pain responses.
Both astrocytes and microglia can release anti-inflammatory factors that facilitate the clearance of apoptotic cells and tissue debris and that increase the expression of self-associated proteins to dampen and halt continued pro-inflammatory actions. For example, despite the finding that exogenous TNFα application worsened NO-induced neurotoxicity in mouse brains100, studies with transgenic TNFα-knockout animals demonstrated that endogenous microglial TNFα was important for the resolution of an inflammatory response and excitotoxic cell death100. Furthermore, although the lack of TNFα decreased microglial activation early on (within 6 hours), an exaggerated microglial activation was measured 4 days later. The authors suggested that the timing of TNFα action is crucial for neuroprotection, and that the lack of TNFα produced a late, uncontrolled activation of microglia after neuroexcitotoxic events. In a separate study, the purinergic receptor P2RX7, activated by ATP on microglial cells, decreased glutamate-induced cell death in neuron–microglia co-cultures through the actions of TNFα101. Although microglial P2X4 receptor activation by ATP unequivocally mediates neuropathic pain, microglial P2RX7 may exert neuroprotective effects through the same signalling molecule, TNFα102. This study supports the possibility that the manner in which microglia are activated is paramount to the net outcome on surrounding cells.
Several microglial factors have protective roles that are dependent on the timing of the factors’ release and possibly on the severity of the CNS injury. In an in vitro neuronal model of differing levels of hypoxic injury due to glutamate and ATP, microglia that were activated with media from moderately damaged neurons and co-cultured with hypoxic neurons were found to be neuro-protective. Neither media from mildly hypoxia-damaged neurons nor media from severely hypoxia-damaged neurons resulted in microglial neuroprotection103. In a separate study of CNS damage produced by LPS in combination with a surfactant compound that leads to cell lysis, TLR4 was shown to mediate microglial neuroprotection94 and lead to increased production of anti-inflammatory cytokine mRNA levels104. Notably, in these studies both IL-6 and IL-10 were strongly upregulated following immune stimulation39. IL-10 is a well-described anti-inflammatory cytokine105 that has been shown to prevent and reverse pathological pain106, whereas IL-6 is a cytokine that has been shown to have both nociceptive91 and anti-nociceptive effects107. However, the preponderance of evidence indicates that IL-6 stimulates nociceptive transmission99.
The balance between the protective and harmful effects of activated CNS glia is suspected to involve several anti-inflammatory molecules as well as adaptive immune responses to ‘self ’, ‘altered self ’ and ‘non-self ’ cues that favour the clearance of tissue debris and allow repair to occur92. Thus, providing anti-inflammatory cytokines, such as IL-10, could be a more beneficial approach than administering antagonists of the pro-inflammatory cytokines themselves or blocking the normal function of glia by globally preventing glial activation.
Targeting glia for pain control
Therapeutic strategies to control neuropathic pain states by targeting glial function108 have attracted a lot of attention and are beginning to yield promising results36. Although the animal studies discussed above have described a broad array of compounds that inactivate or disrupt glial function, most of them are not appropriate for human application. For example, fluorocitrate, although highly effective at blocking the onset of neuropathic pain, blocks glial uptake of excitatory amino acids and has a narrow dose range, with higher doses becoming neurotoxic36. However, given that many animal models of neuropathic pain lead to the activation of microglia and astrocytes in the spinal cord, and given that IL-1β and TNFα are crucially important for the initiation and maintenance of neuropathic pain, several compounds that specifically target microglia or the production of glial IL-1 β and TNFα continue to be explored.
The compounds being examined (for a review see REF. 109) fall into three major categories: compounds that attenuate microglia and/or astrocyte activation, compounds that inhibit pro-inflammatory cytokine production, and anti-inflammatory compounds. Minocycline, a second-generation tetracycline antibiotic, selectively targets microglia and can cross the blood–brain barrier. In addition, minocycline prevents the production of pro-inflammatory cytokines and NO, and was recently shown to reduce microglial trafficking to injured neurons, supporting clinical utility36. Propentofylline, a methylxanthine derivative, suppresses astrocyte activation and is known to control pain behaviour in rodent models of enhanced pain states110. Other compounds, such as pentoxifylline and AV411 (ibudilast) are generally nonspecific cytokine and phosphodiesterase inhibitors that stimulate IL-10 production109. Both are therapeutically effective in animal models of neuropathic pain. Antagonists of IL-1β, such as anakinra (Kineret; Amgen), and of TNFα, such as etanercept (Enbrel; Amgen/Wyeth), have demonstrated beneficial effects in neuropathic animal models111. As these drugs do not cross the blood–brain barrier, chronic, indwelling intrathecal catheterization would be required for ongoing drug adminstration to the spinal cord, potentially limiting further clinical development.
Recent reports show that endogenous IL-1β, TNFα and IL-6 oppose systemic and intrathecal opioid analgesia within 5 minutes, leading to reduced pain suppression58. The same study showed that p38 MAPK and NO have critical roles in endogenous pro-inflammatory cytokine-induced abrogation of opioid analgesia. In a separate study, suppressing microglial activation with minocycline enhanced morphine-induced analgesia59. Thus, co-administration of pro-inflammatory cytokine inhibitors with morphine may not only enhance the morphine’s efficacy but also decrease morphine tolerance. Further supporting the role of glia in suppressing opioid analgesia, the opioid antagonists (+)-naloxone and (−)-naloxone were shown to antagonize TLR4 activation56. As noted above, TLR4 is a crucial glial receptor in neuropathic pain conditions36. Thus, by blocking TLR4 signalling in microglia, both (+)-naloxone and (−)-naloxone reversed fully developed neuropathic pain and suppressed neuropathic chronic constriction-induced microglial activation56. Taken together, these reports have important implications for future drug development.
Although small molecules offer promising approaches to treat neuropathic pain, recent studies using gene-transfer techniques offer a unique advantage: targeted drug delivery to discrete areas of the pain pathway. Targeted and chronic spinal cord transgene expression may be an important mechanism by which to achieve long-term neuropathic pain control. Gene therapy has been gaining momentum as a tool by which to target neurons or glia for pain control112 (BOX 3). Intrathecal delivery of IL-10 or IL-2 genes reversed peripheral nerve injury-induced thermal hyperalgesia and mechanical allodynia for up to 4 weeks112 while leaving normal pain thresholds intact. In these studies, viral vectors were administered by direct spinal or intrathecal injection into the cerebrospinal fluid for gene transfer to the CNS. Intramuscular or intrathecal gene transfer using viral vectors encoding IL-4 or IL-10, respectively, reversed neuropathic pain for 4 months112.
Box 3 Gene therapy in the CNS
Multiple methods have been developed to transfer genes to the CNS for therapeutic purposes, including the transplantation of engineered cells expressing the therapeutic gene into the CNS, referred to as ‘ex-vivo’ gene transfer; transgene injection by intramuscular or intraneural injection, referred to as ‘remote’ gene transfer; vector injection directly into the brain or spinal cord, leading to therapeutic gene expression by the recipient’s own cells, referred to as ‘direct’ gene transfer; and silencing endogenous gene expression through nucleotide-based (DNA or RNA) methods, such as using antisense oligonucleotides or RNA interference128, which involves delivering the RNA without a delivery vehicle (‘naked’, using vectors).
Methods used to optimize gene delivery include engineering elements in the plasmid vector (exogenous DNA expression cassettes), electroporation and gene-gun delivery of chemical modifiers in liposomes or synthetic polymers106,112,129,130. Viral vectors for therapeutic gene transfer include recombinant adenovirus, adeno-associated virus, herpes simplex virus and lentivirus131. Plasmid-based gene delivery is attractive because it lacks the dangers associated with some viral vectors, such as insertional mutagenesis that can result in tumours. Therapeutic gene transfer to the CNS for the treatment of chronic neuropathic pain and other pathological pain syndromes has only been examined in the past 10 years, and a preferred treatment strategy has not yet been determined. Both viral and non-viral methods have successfully been used to treat chronic neuropathic pain in rodent models112,132,133. The advantage of non-viral methods is the ease and low cost of manufacturing vectors, as well as the lack of risk of insertional mutagenesis. Direct spinal gene delivery of anti-inflammatory cytokines, such as interleukin-10 (IL-10), leads to a robust and sustained reversal of neuropathic pain in rodent models. Ongoing studies show further improvement of targeted spinal cord IL-10 gene delivery using synthetic polymers that are engineered to encapsulate the transgene and release it on intrathecal injection130. Other anti-inflammatory transgenes delivered by viral methods include ones encoding IL-4 and the soluble tumour-necrosis factor (TNF) receptor that binds TNFα, thereby terminating its action132. These approaches are highly promising, as they all produce prolonged pain control in rodent models of neuropathic pain. Results from these and other experiments suggest that anti-inflammatory cytokine gene transfer may be an effective method of treating refractory pain in humans. Future research should aim to optimize gene delivery while maximizing safety112.
Conclusions and future directions
Although little is understood about the response repertoire and receptor expression of astrocytes and microglia in the pain-modulatory regions of the spinal cord, we are beginning to appreciate that ‘glial activation’ per se is not always bad. The question of whether activated microglia in the CNS are conditioned to display a particular phenotype (for example, pro-inflammatory and/or destructive) is being examined during neuropathic and neurodegenerative conditions. Schwartz and colleagues have proposed that a well-controlled glial response induced by signals from injured neurons, T cells and other microglia determines whether glia will be neuroprotective or excitotoxic113. Furthermore, depending on the context in which they are stimulated, distinct patterns of microglial activation occur. For example, whereas LPS induces pro-inflammatory cytokine release, which kills infected cells as well as non-infected bystander cells, glia activated by growth factors or anti-inflammatory cytokines, such as IL-4, promote neuronal survival114. Thus, in some cases preventing glial activation in the CNS is not desirable as it could amplify or create pathological pain99. Research into targeting specific types of glial activation to promote anti-inflammatory processes (such as cytokine production and/or regulation) for therapeutic purposes is beginning to yield some interesting results. For example, cannabinoid receptors, both type 1 (CB1) and type 2 (CB2), are being explored as therapeutic targets for inflammatory neurodegenerative diseases and neuropathic pain36,115. Whereas CB1 receptors are expressed on neurons, CB2 receptors are expressed on microglia and astrocytes116. Activation of CB2 receptors has been shown to have beneficial effects in animal models of neuropathic pain116. However, one study identified CB2 expression mainly on neurons after chronic neuropathic conditions117. The discrepancies between studies may reflect differences in the specificities of the antibodies used for immunohisto-chemical detection. Despite these differences, a number of studies have reported that activation of the cannabinoid system leads to anti-inflammatory processing, such as increased expression of anti-inflammatory markers (for example, ED2 (also known as CD163)) (see REF. 36 for a review). These results suggest that facilitating anti-inflammatory aspects of glial activation is a more powerful approach to controlling pain signalling than simply preventing glial activation.
Further understanding of the molecular mechanisms that underlie the effects of glia on pain processing should lead to the development of new and more efficient approaches for the treatment of pain. It is important to consider that both astrocytes and microglia are necessary for the homeostasis of the environment surrounding neurons, and also for the regulated clearance of apoptotic cells. The anti-inflammatory contribution from activated astrocytes and microglia is crucial during such processes. A major challenge that new drug-development strategies for the treatment of neuropathic pain face is targeting the pathological actions of pro-inflammatory cytokines and chemokines from astrocytes and microglia in the spinal cord while not altering their protective and recuperative roles.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=geneCCL2 | CCR2 | CX3CL1 | CX3CR1 | ERK | GLAST | GLT1 | IL-1β | IL-6 | JNK | mGluR3 | mGluR5 | MMP2 | MMP9 | neurokinin 1 receptor | P2X4 | P2RX7 | p38 MAPK | substance P | TLR2 | TLR3 | TLR4 | TNFα | TRPA1 | TRPV1
Acknowledgements
This work was supported by NIH grants: DA018156, DA015642 and DA015656.
Abbreviations
- Acute pain
A sensation that results from a transient, high-intensity stimulation that exceeds a threshold for the activation of specialized nerve endings located on nociceptive sensory fibres; it often leads to tissue damage.
- Pathological pain
Pain that is associated with inflammation and/or trauma of peripheral tissue or nerves after injury.
- Neuropathic pain
Pathological pain that is often persistent and is produced by damage to peripheral nerves or by lesions of the CNS.
- Cytokine
A chemical signal released by immune cells that significantly alters many cell types, including other immune cells.
- Chemokine
A class of cytokine that was originally identified as having chemotactic properties. To date, ~50 chemokines have been identified. Chemokines are classified according to the number and spacing of conserved cysteine residues in their amino termini.
- Nociceptive neuron
A sensory neuron that specifically responds to noxious stimuli and contains a bifurcating axon, with one branch projecting to the periphery and the other to the CNS.
- Dorsal root ganglia
(DRG). Clusters of primary sensory neurons of the peripheral somatosensory system. These ganglia are adjacent to the spinal cord and send axons to the dorsal horn of the spinal cord.
- Spinal cord laminae
Laminae (also known as layers) of the spinal cord that are organized by the type of information that they receive from the body. Laminae I and II, located in the superficial portion of the dorsal spinal cord, receive information from peripheral afferent nociceptive fibres. Laminae III–VI receive information about non-noxious stimuli and proprioceptive information. Lamina V plays an important part in the transmission of noxious information to supra-spinal pain centres.
- Chronic pain
Persistent pain with an indefinite duration. It can originate from tissue and/or nerve damage and becomes chronic pain when it is prolonged and no longer has a clearly defined underlying cause.
- Blood–brain barrier
A unique, selective barrier that makes blood vessels in the CNS highly impermeable to substances carried in the blood stream. Drugs must be able to cross the blood–brain barrier in order to achieve therapeutic levels in the CNS.
- Toll-like receptors
(TLRs). Pattern-recognition receptors expressed on cells of the innate immune system that recognize microbial particles, leading to clearance, cell death and inflammation.
- Meninges
Three membranous layers (the dura mater, arachnoid mater and pia mater) that surround the spinal cord and cerebrospinal fluid space.
References
- 1.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051–2058. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 3.Long DM, et al. Persistent back pain and sciatica in the United States: patient characteristics. J. Spinal Disord. 1996;9:40–58. [PubMed] [Google Scholar]
- 4.Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nature Rev. Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 6.Spike RC, et al. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur. J. Neurosci. 2002;15:1306–1316. doi: 10.1046/j.1460-9568.2002.01969.x. [DOI] [PubMed] [Google Scholar]
- 7.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Loeser JD. In: Pain. Cervero F, Jensen TS, editors. Amsterdam: Elsevier; 2006. pp. 11–20. [Google Scholar]
- 11.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 12.Woolf G, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 13.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 15.McMahon SB, Cafferty WBJ, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells, and glia. Nature Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992.This review describes the development of neuropathic pain as a neuroimmune disorder that involves neuronal pathways as well as glia in the dorsal root ganglia and spinal cord.
- 17.Watkins LR, Wieseler-Frank J, Milligan ED, Johnston I, Maier SF. In: Handbook of Clinical Neurology. Cervero F, Jensen TS, editors. Elsevier; 2006. pp. 309–323. [DOI] [PubMed] [Google Scholar]
- 18.Halassa MM, Fellin T, Hatdon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Haydon PG. Glia: listening and talking to the synapse. Nature Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 21.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration. Nature Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 23.Emery B, Butzkueven H, Snell C, Binder M, Kilpatrick TJ. Oligodendrocytes exhibit selective expression of suppressor of cytokine signaling genes and signal transducer and activator of transcription 1 independent inhibition of interferon-gamma-induced toxicity in response to leukemia inhibitory factor. Neuroscience. 2006;137:463–472. doi: 10.1016/j.neuroscience.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Slaets H, et al. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up-regulation of 14-3-3. Proteomics. 2008;8:1237–1247. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- 25.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog.Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 26.Benveniste EN. In: Immune and Glial Regulation of Pain. DeLeo JA, Sorkin LS, Watkins LR, editors. Seattle: IASP; 2007. pp. 43–63. [Google Scholar]
- 27.Fellin T, et al. Novartis Foundation Symposium. Chichester, New York: Wiley; 2006. pp. 208–217. [DOI] [PubMed] [Google Scholar]
- 28.Parpura V, et al. Glutamate-mediated astrocyteneuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Tawfik VL, et al. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- 31.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 34.Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero-Sandoval EA, Horvath RJ, Deleo JA. Neuroimmune interactions and pain: focus on glialmodulating targets. Curr. Opin. Investig. Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- 37.Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int. Rev.Neurobiol. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. [DOI] [PubMed] [Google Scholar]
- 38.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 39.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 40.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav. Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdallah B, et al. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum. Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 43.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 44.Allan SM, Tyrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nature Rev. Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 45.Aronica E, Gorter JA, Rozemuller AJ, Yankaya B, Troost D. Activation of metabotropic glutamate receptor 3 enhances interleukin (IL)-1β-stimulated release of IL-6 in cultured human astrocytes. Neuroscience. 2005;130:927–933. doi: 10.1016/j.neuroscience.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 47.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 48.Murphy S, editor. Astrocytes: Pharmacology and Function. San Diego: Academic; 1993. [Google Scholar]
- 49.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 51.Obata K, et al. Toll-like receptor 3 contributes to spinal glial activation and tactile allodynia after nerve injury. J. Neurochem. 2008 Apr 9; doi: 10.1111/j.1471-4159.2008.05353.x. [DOI] [PubMed] [Google Scholar]
- 52.Obata K, et al. Toll-like receptor 3 contributes to spinal glial activation and tactile allodynia after nerve injury. J. Neurochem. 2008;105:2249–2259. doi: 10.1111/j.1471-4159.2008.05353.x. [DOI] [PubMed] [Google Scholar]
- 53.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl Acad. Sci. USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 55.Hutchinson MR, et al. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence and reward. Sci.World J. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutchinson MR, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur.J. Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x.The first demonstration that TLRs bind opioids. These data support an entirely new approach to controlling pain.
- 57.Johnston IN, et al. A role for pro-inflammatory cytokines and fractalkine in analgesia, tolerance and subsequent pain facilitation induced by chronic intrathecal morphine. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutchinson MR, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 2008 Jul 1; doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutchinson MR, et al. Minocycline supresses morphine-induced respiratory depression, supresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav. Immun. 2008 Jul 31; doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costigan M, et al. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J. Neurosci. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh TK. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell. Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Lissitsyn Y, Becker AB, Kozyrskyj AL, HayGlass KT. Level of Toll-like receptor agonist exposure differentially determines chemokine production in humans. Can. J. Physiol. Pharmacol. 2007;85:739–746. doi: 10.1139/Y07-064. [DOI] [PubMed] [Google Scholar]
- 63.van Noort JM. Stress proteins in CNS inflammation. J. Pathol. 2008;214:267–275. doi: 10.1002/path.2273. [DOI] [PubMed] [Google Scholar]
- 64.Roelofs MF. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J. Immunol. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 65.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small’ glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002.This review describes the role of microglia in the development of neuropathic pain.
- 66.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature Rev. Neurosci. 2005;6:521–530. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 67.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 68.Coull JA. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:923–925. doi: 10.1038/nature04223.The first report to demonstrate a mechanism for enhanced pain signalling that is mediated by microglia.
- 69.Svensson CI, et al. Spinal p38β isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J. Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- 70.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nature Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 72.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 73.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nature Rev. Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 74.White FA, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl Acad. Sci. USA. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x.This study demonstrated that peripheral immune–CNS interactions lead to neuropathic pain through chemokine action.
- 76.Dansereau MA, et al. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J. Neurochem. 2008;106:757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- 77.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thacker MA, et al. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur. J. Pain. 2008 Jun 11; doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 79.Clark AK, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc.Natl Acad. Sci. USA. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindia JA, McGowan E, Jochnowitz N, Abbadia C. Induction of CX3CL1 expression in astocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J. Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 81.Milligan ED, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 82.Milligan ED, et al. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur.J. Neurosci. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhuang Z-Y, et al. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav. Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc. Natl Acad. Sci. USA. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104.A comprehensive review that describes the role of chemokines in pain signalling.
- 85.Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur. J. Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 86.Beattie EC, et al. Control of synaptic strength by glial TNFα. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 87.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 88.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth. Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 89.Viviani B, et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium release through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang RX, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6,and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozaktay AC, et al. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur. Spine J. 2006;15:58–66. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 93.Besson JM. The neurobiology of pain. Lancet. 1999;353:1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- 94.Glezer A, Simard RS, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. doi: 10.1016/j.neuroscience.2007.02.055.This review considers the beneficial role of activated microglia in the CNS.
- 95.Elward K, Gasque P. “Eat me” and “don’t eat me”signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol. Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 96.Tassiulas I, et al. Apoptotic cells inhibit LPS-induced cytokine and chemokine production and IFN responses in macrophages. Hum. Immunol. 2007;68:156–164. doi: 10.1016/j.humimm.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol.Neurobiol. 2006;34:221–242. doi: 10.1385/MN:34:3:221. [DOI] [PubMed] [Google Scholar]
- 98.Tibrewal N, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-κB transcriptional activation. J. Biol. Chem. 2008;283:3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 99.DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle: IASP; 2007. A book dedicated to understanding the current evidence and ideas regarding the roles that glia play in pain processing.
- 100.Blais V, Rivest S. Effects of TNF-α and IFN-γ on nitric oxide-induced neurotoxicity in the mouse brain. J. Immunol. 2004;172:7043–7052. doi: 10.4049/jimmunol.172.11.7043. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki T, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor activated microglia. J. Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol. Therapeut. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001.A comprehensive review that addresses the role of purinoceptors on microglia in neuropathic pain.
- 103.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 105.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 106.Milligan EM, et al. In: Immune and Glial Regulation of Pain. De Leo JA, Sorkin LS, Watkins LR, editors. Seattle: IASP; 2007. pp. 319–337. [Google Scholar]
- 107.Flatters SJ, Fox AJ, Dickenson AH. Nerve injury alters the effects of interleukin-6 on nociceptive transmission in peripheral afferents. Eur.J. Pharmacol. 2004;484:183–191. doi: 10.1016/j.ejphar.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nature Rev. Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 109.Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol. Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- 110.Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav. Immun. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Watkins LR, Maier SF. Targeting glia to control clinical pain: an idea whose time has come. Drug Discov. Today Ther. Strat. 2004;1:83–88. [Google Scholar]
- 112.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev. Neurother. 2007;7:487–506. doi: 10.1586/14737175.7.5.487.A comprehensive, up-to-date review describing the application of gene therapy to the CNS for chronic pain control.
- 113.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005.Reviews the many factors that act in the CNS to alter the way in which microglia are activated, with an emphasis on the varying chemical signals that significantly influence microglial phenotype.
- 114.Butovsky O. Morphological aspects of spinal cord autoimmune neuroprotection: colocalization of T cells with B7-2 (CD86) and prevention of cyst formation. FASEB J. 2001;15:1065–1067. doi: 10.1096/fj.00-0550fje. [DOI] [PubMed] [Google Scholar]
- 115.Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006;4:239–257. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884.This article reviews the in vitro and in vivo evidence that glia are regulated by cannabinoids in neuroprotection and neurodegeneration, with a particular focus on the cannabinoid CB2 receptor, which has emerged as a potential target for the treatment of neuropathic pain.
- 117.Beltramo M, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 118.Gebhart GF. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Millan MJ. Descending control of pain. Prog.Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 120.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol. Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360.A clear and thorough review that describes intracellular signalling and the role that microglia have in the development of neuropathic pain.
- 121.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;6:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma JY, Zhao ZQ. The involvement of glia in long-term plasticity in the spinal dorsal horn of the rat. Neuroreport. 2002;13:1781–1784. doi: 10.1097/00001756-200210070-00017. [DOI] [PubMed] [Google Scholar]
- 123.Ledeboer A, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 124.Tanga FY, Raghavendra V, De Leo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic markers in a rat model of neuropathic pain. Neurochem. Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 125.Wieseler-Frank J, et al. A novel immune-to-CNS communication pathway: cells of the meninges surrounding the spinal cord CSF space produce proinflammatory cytokines in response to an inflammatory stimulus. Brain Behav. Immun. 2007;21:711–718. doi: 10.1016/j.bbi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 127.Raghavendra V, Tanga FY, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 128.Federici T, Boulis NM. Ribonucleic acid interference for neurological disorders: candidate diseases, potential targets and current approaches. Neurosurgery. 2007;60:3–16. doi: 10.1227/01.NEU.0000249214.42461.A5. [DOI] [PubMed] [Google Scholar]
- 129.Ledeboer AM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav. Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Milligan ED, et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaplitt MG, During MJ. Gene Therapy in the Central Nervous System: From Bench to Bedside. San Diego, USA: Elsevier; 2006. [Google Scholar]
- 132.Mata M, Hao S, Fink DJ. Gene therapy directed at the neuroimmune component of chronic pain with particular attention to the role of TNFα. Neurosci. Lett. 2008;437:209–213. doi: 10.1016/j.neulet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Storek B, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc. Natl Acad. Sci. USA. 2007;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br. J. Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eljaschewitsch E, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]