Abstract
Design
We assessed foreskin inflammation associated with HIV and herpes simplex virus type 2 (HSV-2) in circumcised men.
Methods
Foreskin tissues were assessed in 97 HIV-infected and 135 HIV-uninfected men enrolled in randomized trials of circumcision in Rakai, Uganda. Inflammation was quantified using an ordinal score evaluating extent, intensity, and cellular composition of infiltrates in the epithelium and stroma. Prevalence rate ratios of inflammation were estimated by multivariate Poisson regression.
Results
Foreskin inflammation was primarily focal. Epithelial inflammation was present in 4.2% of men with neither HIV nor HSV-2 infection; 7.8% of men with only HSV-2; 19.0% with HIV alone (P=0.04); and 31.6% in HIV/HSV-2 coinfected men [prevalence rate ratio (PRR) 7.5, 95% confidence interval (CI) 2.3-23.8, P<0.001]. Stromal inflammation was present in 14.1% of HIV/HSV-2 uninfected men, compared with 29.7% in men with HSV-2 alone (P=0.03), 33.3% in men with HIV alone (P=0.04), and 61.0% in men with HIV/HSV-2 coinfection (PRR 4.3, 95% CI 2.3-7.9, P<0.001). In HIV-infected men, epithelial inflammation was associated with higher HIV viral load. Epithelial inflammation was more frequent among men reporting recent genital ulceration. Both epithelial and stromal inflammation were more common among men with smegma on physical examination.
Conclusion
Foreskin inflammation is increased with HIV and HSV-2 infections, higher HIV viral load and presence of smegma. Foreskin inflammation may have implications for HIV transmission and acquisition in uncircumcised men.
Keywords: circumcision, foreskin, HIV, herpes simplex virus type 2, inflammation, Uganda
Introduction
Three trials in sub-Saharan Africa demonstrated that adult male circumcision reduces male heterosexual HIV acquisition [1-3]. Circumcision also reduced symptomatic genital ulcer disease [2-4] in HIV-uninfected men and HIV-infected men, and herpes-simplex virus-type 2 (HSV-2) acquisition in HIV-negative men [5].
Epidemiologic studies suggest that HIV acquisition and transmission are facilitated by bacterial and viral sexually transmitted infections (STIs) [6-10]. HSV-2 is widely prevalent and is associated with HIV acquisition [7,11-13], particularly with recently incident HSV-2 infection [13]. It has been suggested that localized mucosal inflammation, ulcerative lesions, and recruitment of HIV-target lymphocytes to the genital tract affect HIV susceptibility and infectivity [14,15].
Epidemiologic data and foreskin specimens collected from circumcision trials in Rakai, Uganda provided an opportunity to assess whether HIV infection, HIV viral load, and HSV-2 infection are associated with foreskin inflammation.
Materials and methods
The Rakai circumcision trials have been described previously [3,4]. Briefly, uncircumcised men aged 15-49 were enrolled in two trials of male circumcision for HIV and STI prevention in Rakai District, Uganda. One trial evaluated the effect of circumcision on the risk of HIV acquisition among initially HIV-uninfected men [3]. The second trial evaluated the effect of circumcision of HIV-infected men on transmission of HIV to their initially uninfected female partners [4]. Although the two study protocols were separated by funding sources, they were procedurally identical. The two trials were conducted concurrently with the same surgeons, consent forms, questionnaires, and follow-up teams who were blinded to the HIV status of participants. Uncircumcised HIV-infected men were eligible if they had a CD4 cell count at least 350 cells/μl and no AIDS-defining illness. Men were randomized to either the intervention arm that received immediate circumcision or the control arm that received circumcision delayed for 24 months [3,4]. Information on sexual risk behaviors, hygiene practices, symptoms and signs of genitourinary infections, and physical examinations were collected at enrollment and follow-up visits. This study examined foreskin tissues obtained after circumcision from both intervention arm and control arm men.
The trials were approved by four institutional review boards: the Science and Ethics Committee of the Uganda Virus Research Institute (Entebbe, Uganda), the HIV subcommittee of the National Council for Science and Technology (Kampala, Uganda), the Committee for Human Research at Johns Hopkins University Bloomberg School of Public Health (Baltimore, Maryland, USA) and the Western Institutional Review Board (Olympia, Washington, USA). The trials were overseen by two independent Data Safety Monitoring Boards [3,4] and were registered with Clinical. Trials. Gov numbers NCT00425984 and NCT00124878. All participants consented to use of left-over foreskin tissues for research.
Histopathology
Following surgery, the foreskins were immediately immersed and fixed in either SafeFix II (a formalin-free, glyoxal based preservative (Fisher Scientific, Fairlawn, New Jersey, USA) or 100% ethanol and stored at -80°C. We obtained systematic samples of foreskins by unrolling the tissue and exposing the epithelial surface. Tissue thawing was not necessary, as ethanol remains a liquid at -80°C. Using a razor blade, cross-sections from the epithelial to subepithelial connective tissue spaced roughly equally across the tissue surface were cut into approximately 1-mm-thick sections. A minimum of three sections per foreskin were taken. Sections were embedded in paraffin blocks on the cut edge. Five-μm-thick paraffin sections were cut using a microtome, mounted and stained with hematoxylin & eosin (H&E) for differentiation of the epithelial and stromal microanatomy and assessment of inflammatory infiltrates. Inflammatory infiltrates were defined as accumulations of lymphocytes (identified by their mononuclear morphology), neutrophils (identified by their multilobulated nuclei), or ‘other’ (plasma cells, eosinophils) by methods previously described [16]. Tissue was examined at 20× magnification initially and cellular detail was assessed at 400×.
Following methods previously described [17,18], we developed an ordinal scoring system to assess the presence and distribution (extent), intensity and cellular composition of inflammation in the epithelium and stroma. Scores were assigned for epithelial and stromal compartments separately based upon the extent of inflammation scored as no or minimal cells=0; at least one cluster of cells=1 (focal); moderately diffuse distribution=2; highly diffuse=3; intensity as rare cells=0; cells present=1; moderately dense cells=2; prominent (highly dense cells)=3; and cellular composition [mononuclear=1; polymorphonuclear=2; mixed mononuclear and polymorphonuclear=3; ‘other’ (plasma cells, eosinophils)=4]. Stromal inflammation was defined as the presence of inflammatory infiltrates in the subepithelial tissue from the deepest connective tissue to the basement membrane of the surface epithelium. Epithelial inflammation was defined as the presence of inflammatory infiltrates in the visualized tissue from the basement membrane to the border of the keratinized epithelium. Inflammation severity was assessed by two experienced pathologists (M.E.S. and M.A.D.) who were masked to HIV, HSV-2 status and all other subject data.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using standardized techniques on automated staining machines [BondmaX-Leica; Mount Waverly, Victoria, Australia] for qualitative lymphocyte phenotyping, as previously described [19]. Tissue was deparaffinized using xylene and graded alcohols. Epitopes were retrieved with heated buffer ethylenediaminetetraacetic acid. After incubation with primary antibody using an optimized protocol, tissue was incubated with horseradish-peroxidase enzyme-labeled, commercially available secondary antibodies (CD4; Novacastra, Newcastle upon Tyne, UK; CD8; Ventana, Oro Valley, Arizona, USA) at temperature and an antibody dilution optimized on control tissues. Tissue was counterstained with hematoxylin. The antibody/horseradish-peroxidase complex was visualized as a bright red precipitate using nuclear fast red (Kernechtrot) solution. CD4 and CD8 cells were identified via light microscopy.
HIV and herpes simplex virus type 2 detection
HIV status at enrollment was determined using two separate enzyme-linked immunosorbent assays (Vironostika HIV-1, Organon Teknika, Charlotte, North Carolina, USA and Welcozyme HIV 1+2, Murex Diagnostics, Dartford, UK). If results were discordant or the participant was an HIV seroconverter, tests were confirmed by western blot as previously described (Cambridge Biotech HIV-1 western blot; Caltype Biomedical Corp, Rockville, Maryland, USA) [3]. HIV viral load was quantified by reverse-transcriptase (RT) PCR, Amplicor v1.5 assay (Amplicor HIV-1 Monitor version 1.5; Roche Molecular Systems, Branchburg, New Jersey, USA). HSV-2 serostatus was determined using an IgG glycoprotein EIA assay (Kalon Biological, Aldershot, UK). This assay has been previously validated for use in the Rakai population [20]. HSV-2 seropositive status was defined as a Kalon index value of at least 1.5. HSV-2 seroincident infections were defined as positive Kalon test confirmed by western blot (Euroimmune, Lubeck, Germany) among previously HSV-2 seronegative men (enrollment index value <0.9.)
Statistical analysis
We compared the proportion of specimens with any inflammatory changes (focal or diffuse) in the epithelial and stromal tissue by HIV and HSV-2 serostatus and sociodemographic and behavioral variables. Differences in the prevalence of inflammatory changes by HIV and HSV-2 status were assessed by χ2 and Fisher exact tests. We estimated the prevalence rate ratio (PRR) and 95% confidence intervals (95% CI) of inflammation associated with HIV/HSV-2 serostatus and other covariates using modified Poisson regression. Covariates with a univariate P value of 0.1 or less were included in the multivariate analysis. We assessed the prevalence of inflammation among men with HIV viral loads below and above the median using χ2 and Fisher exact tests.
Results
Tissues from 97 HIV-infected and 135 HIV-uninfected individuals were evaluated for inflammation. The median age was 30 years, and 64.2% (149/232) were married. Nearly one-third of participants (34.9%, 81/232) reported four or more sexual partners in the past 5 years (Table 1). One-third (32.8%, 76/232) were coinfected with HIVand HSV-2, 27.6% (64/232) were infected with HSV-2 alone, and a minority (9.1%, 21/232) with HIV alone. Overall, 53.5% of men (123/230) were HSV-2-seropositive at study enrollment. At 24-month follow-up, there were 15 incident HSV-2 infections among the 69 initially HSV-2-negative participants, increasing the overall proportion of HSV-2 infections among all men studied to 60.0% (140/232). In HIV-infected men, 76 of 97 (78.3%) were HSV-2 seropositive, compared with 47 of 133 (35.3%) of HIV-uninfected men (P=<0.001).
Table 1. Participant characteristics, prevalence of inflammation and univariate prevalence rate ratios for epithelial and stromal inflammation.
| All (n = 232) | Percentage of total | Epithelial inflammation, n (%) | Stromal inflammation, n (%) | Univariatea epithelial inflammation PRR (95% CI) | P | Univariate stromal inflammation PRR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| 15-24 | 64 | 27.6 | 6 (9.4%) | 18 (28.1%) | Referent | Referent | ||
| 25-34 | 99 | 42.7 | 18 (18.2%) | 36 (36.4%) | 1.9 (0.8-4.6) | 0.1 | 1.3 (0.8-2.0) | 0.3 |
| 35-50 | 69 | 29.7 | 12 (17.4%) | 28 (40.6%) | 1.9 (0.7-4.7) | 0.1 | 1.4 (0.9-2.3) | 0.1 |
| HIV and HSV-2 status | ||||||||
| No HIV or HSV infection | 71 | 30.6 | 3 (4.2%) | 10 (14.1%) | 1.0 | 1.0 | ||
| HSV-2 infection alone | 64 | 27.6 | 5 (7.8%) | 19 (29.7%) | 1.8 (0.5-7.5) | 0.4 | 2.1 (1.1-4.2) | 0.03 |
| H1V infection alone | 21 | 9.1 | 4 (19.0%) | 7 (33.3%) | 4.5 (1.1-18.6) | 0.04 | 2.4 (1.0-5.5) | 0.04 |
| HIV/HSV-2 coinfection | 76 | 32.8 | 24 (31.6%) | 46 (60.5%) | 7.5 (2.3-23.8) | <0.001 | 4.3 (2.3-7.9) | <0.001 |
| Currently married | 149 | 64.2 | 23 (15.4%) | 53 (35.6%) | 1.0 (0.5-1.8) | 1.0 | 1.0 (0.7-1.5) | 0.9 |
| Highest education levelb | ||||||||
| Primary | 171 | 79.2 | 31 (18.1%) | 67 (39.2%) | 1.0 | - | 1.0 | - |
| Secondary or postsecondary | 45 | 20.8 | 4 (8.9%) | 9 (20.0%) | 0.5 (0.2-1.4) | 0.2 | 0.5 (0.3-0.9) | 0.03 |
| Primary occupationb | ||||||||
| Agriculture | 131 | 56 | 18 (13.7%) | 42 (32.1%) | 1.0 | - | 1.0 | - |
| Government | 5 | 2.1 | 2 (40.0%) | 2 (40.0%) | 2.9 (0.9-9.2) | 0.07 | 1.2 (0.4-3.8) | 0.7 |
| Fishing | 7 | 3.0 | 3 (42.9%) | 3 (42.9%) | 3.1 (1.2-8.1) | 0.02 | 1.3 (0.5-3.3) | 0.5 |
| Student | 17 | 7.3 | 0 | 2 (11.8%) | N/Ac | - | 0.4 (0.1-1.4) | 0.1 |
| Shopkeeper or trading | 26 | 11.2 | 6 (23.1%) | 14 (53.8%) | 1.7 (0.7-3.8) | 0.2 | 1.7 (1.1-2.6) | 0.02 |
| Number of sexual partners in the past 5 yearsd | ||||||||
| 0-1 | 51 | 22.0 | 4 (7.8%) | 12 (23.5%) | 1.0 | - | 1.0 | - |
| 2 | 54 | 23.3 | 8 (14.8%) | 15 (27.8%) | 1.9 (0.6-5.9) | 0.3 | 1.2 (0.6-2.3) | 0.6 |
| 3 | 46 | 19.8 | 6 (13.0%) | 15 (32.6%) | 1.7 (0.5-5.5) | 0.4 | 1.4 (0.7-2.6) | 0.3 |
| 4+ | 81 | 34.9 | 18 (22.2%) | 40 (49.4%) | 2.8 (1.0-7.9) | 0.05 | 2.0 (1.0-3.7) | 0.04 |
| Transactional sexual intercourse n = 213d | ||||||||
| Yes | 3 | 1.4 | 1 (33.3%) | 1 (33.3%) | 2.1 (0.4-10.5) | 0.4 | 0.9 (0.2-4.5) | 0.9 |
| Used condoms past year n = 208d | ||||||||
| Yes | 116 | 55.8 | 18 (15.5%) | 45 (38.8%) | 0.90 (0.5-1.7) | 0.7 | 1.15 (0.8-1.7) | 0.5 |
| Using condom currently n = 153d | ||||||||
| Yes | 59 | 38.6 | 8 (13.6%) | 20 (33.9%) | 0.8 (0.3-1.6) | 0.5 | 0.8 (0.5-1.3) | 0.4 |
| Self-reported symptoms of STIse n = 231d | ||||||||
| Genital ulceration | 4 | 1.7 | 3 (75.0%) | 3 (75.0%) | 5.2 (2.7-9.9) | <0.001 | 2.2 (1.2-4.0) | 0.01 |
| Urethral discharge | 0 | 0 | 0 | 0 | N/Ac | - | N/Ac | - |
| Dysuria | 1 | 0.4 | 0 | 0 | N/Ac | - | N/Ac | - |
| Examination findings of STDsf | ||||||||
| Genital ulceration | 1 | 0.4 | 0 | 1 (100%) | N/Ac | - | N/Ac | - |
| Urethral discharge | 0 | 0 | 0 | 0 | N/Ac | - | N/Ac | - |
| Smegma | 6 | 2.6 | 3 (50.0%) | 4 (66.7%) | 3.4 (1.4-8.1) | 0.005 | 1.9 (1.1-3.5) | 0.03 |
| Genital hygiene n = 150d | ||||||||
| Washes before sex | 1 | 0.7 | 0 | 0 | N/Ac | - | N/Ac | - |
| Washes after sex | 139 | 92.7 | 15 (10.8%) | 43 (30.9%) | 1.2 (0.2-8.2) | 0.9 | 1.7 (0.5-6.1) | 0.4 |
CI, confidence interval; HSV, herpes simplex virus; PRR, prevalence rate ratio; STD, sexually transmitted disease; STI, sexually transmitted infection.
Univariate log-binomial regression
Report at enrollment
No inflammation present
Denominators reflect only those individuals for whom observations were recorded
During the week prior to circumcision
Preoperative examination.
Table 1 shows the prevalence and univariate PRR estimates for epithelial and stromal inflammation. The frequency of epithelial inflammation was 4.2% in men with neither HIV nor HSV-2 infection, 7.8% in men with HSV-2 alone (P=0.4), 19.0% in HIV-infected men without HSV-2 (PRR 4.5, 95% CI 1.1-18.6, P=0.04), and 31.6% in dually infected HIV/HSV-2 seropositive=men (PRR=7.5, 95% CI 2.3-23.8, P<0.001). In the stromal compartment, the prevalence of inflammation in HIV/HSV-2 seronegative men was 14.1%; in men with HSV-2 alone, the prevalence was 29.7% (PRR 2.1, 95% CI 1.1-4.2, P=0.03); and in men with HIV infection alone the prevalence was 33.3% (PRR=2.4, 95% CI 1.0-5.5, P=0.04). In dually infected men, the prevalence of stromal inflammation was 60.5% (PRR=4.3, 95% CI 2.3-7.9, P<0.001). Among 15 HSV-2 seroconverters, only one had epithelial foreskin inflammation (6.7%) and seven had stromal inflammation (46.7%), and this did not differ significantly from the prevalence of inflammation among all HSV-2 seroprevalent individuals. Epithelial inflammation was only observed when stromal inflammation was present.
There were 82 men with any inflammation (Fig. 1), and mononuclear lymphocytes were observed in 83.3% (30/36) of inflammatory sites in the epithelium and 100% (82/82) of inflammatory sites in the stroma. Neutrophils were seen in only six individuals with inflammation (7.3%, 6/82), and where seen, neutrophils were only present within the epithelium. Two of these latter individuals had a mixture of mononuclear cells and neutrophils. No other cell types were observed. Using IHC, preliminary qualitative phenotypic analysis of T-lymphocytes in inflammatory foci revealed a mixture of both CD4 and CD8 cell subsets (Fig. 2).
Fig. 1.
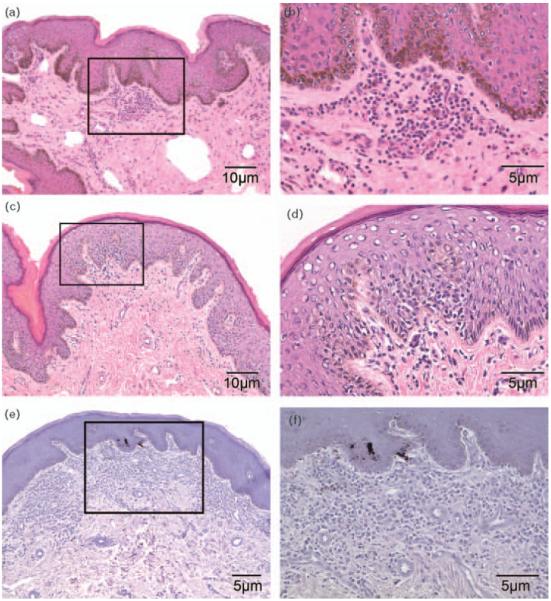
Photomicrographs of (a) hematoxylin and eosin-stained foreskin from an herpes simplex virus type-2-only infected participant demonstrating focal inflammation of foreskin subepithelial stroma (selected area), final magnification, 40×; (b) higher power view of selected area, final magnification, 100×; (c) hematoxylin and eosin-stained foreskin from an HIV/herpes simplex virus type-2 coinfected participant showing focal epithelial lymphocytic infiltrate (selected area), final magnification, 40×; (d) higher power view of selected area demonstrating a mononuclear infiltrate at and above the basal epithelium, final magnification, 100×; (e) hematoxylin and eosin-stained foreskin from an HIV/herpes simplex virus type-2 coinfected participant demonstrating diffuse inflammation of the foreskin stroma (selected area), final magnification, 64×; (f) higher power view of selected area, final magnification, 100× (hematoxylin and eosin of specimens fixed in SafeFix II is limited by decreased eosin uptake, rendering stains blue-purple).
Fig. 2.
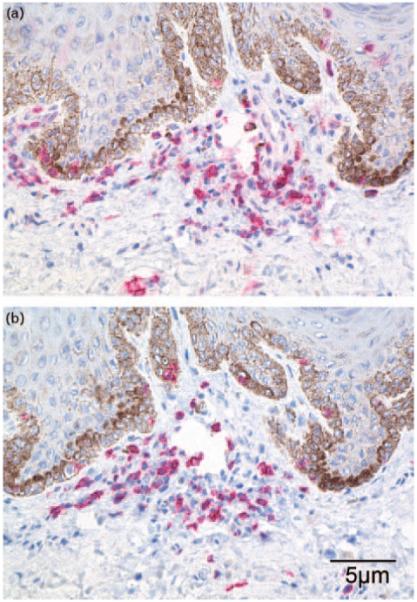
Immunophenotypic characterization of cell types expressing (a) CD4 and (b) CD8 receptors in the lymphocyte focus shown in Figure 1a and b. Positively staining cells appear red in sections counterstained with hematoxylin (blue), final magnification, 100×.
Table 2 shows the multivariate PRRs for epithelial and stromal inflammation. Dual infection with HIV and HSV-2 was strongly associated with epithelial inflammation (adjusted PRR 6.1, 95% CI 1.8-20.1, P=0.003) and stromal inflammation (adjusted PRR 3.6, 95% CI 2.0-6.9, P<0.001), relative to HIV/HSV-2 uninfected men. Among men with only HSV-2 or only HIV infection, the adjusted PRRs of inflammation were increased, but this was not statistically significant. Higher education was associated with a lower risk of stromal inflammation (adjusted PRR 0.4, 95% CI 0.2-0.9, P=0.03). Self-report of four or more sexual partners in the past 5 years was associated with more frequent inflammation, but this was not statistically significant. Epithelial, but not stromal, inflammation was associated with genital ulcer symptoms within the week prior to circumcision (adjusted PRR 2.4, 95% CI 1.1-5.3, P=0.03), although no genital ulceration was observed clinically at the preoperative examination. The presence of smegma on examination was associated with greater frequency of epithelial (adjusted PRR 5.9, 95% CI 1.4-24.4, P=0.01) and stromal inflammation (adjusted PRR 3.0, 95% CI 1.2-7.2, P=0.02). Age, primary occupation, marital status, transactional sex, condom use, and washing before or after sex were not associated with foreskin inflammation.
Table 2. Multivariate prevalence rate ratios for epithelial and stromal inflammationa.
| Epithelial inflammation adjusted PRR (95% CI) | P | Stromal inflammation adjusted PRR (95% CI) | P | |
|---|---|---|---|---|
| Age (years) | ||||
| 15-24 | 1.0 | 1.0 | ||
| 25-34 | 1.1 (0.4-2.5) | 0.9 | - | |
| 35-50 | 0.8 (0.3-2.1) | 0.7 | 0.9 (0.5-1.5) | 0.6 |
| HIV and HSV-2 status | ||||
| No HIV or HSV infection | 1.0 | 1.0 | ||
| HSV-2 infection alone | - | 1.6 (0.6-4.2) | 0.3 | |
| H1V infection alone | 1.9 (0.5-7.0) | 0.4 | 1.8 (0.9-3.6) | 0.08 |
| HIV/HSV-2 coinfection | 6.1 (1.8-20.1) | 0.003 | 3.6 (2.0-6.9) | <0.001 |
| Highest education levelb | ||||
| Primary | 1.0 | |||
| Secondary or postsecondary | - | 0.4 (0.2-0.9) | 0.03 | |
| Primary occupationb | ||||
| Agriculture | 1.0 | 1.0 | ||
| Government | 1.9 (0.5-7.1) | 0.4 | - | |
| Fishing | 2.2 (0.7-7.1) | 0.2 | - | |
| Student | - | 0.9 (0.2-4.0) | 0.9 | |
| Shopkeeper or trading | 1.3 (0.6-2.9) | 0.5 | 1.4 (0.9-2.2) | 0.1 |
| Number of sexual partners in the past 5 yearsb | ||||
| 0-1 | 1.0 | 1.0 | ||
| 2 | 1.7 (0.6-4.9) | 0.3 | 1.0 (0.6-1.9) | 0.9 |
| 3 | 1.5 (0.5-4.9) | 0.5 | 1.2 (0.6-2.2) | 0.6 |
| 4+ | 2.1 (0.8-5.4) | 0.1 | 1.6 (0.9-2.8) | 0.1 |
| Self-reported symptoms of STIsc | ||||
| Genital ulceration | 2.4 (1.1-5.3) | 0.03 | 1.3 (0.7-2.7) | 0.4 |
| Examination findings of STDsd | ||||
| Smegma | 5.9 (1.4-24.4) | 0.01 | 3.0 (1.2-7.2) | 0.02 |
CI, confidence interval; HSV, herpes simplex virus; PRR, prevalence rate ratio; STD, sexually transmitted disease; STI, sexually transmitted infection.
Multivariate Poisson regression; covariates meeting a P value cut-off of 0.1 or less on univariate analysis were included in the final model.
Report at enrollment.
During the week prior to circumcision.
Preoperative examination.
As shown in Table 3, inflammation was more commonly observed in HIV-infected than in HIV-uninfected men, both in the epithelium (28.9 vs. 5.9%, respectively, P<0.001) and the stroma (54.6 vs. 21.5%, respectively, P<0.001). The inflammation was primarily focal (Fig. 1a-d), and no HIV-uninfected men had diffuse inflammatory changes (Fig. 1e and f), whereas among HIV-infected men, 13.4% had moderately diffuse inflammation confined to the stroma. Where present, moderate or prominent lymphocytic infiltrates were observed more frequently in the stroma of HIV-infected men (22.7%, 22/97), but were uncommon in the stromal compartment of HIV-negative men (2.2%, 3/135, P=<0.001). Overall, prominent intensity was infrequent and where seen, it was only found in HIV-infected men (3.1%, 3/97).
Table 3. The prevalence, extent and intensity of inflammation in the foreskins of HIV-infected and HIV-uninfected men.
| Epithelium |
Stromaa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV+N=97 |
HIV-N=135 |
HIV+N=97 |
HIV-N=135 |
|||||||
| Extent of inflammationb | n | % | n | % | Pc | n | % | n | % | P |
| Any inflammationd | 28 | 28.9 | 8 | 5.9 | <0.001 | 53 | 54.6 | 29 | 21.5 | <0.001 |
| Focal | 28 | 28.9 | 8 | 5.9 | <0.001 | 40 | 41.2 | 29 | 21.5 | 0.001 |
| Moderately diffuse | 0 | - | 0 | - | - | 13 | 13.4 | 0 | - | N/A |
| Intensity of inflammatione | ||||||||||
| Moderate | 9 | 9.3 | 7 | 5.2 | 0.22 | 19 | 19.6 | 3 | 2.2 | <0.001 |
| Prominent | 0 | - | 0 | - | - | 3 | 3.1 | 0 | - | N/A |
Defined as the subepithelial connective tissue compartment.
Distribution of inflammatory infiltrate throughout the tissue.
As determined by Fisher exact test.
Prevalence of inflammation within the foreskin at time of circumcision by tissue compartment and HIV status.
Relative density of inflammatory infiltrate.
Immediate precircumcision HIV viral loads were available for 70% (68/97) of HIV-infected individuals. Epithelial inflammation was present in 44.1% (15/34) of men with log viral loads above the median of 4.5 log10 cps/ml, and 14.7% (5/34) in those with viral loads below the median (Fisher exact test, P=0.02).
Discussion
Foreskin inflammation was associated with both HIV and HSV-2 infections in unadjusted analyses (Table 1), and the highest prevalence of inflammation in the foreskin epithelium and stroma was observed in coinfection with HIV and HSV-2, in the adjusted analysis (Table 2). The inflammation was primarily mononuclear, suggesting local immune activation and cellular recruitment, possibly resulting from chronic viral infection [21]. The greater prevalence of foreskin inflammation in HIV-positive men with higher HIV viral loads supports this contention. In situ replication of T cells could play a role in presence or severity of inflammation. However, replication in the skin would most likely occur at sites of preexisting host response, where secreted cytokines lead to T-cell recruitment and activation [22].
Coinfection with HSV-2 and HIV was associated with the highest prevalence of inflammatory changes (Table 1), which may influence the infectivity of HIV-positive men. This is consistent with epidemiologic studies suggesting that HSV-2 enhances HIV genital shedding [23,24] and that coinfection potentiates the clinical severity and infectiousness of the two viruses [25]. These findings may provide insight into the potential mechanisms by which the foreskin may facilitate HIV transmission.
The foreskin mucosal surface is histologically similar to the cervical mucosa [19], and the latter may provide insights into foreskin inflammatory changes. HIV-infected women have depleted HIV-specific CD4 and dendritic cells [16,17,26-28], and viral coinfection (in particular, human papillomavirus) is associated with greater cervical inflammation and immune activation among HIV-infected women [29]. Dermatologic studies show similar responses in the skin of HIV-infected individuals [30,31]. We found that self-report of recent genital ulceration was associated with epithelial but not stromal foreskin inflammation, despite the absence of visible lesions at the preoperative examination. This is consistent with prior reports that subclinical inflammation can persist for many weeks after resolution of HSV-2 induced genital ulceration [32]. The association between higher education and reduced risk of stromal inflammation (Table 2) is consistent with lower HIV and HSV-2 prevalence in these individuals [33].
The presence of smegma on preoperative examination was associated with inflammation and may be a surrogate marker of poor genital hygiene. However, the role of smegma in foreskin inflammation is unclear. It may be a result of local inflammation or may contribute to the inflammation we observed. Poor hygiene (as measured by daily washing of the genitalia or washing after sex) has been associated with an increased risk of HIV and STI acquisition in some [34-36], but not all studies [37].
The major limitation of this study is that of necessity it was cross-sectional because foreskin tissues were only available at time of surgery. However, the viral infections must have preceded the observed inflammatory changes. Furthermore, our study does not include data regarding other STIs at the time of circumcision, and because infection with HIV and/or HSV-2 is associated with a higher risk for other STIs, the latter could contribute to genital inflammation. A strength of this study is the sample size and the fact that the participants did not have medical indication for circumcision. Previous studies of foreskin immunology had small sample sizes, little or no epidemiologic data or only individuals needing medically indicated circumcision due to phimosis or other genitourologic problems [19,38-41]. Moreover, these prior studies did not include HIV+ men and could not assess infection with HSV-2.
Our findings suggest that HIV and HSV-2 are associated with dramatically enhanced foreskin inflammation.
Acknowledgements
The trials were funded by the National Institutes of Health (NIH) (U1AI51171), the Bill & Melinda Gates Foundation (22006.02), and the Fogarty International Center (5D43TW001508 and D43TW00015). This study was supported in part by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, NIH. Authors include Andrew D. Redd, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland. K.E.J., M.E.S., C.S.R. designed the study. K.E.J. conducted the study, gathered the data, performed the analysis and wrote the article. M.E.S. and M.A.D. interpreted the histopathology of the foreskin tissues. R.H.G. and V.S. assisted with the statistical analysis. A.A.R.T. performed the HSV-2serologic assays.R.H.Q.,M.W.,D.S.,G.K.designed and conducted the circumcision studies. R.H.G., J.Z., A.D.R., A.A.R.T., T.C.Q. assisted in writing the article.
We thank Dr Rajni Sharma, Department of Pathology, Johns Hopkins University, for her development of IHC protocols and staining and Alexander P. Rabkin for his assistance with tissue processing. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the article. The authors wish to thank the circumcision trial study participants for their commitment and cooperation.
This work was presented in part at the joint meeting of the American Sexually Transmitted Disease Association/British Association for Sexual Health and Hygiene (ASTDA/BASHH), Brooklyn, NY, 2008 and published as an abstract in the meeting summary.
K.E.J. has received research funding support through a Bristol-Myers Squibb virology fellowship.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey R, Moses S, Parker C, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.Wawer MJ, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, et al. Trial of male circumcision in HIV+ men, Rakai, Uganda: effects in HIV+ men and in women partners; The 15th Annual Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2 April 2008. [Google Scholar]
- 5.Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: under-standing the implications of the Mwanza and Rakai trials. Lancet. 2000;355:1981–1987. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 7.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA. 1988;260:1429–1433. [PubMed] [Google Scholar]
- 9.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 10.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Nonulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Wald A, Link K. Risk of human immunodeficiency virus infec-tion in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 12.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brook-meyer RS, Paranjape RS, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 14.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 15.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Castle PE, Hillier SL, Rabe LK, Hildesheim A, Herrero R, Bratti MC, et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV) Cancer Epidemiol Biomarkers Prev. 2001;10:1021–1027. [PubMed] [Google Scholar]
- 17.Kovacic M, Katki HA, Kreimer A, Sherman ME. Epidemiologic analysis of histologic cervical inflammation: relationship to human papillomavirus infections. Human Pathol. 2008;39:1088–1095. doi: 10.1016/j.humpath.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Bhoopat L, Eiangleng L, Rugpao S, Frankel SS, Weissman D, Lekawanvijit S, et al. In vivo identification of Langerhans and related dendritic cells infected with HIV-1 subtype E in vaginal mucosa of asymptomatic patients. Mod Pathol. 2001;14:1263–1269. doi: 10.1038/modpathol.3880472. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamiel JL, Tobian AA, Laeyendecker OB, Reynolds SJ, Morrow RA, Serwadda D, et al. Improved performance of enzyme-linked immunosorbent assays and the effect of human immunodeficiency virus coinfection on the serologic detection of herpes simplex virus type 2 in Rakai, Uganda. Clin Vaccine Immunol. 2008;15:888–890. doi: 10.1128/CVI.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janeway CA, Travers P. Immunobiology: the immune system in health and disease. Garland; New York: 1997. pp. 9:13–9:20. [Google Scholar]
- 22.Luster AD. Chemokines - chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 23.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 24.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L, et al. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 26.Levi G, Feldman J, Holman S, Salarieh A, Strickler HD, Alter S, Minkoff H. Relationship between HIV viral load and Langerhans cells of the cervical epithelium. J Obstet Gynaecol Res. 2005;31:178–184. doi: 10.1111/j.1341-8076.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 27.Quayle AJ, Kourtis AP, Cu-Uvin S, Politch JA, Yang H, Bowman FP, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–298. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 28.Taube JM, Nichols AD, Bornman LS, Bornman DM, Jackson JB. Langerhans cell density and high-grade vulvar intraepithelial neoplasia in women with human immunodeficiency virus infection. J Cutan Pathol. 2007;34:565–570. doi: 10.1111/j.1600-0560.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 29.Behbahani H, Walther-Jallow L, Klareskog E, Baum L, French AL, Patterson BK, et al. Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. J Acquir Immune Defic Syndr. 2007;45:9–19. doi: 10.1097/QAI.0b013e3180415da7. [DOI] [PubMed] [Google Scholar]
- 30.Weier S, Muller H, Stutte HJ, Kappus R, Berger S, Shah PM. Lymphocytes, Langerhans cells and CD68-positive monocytes/macrophages in the skin of HIV-infected patients and normal controls. Verh Dtsch Ges Pathol. 1991;75:114–118. [PubMed] [Google Scholar]
- 31.Dreno B, Milpied B, Dutartre H, Litoux P. Epidermal interleukin 1 in normal skin of patients with HIV infection. Br J Dermatol. 1990;123:487–492. doi: 10.1111/j.1365-2133.1990.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobian AA, Charvat B, Ssempijja V, Kigozi G, Serwadda D, Makumbi F, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;199:945–949. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier AS, Bukusi EA, Cohen CR, Holmes KK. Independent association of hygiene, socioeconomic status, and circumcision with reduced risk of HIV infection among Kenyan men. J Acquir Immune Defic Syndr. 2006;43:117–118. doi: 10.1097/01.qai.0000224973.60339.35. [DOI] [PubMed] [Google Scholar]
- 35.Moore JE. The diagnosis of chancroid and the effect of prophylaxis upon its incidence in the American Expeditionary Forces. J Urol. 1920;4:169–176. [Google Scholar]
- 36.O’Farrell N. Soap and water prophylaxis for limiting genital ulcer disease and HIV-1 infection in men in sub-Saharan Africa. Genitourin Med. 1993;69:297–300. doi: 10.1136/sti.69.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makumbi F, Gray RH, Wawer M, Nakigozi FG, Serwadda D, Kigozi G, et al. Male postcoital penile cleansing and the risk of HIV-acquisition in rural Rakai district, Uganda; The 4th International AIDS Society Meeting Abstract # WEAC1LB; Sydney, Australia. 25 July 2007. [Google Scholar]
- 38.Donoval BA, Landay AL, Moses S, Agot K, Ndinya-Achola JO, Nyagaya EA, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–391. [PubMed] [Google Scholar]
- 39.Tokgoz H, Polat F, Tan MO, Erdem O, Bozkirli I. Histopathological evaluation of the preputium in preschool and primary school boys. Int Urol Nephrol. 2004;36:573–576. doi: 10.1007/s11255-004-0857-6. [DOI] [PubMed] [Google Scholar]
- 40.Feng JY, Peng ZH, Tang XP, Geng SM, Liu YP. Immunohistochemical and ultrastructural features of Langerhans cells in condyloma acuminatum. J Cutan Pathol. 2008;35:15–20. doi: 10.1111/j.1600-0560.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 41.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]


