Abstract
Bortezomib is a proteasome inhibitor that can synergize with interferon-alpha (IFN-α) to induce apoptosis in melanoma cells in vitro and inhibit tumor growth in vivo. We hypothesized that proteasome inhibition may be an effective means to sensitize melanoma cells to the direct effects of IFN-α. Pre-treatment of human melanoma cells with bortezomib led to significantly increased transcription of interferon-stimulated genes as determined by real-time PCR. Flow cytometric and immunoblot analyses indicated that the enhanced direct actions of IFN-α on melanoma cells were the result of prolonged phosphorylation of STAT1 (P-STAT1) on both the Tyrosine701 and Serine727 residues. In contrast, the enhanced IFN-α-induced P-STAT1 was not observed in peripheral blood mononuclear cells that were pre-treated with bortezomib. These data suggest that proteasome inhibition represents a mechanism to enhance the direct effects of IFN-α on melanoma cells thereby complementing its immunostimulatory properties.
Keywords: Bortezomib, Proteasome inhibition, Interferon, STAT1, Melanoma
Introduction
Malignant melanoma is the most deadly form of skin cancer, and its incidence is rising faster than that of any other cancer [1]. The prognosis for patients with metastatic disease is poor, and current chemotherapeutic and biologic therapies are only marginally effective. Melanoma tumors are genetically heterogeneous and have the ability to promote immunosuppression in a tumor-bearing host [2]. Therefore, one approach to the treatment of this malignancy may be to combine targeted anti-tumor compounds with immunostimulatory agents. Treatment with cytokines such as interferon-alpha (IFN-α) has been a mainstay of therapy for patients with malignant melanoma [3]. This cytokine remains the only FDA-approved agent for the adjuvant therapy of patients who have undergone complete excision of their tumor but are at high-risk for recurrence. Recent evidence suggests that the anti-tumor effects of IFN-α are likely a result of its immunostimulatory properties [4–6]. However, many melanoma cell lines have been shown to exhibit decreased cell proliferation and increased apoptosis in response to IFN-α, suggesting that a direct effect on the tumor could account for a portion of its activity [7–9].
The level of IFN-α-induced signal transduction in melanoma cells is highly variable, and in some cases it is remarkably low despite abundant expression of the Type I IFN receptor and other key signal transduction intermediates [10, 11]. We were therefore interested in developing strategies to enhance the direct anti-tumor actions of IFN-α on melanoma cells.
Our laboratory has recently demonstrated that the proteasome inhibitor bortezomib can synergize with IFN-α to induce apoptosis in melanoma cells. This drug combination also had anti-tumor activity in vivo [12]. These data led us to hypothesize that proteasome inhibition may be an effective means to sensitize melanoma cells to the direct effects of IFN-α. In the present report, we demonstrate that pre-treatment of melanoma cells with bortezomib leads to prolonged STAT1 phosphorylation and increased transcription of interferon-stimulated genes. Conversely, the enhanced activation of STAT1 in response to IFN-α was not observed in peripheral blood mononuclear cells (PBMCs) that were pre-treated with bortezomib. These data suggest that proteasome inhibition represents a novel way to enhance the direct effects of IFN-α on melanoma cells.
Methods
Cell lines
The human A375 melanoma cell line was purchased from American Type Cell Culture Collection (ATCC, Manassas, VA, USA). The 18105 MEL human melanoma cell line was obtained from Dr Soldano Ferrone (Roswell Park Cancer Institute, Buffalo, NY, USA).
Reagents
Recombinant human (hu) IFN-α was purchased from Schering-Plough, Inc. (Nutley, NJ, USA). Recombinant huIFN-γ was purchased from R & D Systems, Inc. (Minneapolis, MN, USA). Bortezomib (Velcade®, PS-341) was obtained from Millennium Pharmaceuticals Inc, (Cambridge, MA, USA).
Analysis of apoptosis via annexin V/propidium iodide staining
Phosphatidyl serine exposure was assessed in tumor cells by flow cytometry using APC-Annexin V and propidium iodide (PI; BD Pharmingen, San Diego, CA, USA) as previously described [13]. Each analysis was performed utilizing at least 10,000 events.
Flow cytometric analysis of STAT1 phosphorylation
Intracellular levels of Tyr701-phosphorylated STAT1 (P-STAT1) in melanoma cell lines was performed as previously described [11] using alexafluor488-labeled antibodies (BD Biosciences, Inc.). Data were expressed as the percentage of cells positive for P-STAT1 with appropriate isotype control antibodies used as negative controls.
Real-time PCR
Real-time reverse transcription-polymerase chain reaction (RT-PCR) was used to quantitate levels of mRNAs expressed by known IFN-stimulated genes. Total RNA was isolated by TRIzol and purified using the RNeasy RNA Isolation Kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed using 2 μg of total RNA and random hexamers (Invitrogen) as primers for first-strand synthesis of cDNA under the following conditions: 70°C for 2 min, 42°C for 60 min, and 94°C for 5 min. We used 2 μl of the resulting cDNA as a template to measure the levels of mRNA for ISG-15 and IFIT2 by real-time RT-PCR with pre-designed primer/probe sets (Assays On Demand; Applied Biosystems, Foster City, CA, USA) and 2× Taqman Universal PCR Master Mix (Applied Biosystems). Pre-designed primer/probe sets for human β-actin (Applied Biosystems) were used as an internal control in each reaction well. Real-time RT-PCR reactions were performed in triplicate in capped 96-well optical plates. The following amplification scheme was used: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. Real-time RT-PCR data were analyzed using Sequence Detector software, version 1.6 (PE Applied Biosystems, Foster City, CA, USA).
Immunoblot analysis
Immunoblots were prepared as previously described and probed with antibodies specific for Tyr701- and Ser727-phosphorylated STAT1 (Cell Signaling Technology), total STAT1 (BD Biosciences), or β-actin (Sigma) [11]. Following incubation with the appropriate horseradish-peroxidase-conjugated secondary Ab, immune complexes were detected using the ECL Plus detection kit (Amersham Biosciences, Ayesbury, UK).
Statistical analysis
Mixed effects regression models were used to analyze the outcomes, including a random effect for each experiment. The real-time PCR outcomes (fold change in gene expression) were log-transformed to meet the necessary model assumptions of normality and constant variance. P value adjustments were performed within each set of experiments via a Bonferroni correction if the number of prescribed comparisons was small (i.e., <6) and a Tukey–Kramer [14] adjustment otherwise. P values <0.05 were considered to be statistically significant.
Results
Bortezomib sensitizes melanoma cells to IFN-α
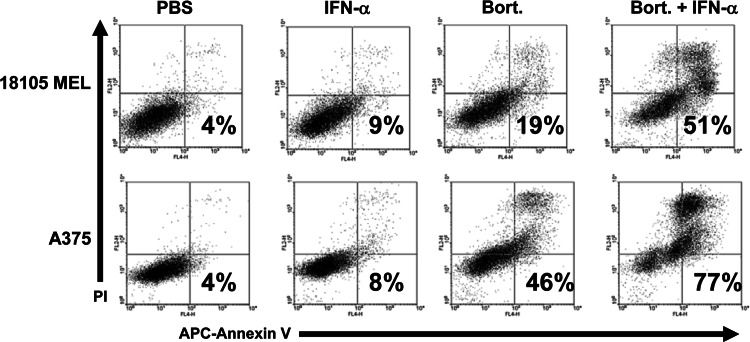
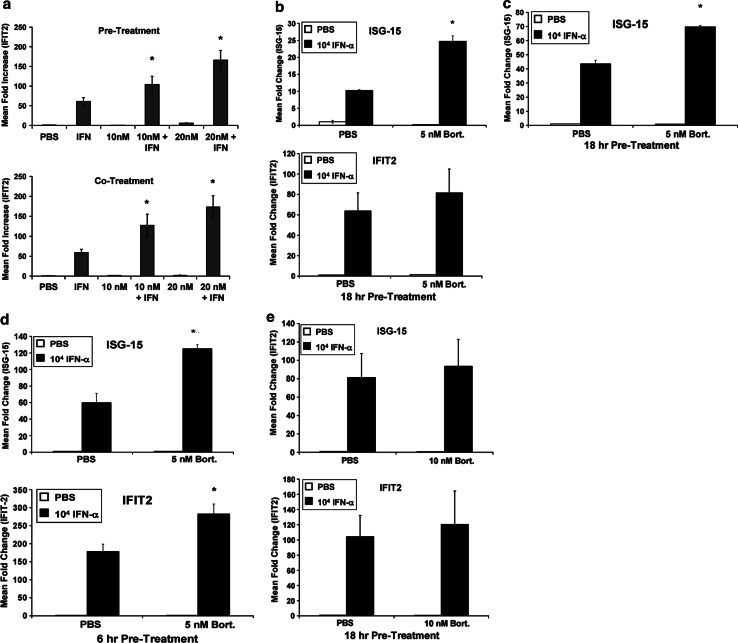
Bortezomib and IFN-α have been previously shown to induce synergistic apoptosis of melanoma cells [12]. These observations were reproducible in the present study, as a 48-h treatment of the human A375 and 18105 MEL cell lines with bortezomib (10 nM) significantly enhanced IFN-α-induced apoptosis (Fig. 1). We therefore evaluated whether pre-treatment with bortezomib could sensitize melanoma cells to the direct actions of IFN-α. Following an 18-h treatment with bortezomib (10–20 nM) or PBS (vehicle), A375 melanoma cells were stimulated with IFN-α for an additional 4 h and the transcription of IFN-α-responsive genes was measured by real-time PCR. Proteasome inhibition resulted in a statistically significant dose-dependent increase in the expression of the well-characterized IFN-α-responsive gene IFIT2 (Fig. 2a). Combined treatment of A375 melanoma cells with IFN-α and bortezomib at the 10 and 20 nM doses resulted in a 71% increase (P = 0.0161 vs. IFN-α alone) and 131% increase (P = 0.0345 vs. 10 nM combination group) in gene expression respectively, over the IFN-α alone group. Importantly, this effect was observed at a time point prior to the onset of cell death. A similar effect was also evident following an 18-h co-treatment with bortezomib and IFN-α (Fig. 2a, P < 0.005 for each combination group vs. IFN-α alone). The enhanced transcription of IFIT2 and other IFN-α-stimulated genes (ISG-15) was also achieved using lower doses of bortezomib (5 nM) in both the A375 and 18105 MEL human melanoma cell lines (Fig. 2b: ISG-15 P = 0.0178, IFIT2 P = 0.1573; Fig. 2c: ISG-15 P = 0.0006). Finally, a significant increase in IFN-α stimulated gene transcription could also be reproduced following a 6-h pre-treatment of A375 cells with bortezomib (Fig. 2d: ISG-15 P = 0.0003, IFIT2 P = 0.0004). A similar trend was observed following a 1-h pulse stimulation of A375 cells with bortezomib, although these data were not statistically significant and the enhanced responsiveness was more pronounced following longer durations of pre-treatment (Fig. 2e: ISG-15 P = 0.157, IFIT2 P = 0.472).
Fig. 1.
Bortezomib and IFN-α induce apoptosis in melanoma cells. The pro-apoptotic effects of IFN-α (104 U/ml), bortezomib (10 nM) or both agents combined were evaluated in human melanoma cell lines (18,105 MEL and A375) at 48 h post-treatment by annexin V/propidium iodide (PI) staining and flow cytometric analysis
Fig. 2.
Bortezomib enhances IFN-α-induced gene transcription in melanoma cells. a Real-time PCR was used to analyze the transcript levels of IFIT2, a known IFN-α-responsive gene in A375 cells following an 18-h pre-treatment with bortezomib, followed by a 4-h stimulation with IFN-α (top panel) or an 18-h co-treatment with bortezomib and IFN-α (bottom panel). Data are presented as the mean fold increase in IFIT2 or ISG-15 vs. PBS-treated cells. Data are normalized to β-actin (housekeeping gene). Error bars are derived from triplicate wells. b Real-time PCR analysis of ISG-15 and IFIT2 transcripts following a 18-h pre-treatment of A375 melanoma cells with lower doses of bortezomib (5 nM) and subsequent IFN-α stimulation. c Real-time PCR analysis of ISG-15 transcript following an 18-h pre-treatment of 18105 MEL melanoma cells with 5 nM bortezomib. d A 6-h pre-treatment of A375 melanoma cells with 5 nM bortezomib enhanced the transcription of IFIT2 and ISG-15 following IFN-α stimulation as measured by Real-time PCR. e Fold increase in ISG-15 and IFIT2 transcripts were measured by Real-time PCR following a 1-h pulse stimulation with bortezomib (10 nM), and subsequent 4 h treatment with IFN-α. Asterisk denotes significant difference as compared to IFN-α-treated cells
Bortezomib sensitizes melanoma cells to IFN-γ-induced gene regulation
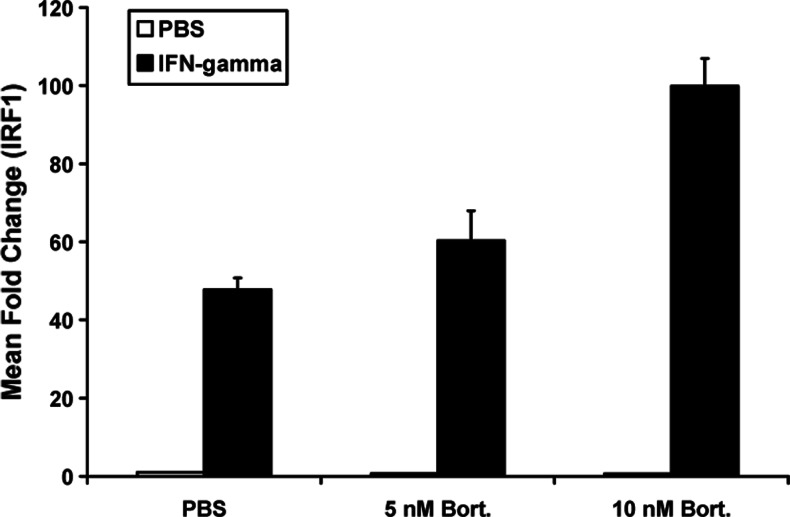
Although IFN-α represents a clinically relevant cytokine used in adjuvant therapy, melanoma cells also express functional interferon-gamma (IFN-γ) receptors. Because IFN-γ is thought to play a role in mediating apoptosis and immune recognition of tumors [15], we next examined whether bortezomib would also sensitize melanoma cells to IFN-γ-induced gene regulation. Real-time PCR analysis revealed that pre-treatment of A375 melanoma cells with bortezomib enhanced the transcription of interferon-regulatory factor-1 (IRF1), an IFN-γ-responsive gene that regulates cell cycle progression and apoptosis [16]. In contrast to data obtained with IFN-α, pre-treatment of melanoma cells with lower doses of bortezomib (5 nM) did not lead to a statistically significant increase in IRF1 expression as compared to treatment with IFN-γ alone (adjusted P = 0.1755). However, pre-treatment with bortezomib at the 10-nM dose resulted in a significant increase in IFN-γ-induced IRF1 expression as compared to either IFN-γ alone (P < 0.0001) or pre-treatment with 5 nM and subsequent IFN-γ stimulation (Fig. 3: P = 0.0024).
Fig. 3.
Bortezomib enhances IFN-γ-induced gene transcription in melanoma cells. Real-time PCR was used to analyze the transcript levels of IRF1, a known IFN-γ-responsive gene in A375 cells following an 18-h pre-treatment with bortezomib, followed by a 4-h stimulation with IFN-γ (10 ng/ml). Data are presented as the mean fold increase in IRF1 vs. PBS-treated cells. Data are normalized to β-actin (housekeeping gene). Error bars are derived from triplicate wells. Asterisk denotes significant difference as compared to IFN-γ-treated cells
Bortezomib does not upregulate the expression level of Jak-STAT signal transduction intermediates
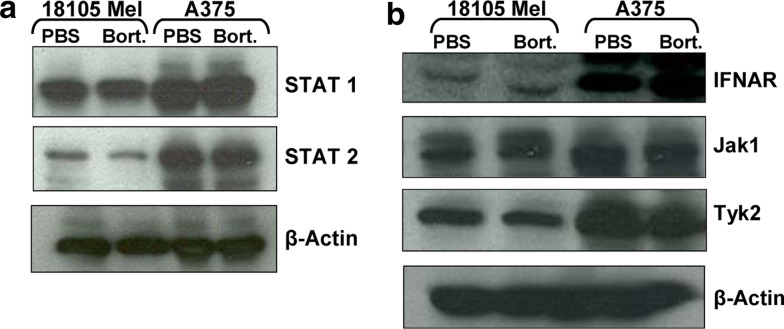
We next tested whether enhanced IFN-α stimulated gene transcription was a result of increased levels of proteins involved in Jak-STAT signal transduction. Immunoblot analysis revealed that total levels of non-phosphorylated STAT1 and STAT2 protein expression were variable between individual melanoma cell lines. However, treatment with bortezomib did not lead to any difference in the expression of these or other proteins required for IFN-α-induced Jak-STAT signal transduction including IFNAR, Jak1 or Tyk2 (Fig. 4a, b).
Fig. 4.
Effects of bortezomib pre-treatment on protein expression of Jak-STAT signal transduction intermediates. 18105 MEL and A375 human melanoma cells were pre-treated for 18 h with bortezomib or PBS (negative control). Expression of STAT1, STAT2, Jak1, Tyk2 or IFNAR1 were evaluated by immunoblot analysis. Membranes were probed with anti-β-actin antibodies as a control
Bortezomib pre-treatment leads to increased phosphorylation of STAT1
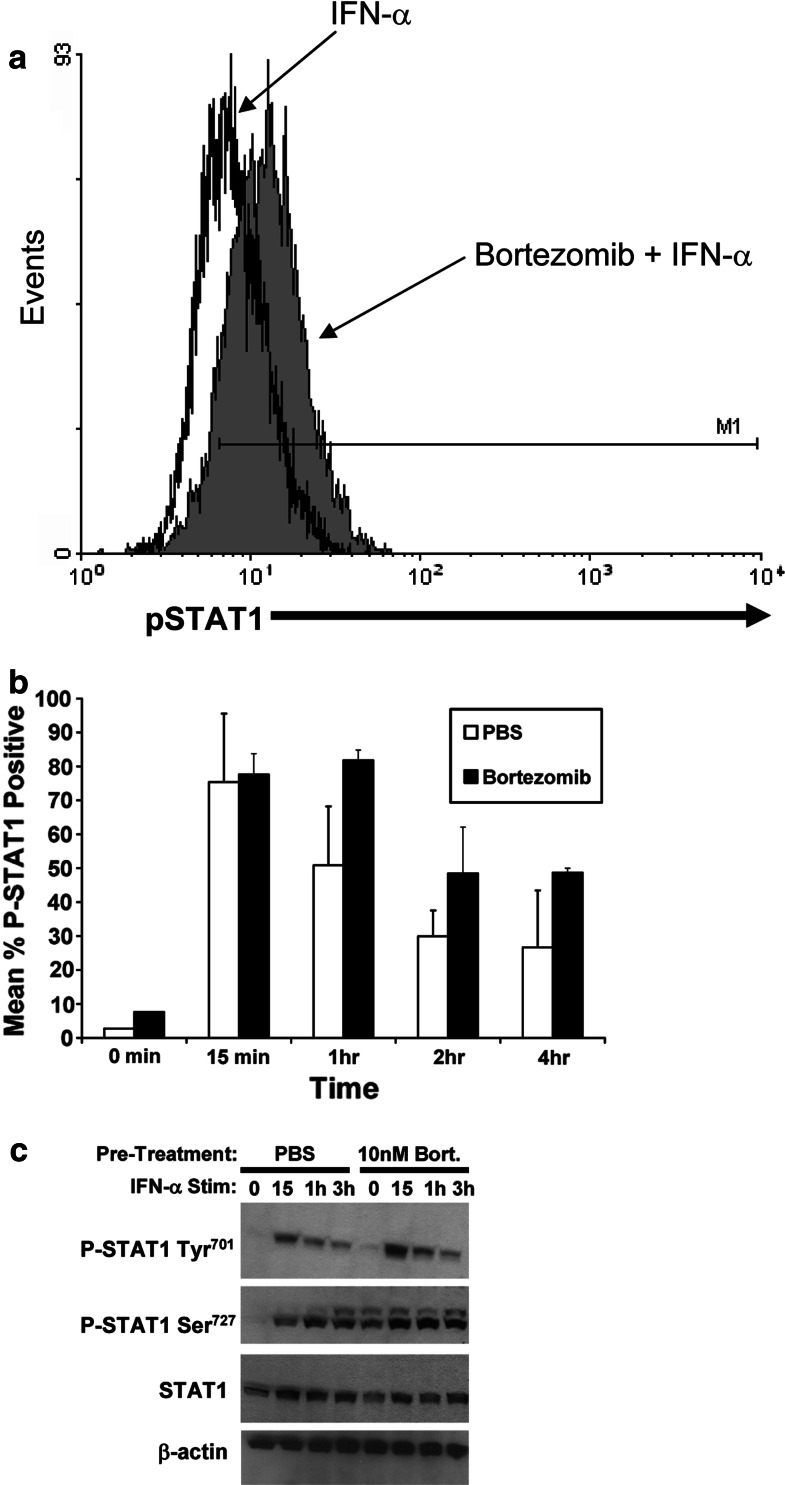
To further investigate whether bortezomib might be acting at the level of STAT1 signal transduction, we measured the phosphorylation of STAT1 (P-STAT1) at Tyr701 in response to IFN-α stimulation at various time points (15 min, 1, 2, 4 h) by flow cytometry following a 18-h pre-treatment with bortezomib (10 nM). This dose of bortezomib was previously shown to result in apoptosis of melanoma cells at a 24-h time point [12]. Results from these experiments indicated that the duration and level of IFN-α-induced P-STAT1 was enhanced as a result of bortezomib pre-treatment in both melanoma cell lines (Fig. 5a, b). These results were confirmed by immunoblot analysis which also revealed that proteasome inhibition led to increased levels of Ser727 phosphorylated STAT1 at the 3-h time point following IFN-α treatment (Fig. 5c). These data suggest that the enhanced IFN-α stimulated gene expression in melanoma cells correlates with enhanced STAT1 phosphorylation events.
Fig. 5.
Pre-treatment of melanoma cells with bortezomib leads to enhanced phosphorylation of STAT1 in response to IFN-α. A375 melanoma cells were pre-treated for 18 h with bortezomib (Bort; 10 nM) or PBS (Vehicle). Fresh media containing PBS or IFN-α (104 U/ml) was subsequently added for various time points and phosphorylation of STAT1 at Tyr701 (P-STAT1) was measured by flow cytometry. a Representative histogram of P-STAT1 is shown at the 1-h time point. b Summary of P-STAT1 staining from n = 2 experiments with similar results. Data are presented as the percentage of cells staining positive for P-STAT1. Voltage was set using an isotype control antibody and a minimum of 10,000 gated events were collected for each condition. Error bars are derived from two experiments with similar results. c Phosphorylation of STAT1 at Tyr701 and Ser727 residues were also measured by immunoblot. Total levels of unphosphorylated STAT1 protein and β-actin were measured to control for equal loading
Proteasome inhibition does not modulate IFN-α-responsiveness of PBMCs
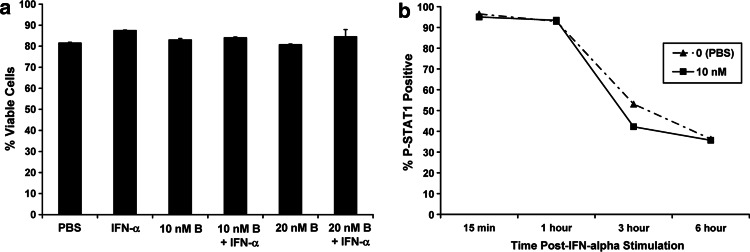
In addition to its direct effects on the tumor, IFN-α is postulated to mediate many of its anti-tumor actions via stimulation of host immune effector cells [4–6]. We therefore tested whether bortezomib could also sensitize PBMCs to the actions of IFN-α. Importantly, combined treatment of PBMCs from healthy donors with bortezomib and IFN-α did not induce cell death as measured by Annexin V/PI staining (Fig. 6a). In contrast to data obtained in melanoma cells, an 18-h pre-treatment of PBMCs did not enhance IFN-α-induced P-STAT1 (Fig. 6b).
Fig. 6.
Effects of bortezomib on IFN-α-induced signal transduction in PBMCs. a The effects of IFN-α (104 U/ml), bortezomib (10–20 nM; B = Bortezomib), or both agents combined were evaluated in PBMCs from healthy adult donors at 48 h post-treatment by annexin V/propidium iodide staining and flow cytometric analysis. Data are presented as the percentage of viable PBMCs. b Phosphorylation of STAT1 at Tyr701 (P-STAT1) was measured in PBMCs by flow cytometry following a 18-h pre-treatment with bortezomib (10 nM) and a subsequent 15 min pulse-stimulation with IFN-α. Cells were placed in cytokine-free media and harvested for staining at various time points following IFN-α stimulation. Data are presented as the percentage of cells staining positive for P-STAT1. Voltage was set using an isotype control antibody and a minimum of 10,000 gated events were collected for each condition. Similar results were obtained upon co-treatment of PBMCs with bortezomib and IFN-α (data not shown)
Discussion
Stimulation of human melanoma cells with IFNs can induce the expression of numerous genes that regulate apoptosis [17, 18]. However, IFN-α as a single agent is largely ineffective at overcoming the numerous cellular mechanisms that mediate tumor cell resistance to pro-apoptotic agents. Prior studies by our group have shown that melanoma cells exhibit a highly variable response to IFN-α stimulation. In many cell lines and patient-derived melanoma cells, the level of IFN-α-induced P-STAT1 was remarkably low [11]. Although single agent bortezomib produced disappointing results in the setting of a Phase II clinical trial of malignant melanoma, we have recently demonstrated that combined treatment with bortezomib and IFN-α induces synergistic apoptosis in a number of human tumor cell lines in a manner that was dependent upon caspase-8 activation and an interaction between Fas and FADD [12]. Importantly, proteasome inhibition enhanced the anti-tumor activity of IFN-α in two separate murine models of melanoma. In the present report, we further demonstrate that bortezomib can enhance IFN-induced gene expression in human melanoma cells by prolonging the duration and level of STAT1 phosphorylation. Conversely the IFN-responsiveness of PBMCs was not affected following proteasome inhibition. These data suggest that proteasome inhibition could specifically sensitize melanoma cells to the direct actions of interferons.
The proteasome is responsible for the degradation of ubiquitinated cellular proteins, including numerous kinases and transcription factors [19]. Mechanistically, our data suggest that the enhanced IFN-responsiveness of melanoma cells occurred as a result of elevated levels of phosphorylated STAT1 following proteasome inhibition. These findings are consistent with observations that proteasome inhibition by bortezomib can lead to reduced turnover of transcription factors and other cellular proteins. Thus, treatment with bortezomib may have led to an increased cellular pool of phosphorylated transcription factors that would normally be subject to degradation in malignant cells. These results indicated that bortezomib could prime malignant cells to signal more efficiently in response to cytokine stimulation. One potential limitation of the in vitro studies with continuous bortezomib is that this design may not directly reflect the clinical pharmacokinetic profile of bortezomib where maximum proteasome inhibition is achieved within the first hour of administration. However, at the 24-h time point, proteasome activity is still reduced by 50% [20]. These observations suggest that data obtained following a 6-h (Fig. 2a–c) or even an 18-h (Fig. 2b) pre-treatment may still be clinically relevant.
The present data suggest that proteasome inhibition may be a good strategy to augment the direct anti-tumor effects of interferons or other cytokines produced by the innate immune system. Other studies have shown that low doses bortezomib can upregulate MICA and MICB protein expression in hepatoma cells and render them more susceptible to natural killer (NK) cell cytotoxicity [21]. In a similar manner, Vales-Gomez and colleagues have demonstrated that human fibroblasts, Jurkat, colon carcinoma and HeLa cells treated with proteasome inhibitors can become more susceptible to NK cell-mediated cytotoxicity due to increased expression of the ULBP2 protein, a ligand for the NKG2D activating receptor. Finally, bortezomib pre-treatment has been shown to sensitize human bladder and lung cancer cells to TRAIL-mediated apoptosis [22, 23].
In contrast to the observation of synergistic apoptosis in melanoma cells by this drug combination, pre-treatment of PBMCs from healthy donors with bortezomib did not modulate their cellular response to IFN-α. These data are consistent with previous observations that non-transformed cells are relatively insensitive to the effects of proteasome inhibition. However, these data also suggest that bortezomib is unlikely to further enhance the immunomodulatory effects of IFN-α on lymphocytes, which are thought to be an important component to the anti-tumor activity of this cytokine [4–6]. Together these data suggest that bortezomib can be effectively administered with therapies that modulate recognition of tumors by the innate immune system. We are currently evaluating the safety of this treatment combination in an investigator-initiated phase I clinical trial at our institution.
Acknowledgments
The authors thank the Ohio State University Comprehensive Cancer Center Analytical Cytometry and Nucleic Acid Shared Resources. The Harry J. Lloyd Charitable Trust, The Melanoma Research Foundation, The Valvano Foundation for Cancer Research Award (to G.B. Lesinski), National Institutes of Health (NIH) Grants CA84402, K24 CA93670 (to W.E. Carson), K22 CA134551 (to G.B. Lesinski), P30-CA16058, P01-CA95426 (to M.A. Caligiuri), and Millennium Pharmaceuticals, Inc., and Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
References
- 1.American Cancer Society (2007) Cancer facts and figures for 2006. American Cancer Society, Atlanta
- 2.Kabbarah O, Chin L. Revealing the genomic heterogeneity of melanoma. Cancer Cell. 2005;8(6):439–441. doi: 10.1016/j.ccr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Belardelli FM, Ferrantini E, Proietti, et al. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi: 10.1016/S1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 5.Lesinski GB, Anghelina M, Zimmerer J, et al. The anti-tumor effects of interferon-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112(2):170–180. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24(19):3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 7.Levy DE, Gilliland DG. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene. 2000;19(21):2505–2510. doi: 10.1038/sj.onc.1203480. [DOI] [PubMed] [Google Scholar]
- 8.Pansky A, Hildebrand P, Fasler-Kan E, et al. Defective Jak-STAT signal transduction pathway in melanoma cells resistant to growth inhibition by interferon-alpha. Int J Cancer. 2000;85(5):720–725. doi: 10.1002/(SICI)1097-0215(20000301)85:5<720::AID-IJC20>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Thyrell L, Erickson S, Zhivotovsky B, et al. Mechanisms of interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21(8):1251–1262. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DP, Watling D, Rogers NC, et al. The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line. Melanoma Res. 2003;13(3):219–229. doi: 10.1097/00008390-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lesinski GB, Trefry J, Brasdovich M, et al. Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13(17):5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- 12.Lesinski GB, Raig ET, Guenterberg K, et al. IFN-alpha and bortezomib overcome Bcl-2 and Mcl-1 overexpression in melanoma cells by stimulating the extrinsic pathway of apoptosis. Cancer Res. 2008;68(20):8351–8360. doi: 10.1158/0008-5472.CAN-08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 14.Kramer C. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:309–310. doi: 10.2307/3001469. [DOI] [Google Scholar]
- 15.Gollob JA, Sciambi CJ, Huang Z, et al. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer Res. 2005;65(19):8869–8877. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- 16.Pamment J, Ramsay E, Kelleher M, et al. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene. 2002;21(51):7776–7785. doi: 10.1038/sj.onc.1205981. [DOI] [PubMed] [Google Scholar]
- 17.Caraglia M, Vitale G, Marra M, et al. Alpha-interferon and its effects on signalling pathways within cells. Curr Protein Pept Sci. 2004;5(6):475–485. doi: 10.2174/1389203043379378. [DOI] [PubMed] [Google Scholar]
- 18.Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/A:1023668705040. [DOI] [PubMed] [Google Scholar]
- 19.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton AL, Eder JP, Pavlick AC, et al. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol. 2005;23(25):6107–6116. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 21.Armeanu S, Krusch M, Baltz KM, et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res. 2008;14(11):3520–3528. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 22.Voortman J, Resende TP, Abou El Hassan MA, et al. TRAIL therapy in non-small cell lung cancer cells: sensitization to death receptor-mediated apoptosis by proteasome inhibitor bortezomib. Mol Cancer Ther. 2007;6(7):2103–2112. doi: 10.1158/1535-7163.MCT-07-0167. [DOI] [PubMed] [Google Scholar]
- 23.Papageorgiou A, Kamat A, Benedict WF, et al. Combination therapy with IFN-alpha plus bortezomib induces apoptosis and inhibits angiogenesis in human bladder cancer cells. Mol Cancer Ther. 2006;5(12):3032–3041. doi: 10.1158/1535-7163.MCT-05-0474. [DOI] [PubMed] [Google Scholar]