Abstract
The awareness of the beneficial effects of Mn porphyrin-based superoxide dismutase (SOD) mimics and peroxynitrite scavengers on decreasing oxidative stress injuries has increased the use of these compounds as mechanistic probes and potential therapeutics. Simple Mn2+ salts, however, have SOD-like activity in their own right both in vitro and in vivo. Thus, quantification/removal of residual Mn2+ species in Mn-based therapeutics is critical to an unambiguous interpretation of biological data. Herein we report a simple, sensitive, and specific method to determine residual Mn2+ in Mn-porphyrin preparations that combines a hydrometallurgical approach for separation/speciation of metal compounds with a spectrophotometric strategy for Mn determination. The method requires only common chemicals and a spectrophotometer and is based on the extraction of residual Mn2+ by bis(2-ethylhexyl)hydrogenphosphate (D2EHPA) into kerosene, re-extraction into acid, and neutralization followed by UV-vis determination of the Mn2+ levels via a Cd2+-catalyzed metallation of the H2TCPP4− porphyrin indicator. The overall procedure is simple, sensitive, specific, and amenable to adaptation. This quantification method has been routinely used by us for a large variety of water-soluble porphyrins.
Introduction
The beneficial effects of Mn porphyrins as superoxide dismutase (SOD) and peroxynitrite reductase mimics in oxidative stress [1] models, particularly cationic Mn(III) 5,10,15,20-tetrakis-N-alkylpyridylporphyrins [2–5], have motivated their use as mechanistic probes and potential therapeutics and stimulated the commercialization of some of the compounds of the series. However, along with the bigger demand have emerged a variety of issues related to the quality of the samples that have jeopardized some of the biological data reported so far (for details, see [6–8]). Inappropriate synthetic procedures and unsuitable work-up and purification strategies may leave behind non-innocent impurities that may have biological activity in their own right, may mask the in vivo effects of the compounds in study, and could in turn result in questionable conclusions. CalBiochem preparations of Mn(III) 5,10,15,20-tetrakis-N-alkylpyridylporphyrins, for example, were recently found to be actually a complex mixture of compounds of different degree of N-alkylation that affected dramatically the intrinsic antioxidant capacity and biological activity of such compounds [7,8]. In another case, we have also clearly shown that all commercially available preparations of anionic Mn(III) tetrakis(4-carboxylatophenyl)porphyrin, MnTCPP3− (or MnTBAP3−) (from Alexis, Porphyrin Products, MidCentury Chemicals and CalBiochem) carried traces of Mn in the form of non-porphyrin species that possessed high SOD-like activity in its own right, while pure MnTBAP3− itself was not an SOD mimic at all [6,7]; such impurities had little effect on the peroxynitrite scavenging ability of the samples, which made pure MnTBAP3− a suitable probe for peroxynitrite over superoxide in mechanistic studies [9].
The two obvious impurities that may arise in all Mn porphyrin-based preparations are the unmetallated porphyrins (metal-free ligands) and residual Mn2+ salts left from the metallation procedure. While the unwanted presence of metal-free ligands, which are photosensitizers, in Mn porphyrin samples can be conveniently detected and quantified by a variety of techniques [6,7], the quantification of residual Mn2+ species in Mn porphyrin samples (or any other Mn-based antioxidant, for that matter) has never been fully addressed. The catalytic rate constant for the dismutation of O2.− by simple Mn2+ salts ranges from 1.3 × 106 M1 s−1 to ~107 M−1 s−1 [10–12]. Thus, residual Mn2+ salts left in the most common Mn-based antioxidants such as Mn porphyrins (MnP), Mn salens, or Mn cyclic polyamines may eventually have SOD-like biological activity in their own right. The critical role of simple Mn2+ salts as SOD mimics in cell models of oxidative stress have been documented [13–15].
The determination of residual Mn2+ in Mn porphyrin samples is critical for assessing if further purification is required and for granting that the effects observed in vivo are related to the Mn porphyrin itself rather than to residual Mn2+ salts contaminants or their derivatives. We describe herein the first method for determination of residual Mn2+ species in Mn porphyrin samples, by combining a hydrometallurgical approach for separation/speciation of metal compounds with a sensitive spectrophotometric strategy for Mn determination.
Materials and Methods
Materials
The following reagents were used as received: 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (H2TCPP4−, Aldrich, ~85% dye content), HClO4 (Aldrich, 69.0–72.0%), CdCl2 (Aldrich, 99+%), Mn(OAc)2·4H2O(Aldrich, 99.99%), bis(2-ethylhexyl)hydrogenphosphate (D2EHPA, Aldrich, 97%), kerosene (Aldrich), NaCl (Mallinckrodt, 100.2%), NaOH (Mallinckrodt, 98.4%), hydroxymethylaminomethane (Tris, EM, >99.8%). UV-vis measurements were performed on a Shimadzu UV-2501PC spectrophotometer at 0.5 nm resolution.
Preparation of working solutions
H2TCPP4−(aq) solution (2.03 × 10−4 M) was prepared by diluting 8 ml of H2TCPP4− stock solution (10.01 mg of porphyrin in 7 ml of 0.01 M NaOH and 3 ml H2O) with 27.8 ml of water. The concentration of the H2TCPP4− solution was determined spectrophotometrically (ε415nm = 386,000 M−1 cm−1) [6]. Due to light sensitivity, all flasks containing H2TCPP4− solutions were fully wrapped with aluminum foil. Solutions were found indefinitely stable if stored in the dark. D2EHPA solution in kerosene (0.227 M), aqueous solutions of CdCl2 (1.20 mM), HClO4 (1 M); NaOH(aq) (5 M), NaCl (2.00 M) and Tris buffer (pH 7.8; 1.00 M) were prepared. Freshly prepared aqueous solutions of Mn(OAc)2·4H2O of various concentration were used for calibration. The concentration of MnPs in their aqueous solutions was determined spectrophotometrically using reported ε values. The ε468nm = 104,700 M−1 cm−1 of a pure sample of MnTCPP3− was used [6]. Deionized water was used throughout the study.
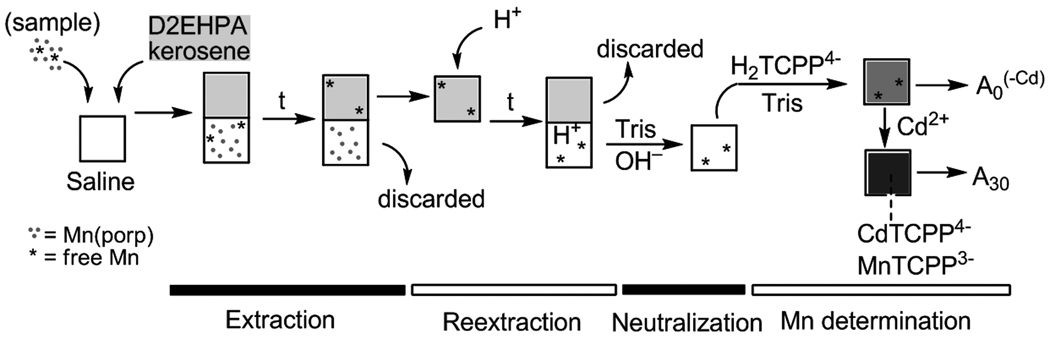
Methods (Figure 1)
Figure 1.
Schematic representation of the procedure for the determination of residual Mn 2+ in Mn porphyrin samples.
Step 1: Extraction
To a 1.7 ml-snap-cap conical plastic microcentrifuge tube were added 100 µl of 2.00 M NaCl solution, (900 − X) µl of water and X µl of the aqueous MnP sample. This solution is referred to as “saline solution”. Then 500 µl of 0.227 M D2EHPA solution in kerosene was added. The biphasic mixture was heavily shaken (manually) for 5.0 min, after which the phases were separated in a Brinkmann Eppendorf 4515 centrifuge (2.5 min at 3,000 rpm). The volume X of the aqueous MnP sample is chosen in order to give a concentration of residual Mn2+ in the saline solution in the range between 1 and 30 µM. For example, if the MnP sample is thought to contain residual Mn2+ in the order of (a) ~1 mol% or (b) ~0.1 mol%, then the volume X of a 5 mM MnP stock should be (a) ~ 80 µl of or (b) ~ 800 µl, respectively, to yield a “saline solution” of ~ 4 µM residual Mn2+.
Step 2: Reextraction
An 450 µl aliquot of the organic phase (from the step 1) was transferred to a microcentrifuge tube, to which were added 900 µl of HClO4(aq) (1.00 M). The biphasic mixture was heavily shaken manually for 5.0 min, after which the phases were separated in a centrifuge (2.5 min at 3,000 rpm). The organic layer was carefully and fully discarded using 100 µl Eppendorf pipettes. The use of 1000 µl Eppendorf tips were found unsuitable to carry this phase separation out.
Step 3: Neutralization
An 800 µl aliquot of the acidic solution (aqueous phase from step 2) was transferred to a microcentrifuge tube. Then were added 40 µl of Tris buffer (pH 7.8; 1.00 M) and ~160 µl of NaOH (~5 M). The exact volume of NaOH solution was determined in advance as that necessary to bring a control solution of HClO4 (800 µl, 1 M) and Tris (40 µl) to pH 7.0–7.1. The resulting neutralized Mn2+– containing solution was used in last step.
Step 4: Mn2+ determination (spectrophotometric measurement)
Into a 1.5 ml quartz cuvette (1 cm optical path) were added 40 µl of Tris buffer (1 M), 120 µl of H2TCPP4− (2.03 × 10−4 M) solution, and 800 µl of the neutral solution from step 3. The UV-vis measurements were carried out in the following manner: (1) the initial absorbance at 468 nm (λmax of MnTCPP3− product) is recorded as A0; (2) addition of 40 µl of CdCl2 (1.20 mM) to the cuvette marks time zero (the absorbance maximum shifts from 413 nm for H2TCPP4− to 433 nm for CdTCPP4−); (3) the formation of MnTCPP3- is marked by a band at 468 nm, whose change in absorbance is monitored for 30 min; (4) the absorbance at 30 min is recorded as A30. The concentration of residual Mn2+ may be expressed in mol % of the MnP by the quotient between the concentrations of the residual Mn2+ and MnP in the “saline solution” times 100%.
Calibration curves
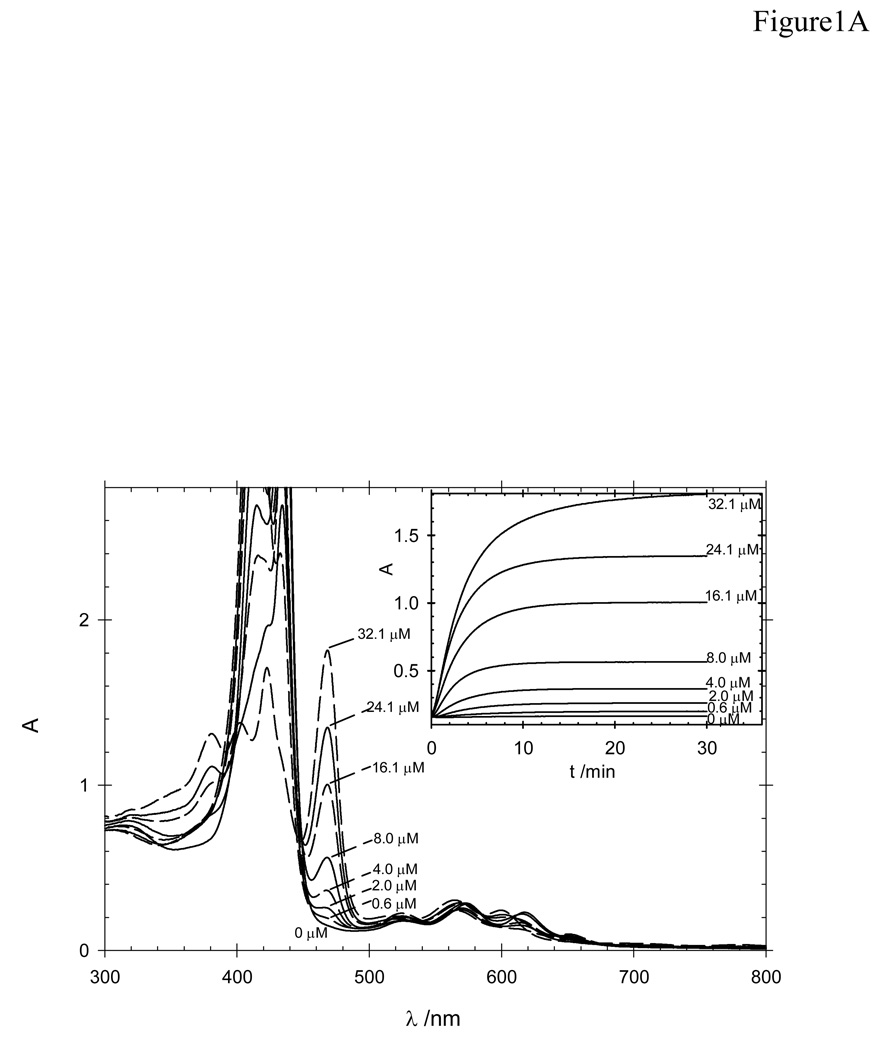
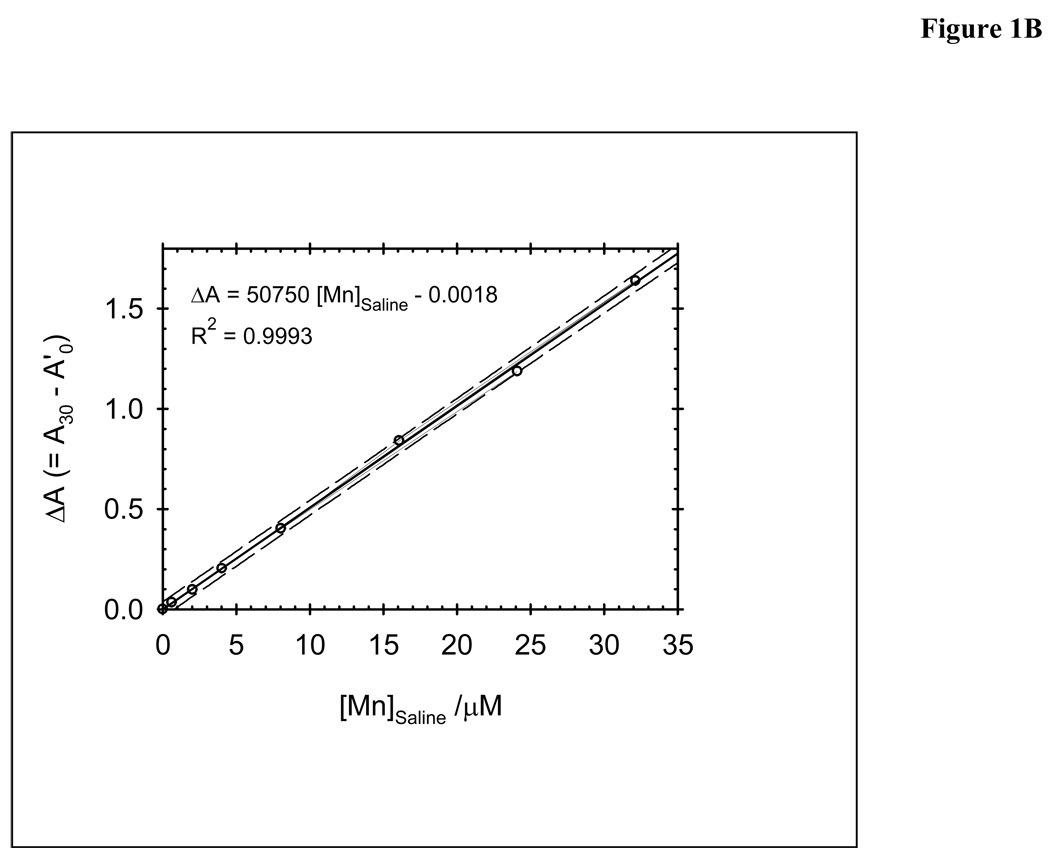
Calibration curve was obtained using the procedure described above with different concentrations of Mn(OAc)2 4H2O (0.6, 2.0, 4.0, 8.0, 16.1, 24.1 and 32.1 µM) (Figure 2A and 1B). The content of Mn2+ in these sample was calculated from the recorded absorbance measurements using the following relationships: (a) A0’ = 0.96 × A0 (where 0.96 corresponds to the dilution factor upon addition of CdCl2 to the cuvette); (b) ΔA = A30 − A0’; (c) a calibration curve constructed from ΔA values versus known concentration of Mn2+ in the “saline” solution.
Figure 2.
Calibration curves for the procedure for the determination of residual Mn 2+ in Mn porphyrin samples: A) UV-vis spectra of the cuvette solution at the end of the reaction time (30 min); insert: time-dependent change in the absorbance at 468 nm at various concentrations of Mn2+. B) Absorbance change as a function of Mn(OAc)2 concentrations: 0.6, 2.0, 4.0, 8.0, 16.1, 24.1 and 32.1 µM.
Results and Discussion
A common hydrometallurgical practice for the separation of metal ions involves the controlled liquid-liquid extraction of the cations into an organic phase containing an appropriate extractant. Among the systems that have been extensively studied for hydrometallurgical Mn2+ extraction are those using di-(2-ethylhexyl)phosphoric acid (D2EHPA) as extractant [16–20]. It follows, therefore, that the separation of the residual Mn2+ salts from the aqueous Mn porphyrin samples was carried out by liquid-liquid extraction using D2EHPA in kerosene. No in situ demetallation of Mn(III) porphyrins by D2EHPA was observed. Of note, common water-soluble Mn(III) porphyrins are indefinitely stable toward metal loss even in concentrated sulfuric acid. The use of a large excess of D2EHPA in kerosene (0.227 M) relates to the extraction equilibrium constant of ~10−3 [16] for the Mn2+/D2EHPA system. Of note, the Mn2+ loading capacity of 0.1 M D2EHPA is 0.026 M [18]. NaCl was added to the aqueous phase to yield a “saline solution” and to avoid, thus, the partition of some of the Mn porphyrins (e.g., PEG-ylated Mn(III) N-pyridylporphyrin [2]) into the kerosene phase. The residual Mn2+ species extracted with D2EHPA/kerosene is re-extracted into an aqueous medium with a strong acid. This re-extracted solution is, then collected and neutralized with NaOH. Perchloric acid was the acid of choice to minimize interference with the spectrophotometric assay (see below).
The use of this extraction method to metal complexes of low stability to acids is precluded. Attemtps to measure the free Mn2+ content of Mn(III) salen samples (EUK-8, Mn(III) N,N′-ethylenebis(salicylideneiminato) resulted in the in situ demetallation of Mn(III) salen. The acidity of D2EHPA prevented also the quantification of residual Mn2+ species in commercial MnTBAP3−, as this porphyrin precipitates in the acidic form and co-precipitation of residual Mn2+ could not be ruled out, as observed with other acids [6].
Although atomic absorption is a classical technique to measure metal levels in water, we chosed to determine the residual Mn2+ by spectrophotometry given the wide availability of spectrophotometers in biochemical and biomedical laboratories. A method utilizing H2TCPP4− (λmax = 415 nm) as indicator [21–23] gives results comparable to those obtained by atomic absorption, is simple and, given the specific nature of the MnTCPP3− complex formed (λmax = 468 nm), is subjected to little interference by other metal ions [21–23]. The insertion of Mn2+ in the H2TCPP4− porphyrin does not occur at room temperature in absence of a catalyst, such as Cd2+. The coordination of Cd2+ to H2TCPP4− occurs readily to yield a deformed CdTCPP4− porphyrin (λmax = 433 nm) that enhances the Mn2+ insertion into the porphyrin by several orders of magnitude via a Cd-to-Mn metathesis reaction [24]. The very low reduction potential of the Mn(II)TCPP4− [6] favors its immediate aerobic oxidation to Mn(III)TCPP3−, which has a sharp, characteristic band at 468 nm of high molar absortivity (log ε = 5.04 [6]). The reaction is, thus, monitored at this wavelength (Fig. 2A-insert) as it is specific to the Mn product and falls in a spectral region that remains unmasked by the high absorptions due to the excess H2TCPP4− and CdTCPP4− species (Fig. 2A). The reaction was carried out in Tris buffer instead of borate and/or imidazole buffers [21–23] because of its common use in biochemical and biomedical laboratories.
The large excess of NaClO4 (derived from the NaOH-neutralization of HClO4) in the cuvette slows the MnTCPP3− formation in a classical kinetic salt effect manner, as the CdTCPP4− complex and the residual Mn2+ species are of opposing charges. The use of HCl instead of HClO4 was prohibited by the high coordination ability of Cl− compared to ClO4−. In excess Cl−, formation of the catalytic intermediate CdTCPP4− was precluded, as indicated by UV-vis spectra (the major species in solution was still H2TCPP4−), and formation of MnTCPP3− did not occur. Excess Cl− competes effectively with H2TCPP4− for the metal ions and inhibits, thus, metalloporphyrin formation [25].
The method gives a linear response of ΔA vs [Mn2+] (Fig. 2B) up to ~35 µM Mn2+ in the “saline solution” (i.e., ~22.4 µM of Mn2+ in the cuvette), after which H2TCPP4−/CdTCPP4− (24.36 µM in the cuvette) is mostly depleted and the system is no longer responsive to excess Mn2+, unless more H2TCPP4− is added. The limits of detection (LOD, 3s) and of quantification (LOQ, 10s) were 0.4 and 1.3µM of Mn2+ in the “saline solution”, respectively. An artificial “saline solution” prepared by deliberate mixing of a PEG-ylated Mn(III) N-pyridylporphyrin (290 µM) and Mn(OAc)2 (24.1 µM) was subjected to the whole extraction/quantification procedure and compared to a control sample of Mn(OAc)2 alone (24.1 µM); the Mn2+ recovery was 99.2 %, which confirmed also that excess MnP does not interfere in neither the extraction of Mn2+ by D2EHPA nor the subsequent quantification steps.
This method has been routinely used by us for a large variety of water-soluble porphyrins. The cationic Mn(III) N-alkylpyridylporphyrins are regularly isolated through double precipitation and extensive washings; both procedures are critical for effective removal of nearly all residual Mn2+ from the preparations. Mn porphyrins that we have prepared via this route [7,26–28] usually have low levels of residual Mn2+, often in the range of 0.05 – 1 mol % on a MnP basis. The extensive ultrafiltration procedure used previously for Mn(III) porphyrins that do not precipitate readily [2,27] gave samples of 0.1 – 1 mol % on a MnP basis.
Acknowledgement
We thank Dr. Wallace D. Fragoso for valuable discussions on the method validation. Financial support of NIAID 5-U19-AI-067798-03 (pilot project to IBH) and The Wallace H. Coulter Translational Partners Grant Program was greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC. Free Radical in Biology and Medicine. 4th Edition. Oxford: Biosciences; 2004. [Google Scholar]
- 2.Batinić-Haberle I, Spasojević I, Stevens RD, Bondurant B, Okado-Matsumoto A, Fridovich I, Vujasković Z, Dewhirst MW. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006:617–624. doi: 10.1039/b513761f. and references therein. [DOI] [PubMed] [Google Scholar]
- 3.Rebouças JS, DeFreitas-Silva G, Idemori YM, Spasojević I, Benov L, Batinić-Haberle I. Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: Protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier. Free Radic. Biol. Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spasojević I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouças JS, St. Clair DK, Batinić-Haberle I. Pharmacokinetics of the potent redox-modulating manganese porphyrin, MnTE-2-PyP5+, in plasma and major organs of B6C3F1 mice. Free Radic. Biol. Med. 2008;45:943–949. doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spasojević I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, St. Clair DK, Batinić-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic. Biol. Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebouças JS, Spasojević I, Batinić-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J. Biol. Inorg. Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 7.Rebouças JS, Spasojević I, Batinić-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J. Pharm. Biomed. Anal. 2008;48:1046–1049. doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard J, Rebouças JS, Durazo A, Kos I, Fike F, Panni M, Gralla EB, Valentine JS, Batinić-Haberle I, Gatti RA. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia–telangiectasia cells. Free Radic. Biol. Med. 2009 doi: 10.1016/j.freeradbiomed.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spasojević I, Batinić-Haberle I, Stevens RD, Hambright P, Thorpe AN, Grodkowski J, Neta P, Fridovich I. Manganese(III) biliverdin IX dimethyl ester: a powerful catalytic scavenger of superoxide employing the Mn(III)/Mn(IV) redox couple. Inorg. Chem. 2001;40:726–739. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]
- 11.Barnese K, Gralla EB, Cabelli DE, Valentine JS. Manganous phosphate acts as a superoxide dismutase. J. Am. Chem. Soc. 2008;130:4604–4606. doi: 10.1021/ja710162n. [DOI] [PubMed] [Google Scholar]
- 12.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: In vitro. Arch. Biochem. Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 13.Al-Maghrebi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch. Biochem. Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez RJ, Srinivasan C, Munroe WH, Wallace MA, Martins J, Kao TY, Le K, Gralla EB, Valentine JS. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J. Biol. Inorg. Chem. 2005;10:913–923. doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 15.Munroe W, Kingsley C, Durazo A, Gralla EB, Imlay JA, Srinivasan C, Valentine JS. Only one of a wide assortment of manganese-containing SOD mimicking compounds rescues the slow aerobic growth phenotypes of both Escherichia coli and Saccharomyces cerevisiae strains lacking superoxide dismutase enzymes. J. Inorg. Biochem. 2007;101:1875–1882. doi: 10.1016/j.jinorgbio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas RK, Habib MA, Islam MN. Some Physicochemical Properties of (D2EHPA). 1. Distribution, Dimerization, and Acid Dissociation Constants of D2EHPA in a Kerosene/0.10 kmol m−3 (Na+,H+)Cl− System and the Extraction of Mn(II) Ind. Eng. Chem. Res. 2000;39:155–160. [Google Scholar]
- 17.Tao W, Nagaosa Y. Evaluation of Some Prediction Models for the Determination of Physicochemical Constants of Dialkylphosphoric Acids. Sep. Sci. Technol. 2003;38:1621–1631. [Google Scholar]
- 18.Devl NB, Nathsarma KC, Chakravortty V. Liquid-Liquid Extraction of Manganese(II) with Binary Mixtures of Sodium Salts of D2EHPA, PC 88A and CYANEX 272. Solvent Extr. Res. Dev. Jpn. 1997;4:117–128. [Google Scholar]
- 19.Biswas RK, Mondal MGK. Kinetics of Mn(II) extraction by D2EHPA. Hydrometallurgy. 2003;69:145–156. [Google Scholar]
- 20.Sato T, Kawamura M, Nakamura T, Ueda M. Extraction of divalent manganese, iron, cobalt, nickel, copper and zinc from hydrochloric-acid solutions by di-(2-ethylhexyl)phosphoric acid. J. Appl. Chem. Biotechnol. 1978;28:85–94. [Google Scholar]
- 21.Ishii H, Koh H, Satoh K. Spectrophotometric determination of manganese utilizing metal ion substitution in the cadmium-α,β,-γ,δ -tetrakis(4-carboxyphenyl)porphine complex. Anal. Chim. Acta. 1982;136:347–352. [Google Scholar]
- 22.Chiswell B, O’Halloran KR. Comparison of three colorimetric methods for the determination of manganese in freshwaters. Talanta. 1991;38:641–647. doi: 10.1016/0039-9140(91)80149-t. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D, Chiswell B. A new method for the evaluation of the oxidizing equivalent of manganese in surface freshwaters. Talanta. 1993;40:533–540. doi: 10.1016/0039-9140(93)80013-h. [DOI] [PubMed] [Google Scholar]
- 24.Hambright P. Chemistry of water soluble porphyrins. In: Radish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. Vol. 3. New York: Academic Press; 2000. pp. 129–210. [Google Scholar]
- 25.Rebouças JS, Cheu ELS, Ware CJ, James BR, Skov KA. Synthetic and Mechanistic Aspects of a New Method for Ruthenium-Metalation of Porphyrins and Schiff-Bases. Inorg. Chem. 2008;47:7894–7907. doi: 10.1021/ic800616q. [DOI] [PubMed] [Google Scholar]
- 26.Batinić-Haberle I, Benov L, Spasojević I, Hambright P, Crumbliss AL, Fridovich I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of Manganese(III) and Iron(III) cationic and anionic porphyrins. Inorg. Chem. 1999;38:4011–4022. [Google Scholar]
- 27.Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Fridovich I. Manganese(III) mesotetrakis ortho N-alkylpyridylporphyrins. Synthesis, characterization and catalysis of O2•− dismutation. J. Chem. Soc., Dalton Trans. 2002:2689–2696. [Google Scholar]
- 28.Rebouças JS, Spasojević I, Tjahjono DH, Richaud A, Méndez F, Benov L, Batinić-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]