Abstract
Aims
One proposed method to diagnose diabetic foot ulcers (DFU) for infection is clinical examination for signs of infection. Twelve different signs of infection have been reported. The purpose of this study was to examine each individual sign, a combination of signs recommended by the Infectious Disease Society of America (IDSA), and a composite predictor based on all signs among DFUs.
Methods
A cross-sectional research design was used. Sixty-four subjects with DFUs were recruited from a Department of Veteran’s Affairs Medical Center and an academic-affiliated-hospital. Each DFU was independently assessed by two research team members using the Clinical Signs and Symptoms Checklist. Tissue specimens were then obtained via wound biopsy and quantitatively processed. Ulcers with more than 106 organisms per gram of tissue were defined as having high microbial load. Individual signs and the IDSA combination were assessed for validity by calculating sensitivity, specificity, and concordance probability. The composite predictor was analyzed using c index and receiver operating curves.
Results
Twenty-five (39%) of the DFUs had high microbial loads. No individual sign was a significant predictor of high microbial load. The IDSA combination was not a significant predictor either. The c index of the composite predictor was .645 with a 95% confidence interval of .559, .732.
Conclusions
Individual signs of infection do not perform well nor does the IDSA combination of signs. However, a composite predictor based on all signs provides a moderate level of discrimination, suggesting clinical utility. Larger sample sizes and alternate reference standards are recommended.
Keywords: wound infection, diabetic foot ulcers, diabetes
Introduction
DFUs are a common type of chronic wound. The prevalence of diabetes is 6.3% in the general population, 8.7 among persons 20 years of age and older (Center for Disease Control and Prevention, 2003), and 17% among veterans enrolled in Veterans Health Administration (VHA) (Reiber et al., 2001). Approximately 15–20% of persons with diabetes will develop a DFU in their lifetime (American Diabetes Association, 1999; Boulton et al., 1999). Persons with diabetes are at higher risk for developing infections (Grossi et al., 1991; Josephs et al., 1993; Reiber et al., 2001; Wymenga et al., 1992) due to the effects of hyperglycemia on leukocyte function (Pecoraro & Chen, 1987). Outcomes associated with infection-related complications among persons with DFUs are striking. For example, 14–24% of persons with DFUs will have an amputation (ADA, 1999), and complications associated with foot ulcers account for 20–25% of all hospital days for persons with diabetes (Reiber, 1995).
Nonetheless, the identification and diagnosis of DFU infection, like chronic wound infection in general, remains a complex and unsolved problem (Bowler, 2003). During the past 10 years, 50 papers in the nursing and medical literature were devoted specifically to identifying and diagnosing infection in the chronic wound. Although a few of these have provided data to advance the knowledge base in this area, most address the problems associated with the uncertainty regarding the definition of infection, the issues surrounding wound cultures, rationales supporting best culturing techniques, and the role of clinical signs of infection. The reiteration of these issues underscores the continued frustration felt by clinicians and experts in screening chronic wounds for localized infection. The problem is that many studies on chronic wound infection have used variable definitions to determine wound infection, variable methods of wound sampling, and variable laboratory procedures for processing wound cultures (Bowler, 1998). Despite the continued attention given to this problem, therefore, there is a critical gap in the knowledge base that centers on the value of wound culture findings in predicting clinical outcomes. To address this limitation in wound healing science, tightly controlled prospective studies based on clearly defined, valid measures of wound bioburden and wound outcomes are needed.
Identification of localized infection in diabetic foot ulcers (DFU) is essential in order to prevent complications, such as amputation (American Diabetes Association, 1999; Williams, Hilton, & Harding, 2004). The optimal method to diagnose localized DFU infection is unknown. Clinical examination of DFUs for signs of infection has been proposed as a method. In this paper, the term “signs” signifies both signs and symptoms.
Clinical Signs of Wound Infection
Twelve clinical signs of localized wound infection have been reported. Erythema, edema, heat, and pain are signs of inflammation. Signs of inflammation plus purulent exudate are known as “classic” signs of infection, traditionally associated with wound infection. Serous exudate, delayed healing, friable granulation tissue, discolored granulation tissue, foul odor, pocketing of the wound base, and wound breakdown are thought of as “signs specific to secondary wounds” (Cutting & Harding, 1994), those healing by secondary, rather than primary intention.
In a heterogeneous sample of chronic wounds, none of the classic signs or signs specific to secondary wounds was found to be a useful diagnostic test for infection (Gardner, Frantz, & Doebbeling, 2001). However, only two wounds were DFUs. The diagnostic validity of clinical signs may differ by chronic wound etiology. In addition, combining signs may be more useful in identifying infection than any one sign.
Combinations of Clinical Signs of Infection
The Infectious Disease Society of America (IDSA) Guidelines (Lipsky, et al., 2004) for diagnosis and treatment of diabetic foot infections recommend a specific combination of clinical signs to diagnosis DFU infection. The IDSA recommends infection be diagnosed based on purulent exudate or two or more signs of inflammation (i.e., pain, erythema, heat, or edema). Persons with diabetes may have diminished inflammatory responses (Gardner, Frantz, & Saltzman, 2005), thus, validity of the IDSA combination as a diagnostic criterion is questionable. Further, the IDSA combination has not been empirically examined.
Composite of Clinical Signs of Infection
Signs specific to secondary wounds are better indicators of infection in chronic wounds than classic signs (Gardner, Frantz, & Doebbeling, 2001). A composite variable based on both classic and signs specific to secondary wounds may improve diagnostic validity of clinical examination in identifying DFU infection.
Purpose and Specific Aims
The purpose of this study was to examine diagnostic validity of clinical signs of localized wound infection in identifying DFU infection, using high microbial load as the reference standard. Microbial load is the number of organisms per gram of wound tissue and is measured using quantitative cultures of wound tissue. Microbial load greater than 106 organisms per gram of tissue is an evidence-based criterion to diagnose infection in chronic wounds (Heggers, 1991). The specific aims of the study were to identify:
sensitivity, specificity, and concordance probability of each sign as compared to microbial load (reference standard),
sensitivity, specificity, and concordance probability of the IDSA combination of signs as compared to microbial load, and
discriminatory accuracy of a composite predictor computed from the classic and signs specific to secondary wounds as compared to microbial load.
Patients and Methods
This study was part of a larger study that examined three swab techniques (Gardner, Frantz, Saltzman, et al., 2006). Subjects from the larger study who had DFUs were the sample in this cross-sectional study. DFUs were clinically assessed for signs of infection without knowledge of microbial load. Specimens of wound tissue were quantitatively processed and each ulcer was categorized by microbial load status. Presence of individual signs of infection, IDSA combination of signs, and a composite predictor computed from all signs of infection were then compared with microbial load status.
Setting and Sample
A Department of Veteran’s Affairs Medical Center and an academic-affiliated tertiary hospital served as settings for data collection. The Institutional Review Board (IRB) approved research protocols. A convenience sample was recruited during who: 1) were ≥18 years of age, and 2) had one or more full-thickness, non-arterial DFUs. Excluded were those with: 1) white blood cell count (WBC) < 1500 cells/mm3, 2) platelet count < 125,000/mm3, 3) coagulopathies, or 4) receiving anticoagulation therapy. Full-thickness diabetic foot ulcers justified acquisition of full-thickness tissue specimens. Non-arterial DFUs controlled the impact of inadequate perfusion on expression of clinical signs of infection. Exclusion of persons with low WBC, low platelet counts, coagulopathies or anticoagulation therapy reduced risks of tissue biopsy. All subjects gave informed consent prior to study procedures.
Study Variables
Clinical Signs of Wound Infection
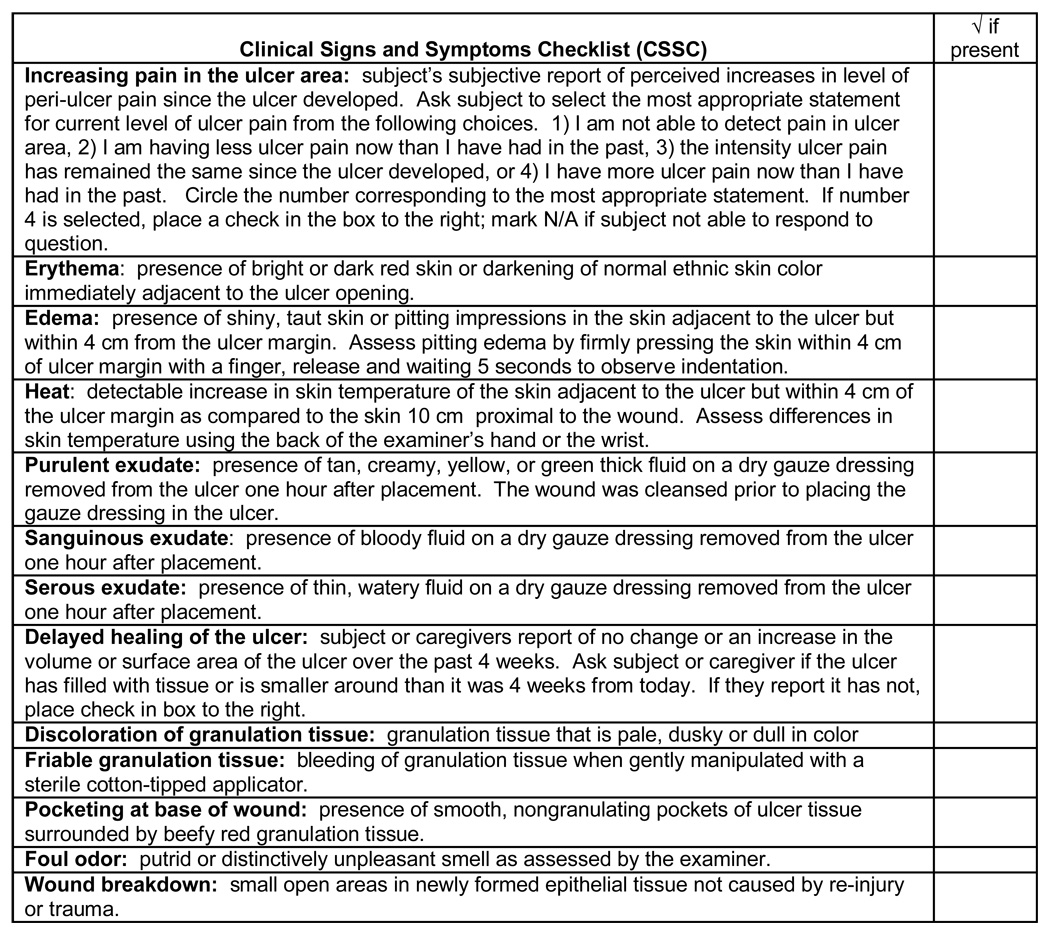
Clinical signs of infection, individually or combined, were index tests in this study. The Clinical Signs and Symptoms Checklist (CSSC) more objectively measures clinical signs of infection in chronic wounds (Gardner, Frantz, Troia, et al., 2001) (see Figure 1). The tool contains an item for each classic sign (i.e., increased pain, erythema, edema, heat, and purulent exudate) and each sign specific to secondary wounds (i.e., serous exudate, sanguinous drainage, delayed healing, discolored granulation tissue, friable granulation tissue, foul odor, wound breakdown). A specific item and descriptor operationalizes each sign. The inter-rater reliability of the items was assessed using simultaneous, but independent assessments from two nurse observers. Kappa’s ranged from .35 to 1.00 (Gardner, Frantz, Park, & Scherubel, 2006). Individual signs of infection are represented by the corresponding item on the CSSC. IDSA combination of signs specifies purulent exudate, or two or more of erythema, edema, heat, and increasing pain must be present. The composite predictor sign is a linear combination of all 12 signs.
Figure 1.
Clinical Signs and Symptoms Checklist
Microbial Load
Quantitative cultures of viable wound tissue measured microbial load. Laboratory procedures for culturing tissue followed methods outlined by Krizek and Robson (1975) and are reported in detail elsewhere (Gardner, Frantz, Saltzman, et al., 2006). Ulcers with high microbial load were defined as ≥1,000,000 organisms/gram of tissue (Heggers, 1991). Ulcers with low microbial load were defined as < 1,000,000 organisms/gram of tissue. Although deemed an important criterion for diagnosing infection in chronic wounds, beta hemolytic streptococcus was present in only one ulcer that also contained > 1,000,000 organisms/gram of tissue.
Secondary Study Variables
Secondary study variables were measured to examine their association with microbial load. These variables included age, sex, race, diabetes type, systemic antibiotics, red blood cell count (RBC), white blood cell count (WBC), albumin, hemoglobin A1c (HbA1c), size, depth and duration of the ulcer, amount of necrotic tissue, wound tissue oxygen (i.e., transcutaneous oxygen measurement), and type of ulcer dressing.
Study Procedures
After enrollment, age, sex, race, type of diabetes, and systemic antibiotic data were collected from medical records and patient/caregiver report. History, type, and location of ulcer were recorded. Blood samples were collected for complete blood count, albumin, and HbA1c.
A transcutaneous oxygen monitor (Novametric Model 840 Novametrix Systems, Inc., Wallingford, CT) measured wound tissue oxygen. The monitor non-invasively measures oxygen partial pressure (TcPO2). Following calibration, the heated (i.e., 45° C) TcPO2 sensor was secured on the dorsum of the foot and equilibrated for 20 minutes. A bath blanket covered the foot to control influence of ambient air temperature on blood flow. After equilibration, the TcPO2 levels were recorded at one-minute intervals over five minutes, while the subject maintained a supine position.
Current type of cover dressing and topical treatment was recorded and the dressing removed. The ulcer margin was outlined on transparent film. A Lasico Planimeter/Digitizer (Lasico, Inc., Los Angeles, CA) measured surface area by gliding a 24 -inch arm over the outline. A cotton-tipped swab placed in the deepest portion measured ulcer depth. The swab was marked at the point level with periwound skin. Distance from mark to swab tip was measured.
The ulcer was then cleansed with non-bacteriostatic saline and rated for amount of necrotic tissue using a Likert-type scale that classified percentage of ulcer bed covered with necrotic tissue (Bates-Jensen, 1997). A digital camera captured an image of the ulcer. The ulcer was dressed with dry gauze to standardize topical wound environment for assessment of wound exudate. It was left in place for one hour.
After one hour, the dressing was removed and saved. Non-bacteriostatic saline was used for ulcer cleansing. The CSSC was used to assess clinical signs of infection by one member of the research team trained by the principal investigator (PI). Exudate items were assessed using the dry gauze dressing. A second member of the research team independently reassessed the ulcer and dressing using CSSC. Ulcer assessments with CSSC were blind to microbial load status.
After cleansing, a specimen of viable wound tissue was removed from center of the ulcer using a 4–6 mm dermal punch instrument and sterile technique. Laboratory technicians, who processed tissue for culture, were blind to study aims and procedures to minimize observer bias.
Statistical Analyses
Descriptive and Comparative Analyses
Chi-square or Fisher’s exact tests were used to compare nominal variables between high and low microbial load groups. Nonparametric Wilcoxon rank-sum test was used to compare continuous variables. A two-tailed alpha level of .05 was used for comparative analyses.
Analyses of Individual Signs of Infection (Aim #1)
The validity of individual signs for identifying infection was assessed by determining each sign’s sensitivity (i.e., TPF: true positive fraction), 1 - specificity (i.e., FPF: false positive fraction), and probability of concordance, as defined by c index (Harrell, 2001). C index is equivalent to the AUC (area under the receiver operating characteristic curve) and when computed using the trapezoidal method is similar to Wilcoxon rank-sum (Bamber, 1975; Hanley, 1982). An individual sign of infection would be a useful discriminator of microbial load if the true sensitivity exceeds 1 – specificity, or equivalently, the population AUC or c index eceeds .5. A one-sided exact Wilcoxon rank-sum test and a p-value of < 0.05 was used to determine if the sign provided significant discrimination between low and high microbial load subjects (i.e., if the c index or AUC exceeds .5). A one-sided test is justifiable, since a “present” outcome is indicative of high microbial load and a “not present” outcome indicative of low microbial load.
Analyses of IDSA Combination of Signs (Aim #2)
The IDSA combination of signs was similarly computed and tested. A “present” outcome included subjects who had either “purulent exudate” or two or more of erythema, edema, heat, or increasing pain.
Analyses of the Composite Predictor (Aim #3)
A composite predictor was computed by regressing microbial load status on presence or absence of all signs of infection using multivariable logistic regression. The resulting prediction equation, which is a linear combination of the signs, was used as the composite predictor.
The composite predictor c index is expected to overestimate discrimination ability for an independently selected sample. Bootstrapping was used to estimate positive bias and to compute a corrected c index (Harrell, 2001). Jackknifing (Shao & Tu, 1995) was then used to obtain standard error estimates (SE) for the corrected c index. A 95% confidence interval was calculated as coverfitting corrected ±1.96SE.
Discriminatory accuracy of the composite predictor was estimated using its receiver operating characteristic (ROC) curve, which is a plot of sensitivity versus 1 - specificity for each possible threshold. An accurate predictor has an ROC curve closer to the top left corner. A non-informative predictor has an ROC curve that lies along the diagonal, known as the chance line where sensitivity = 1 – specificity. For this study, two ROC curves were computed. One curve was the empirical ROC curve based on the full sample; AUC is equal to cfull sample. The second ROC curve was corrected; AUC equal to coverfitting corrected. The latter curve provides a closer approximation of accuracy for an independently selected sample.
Results
Subjects and Wound Characteristics
Of 310 patients screened for the larger study, 102 met study criteria, and 83 agreed to participate in the larger study (Gardner, Frantz, Saltzman, et al., 2006). Of the 83 subjects, 64 had non-arterial DFUs and served as the sample for this study. Descriptive statistics of the sample are presented in Table 1.
Table 1.
Subject and Wound Characteristics by Microbial Load Status
| Total sample (n= 64) | High microbial load (n=25) | Low microbial load (n=39) | p value | |
|---|---|---|---|---|
| Mean age ± SD (years) | 55 ± 11.4 | 53 ± 10.3 | 56 ± 11.9 | 0.215 |
| Gender: | 0.765 | |||
| Male | 77% (49) | 80% (20) | 74% (29) | |
| Female | 23% (15) | 20% (5) | 26% (10) | |
| Race: | 1.000 | |||
| Caucasian, not Hispanic | 98% (63) | 100% (25) | 97% (38) | |
| Black | 2% (1) | 0% (0) | 3% (1) | |
| Diabetes Types | 0.494 | |||
| Type 1 | 16% (10) | 20% (5) | 13% (5) | |
| Type 2 | 84% (54) | 80% (20) | 87% (34) | |
| Systemic antibiotics | 0.112 | |||
| No | 63% (40) | 76% (19) | 54% (21) | |
| Yes | 37% (24) | 24% (6) | 46% (18) | |
| Mean RBC ± SD (million cells/µL) | 4.2 ± 0.61 | 4.2 ± 0.60 | 4.2 ± 0.61 | 0.620 |
| Mean WBC ± SD (cells/µL) | 8670.3 ± | 8672.0 ± | 8669.2 ± | 0.726 |
| 2750. 28 | 2122.99 | 3113.48 | ||
| Mean albumin ± SD (gm/dL) | 3.8 ± 0.47 | 3.8 ± 0.49 | 3.8 ± 0.46 | 0.917 |
| Mean HbA1c ± SD | 7.9 ± 1.66 | 8.0 ± 1.79 | 7.9 ± 1.59 | 0.880 |
| Mean size ± SD (cm2) | 5.9 ± 8.29 | 5.4 ± 10.94 | 6.2 ± 6.17 | 0.058 |
| Median size (cm2) | 2.43 | 1.40 | 4.3 | |
| Mean depth ± SD (cm) | 0.6 ± 0.51 | 0.6 ± 0.52 | 0.6 ± 0.50 | 0.557 |
| Median depth (cm) | 0.40 | 0.40 | 0.40 | |
| Mean duration of wound ± SD (wks) | 33.9 ± 45.15 | 50.6 ± 57.62 | 23.2 ± 31.34 | 0.079 |
| Median duration (wks) | 14.00 | 24.00 | 13.00 | |
| Amount of wound bed with necrotic tissue: | 0.832 | |||
| None | 66% (42) | 68% (17) | 64% (25) | |
| 1 to 50% | 28% (18) | 28% (7) | 28% (11) | |
| >50 to 100 % | 6% (4) | 4% (1) | 8% (3) | |
| Mean wound tissue oxygen ± SD (mmHg) | 59.6 ± 120.26 | 47.0 ± 15.83 | 67.6 ± 153.78 | 0.401 |
| Primary wound dressing | 0.185 | |||
| None | 3% (2) | 8% (2) | 0% (0) | |
| Dry | 28% (18) | 24% (6) | 31% (12) | |
| Moisture retentive | 69% (44) | 68% (17) | 69% (27) |
Validity of Individual Signs of Infection (Aim #1)
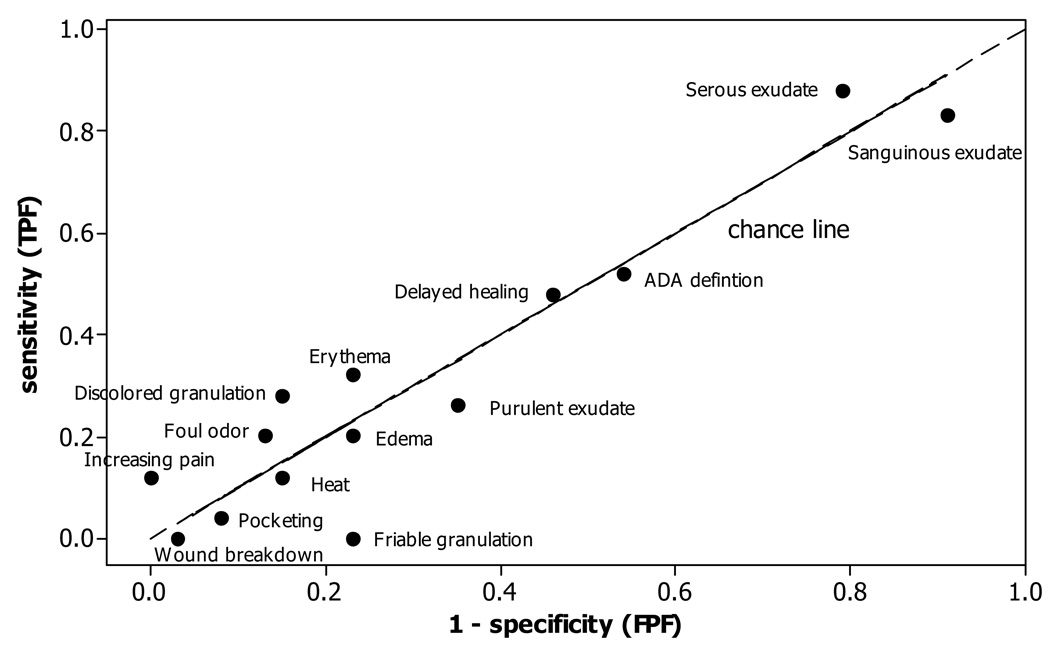
Sensitivity (TPF), 1 – specificity (FPF), and c index (AUC) of each individual sign are presented in Table 2 along with the p-value for testing if the sign is a useful predictor. Pairs (1 – specificity, sensitivity) are plotted in Figure 2. None of the signs was a significant predictor, although increasing pain was close (c = .56, p = .055). None of the signs show discriminatory ability, with sensitivity being only slightly more than 1 – specificity for six of the signs, and less than 1 – specificity for seven signs. Thus, many paired points are lower than the chance line in Figure 2.
Table 2.
Sensitivity, 1-Specificity, and c Index of Clinical Signs and Symptoms and ADA Definition (N=64)
| Sensitivity (TPF) | 1 – Specificity (FPF) | c(AUC) | P-value* | Specificity (1 – FPF) | |
|---|---|---|---|---|---|
| Classical signs of infection: | |||||
| Increasing pain | .12 | .00 | .56 | .055 | 1.00 |
| Erythema | .32 | .23 | .55 | .31 | .77 |
| Edema | .20 | .23 | .48 | >.5 | .77 |
| Heat | .12 | .15 | .48 | >.5 | .85 |
| Purulent exudate | .26 | .35 | .47 | >.5 | .65 |
| Signs specific to secondary wounds: | |||||
| Serous exudate | .88 | .79 | .54 | .30 | .21 |
| Sanguinous exudate | .83 | .91 | .46 | >.5 | .9 |
| Delayed healing | .48 | .46 | .51 | .54 | .54 |
| Discolored granulation | .28 | .15 | .56 | .18 | .85 |
| Friable granulation | .00 | .23 | .38 | >.5 | .77 |
| Pocketing | .04 | .08 | .48 | >.5 | .92 |
| Foul odor | .20 | .13 | .54 | .33 | .87 |
| Wound breakdown | .00 | .03 | .49 | >.5 | .97 |
| ADA definition†: | .52 | .54 | .49 | >.5 | .46 |
The p-value is for testing the one-side alternative hypothesis that a “yes” response is more indicative of high microbial load patients.
Purulent exudate and/or two signs of inflammation
Figure 2.
Scatterplot of Pairs (Sensitivity, 1 – Specificity) for Each Sign and IDSA Definition.
Sensitivity = 1 – specificity along the chance line.
Validity of IDSA Combination of Signs (Aim #2)
Sensitivity, 1 - specificity, c index, and corresponding p-value for the IDSA combination of signs are presented in Table 2. Table 2 and Figure 2 show that sensitivity (.52) is less than 1- specificity (.54), meaning the IDSA combination performed no better than chance (c = .49, p-value > .5).
Validity of the Composite Predictor (Aim #3)
To compute the composite predictor, load status was regressed on eleven sign variables. Friable granulation tissue was excluded because it showed an inverse association from what was expected. Because of multicollinearity, coefficients in the predictor are not easily interpretable, and thus, not reported here. However, how well microbial load can be predicted using all of the information was of interest.
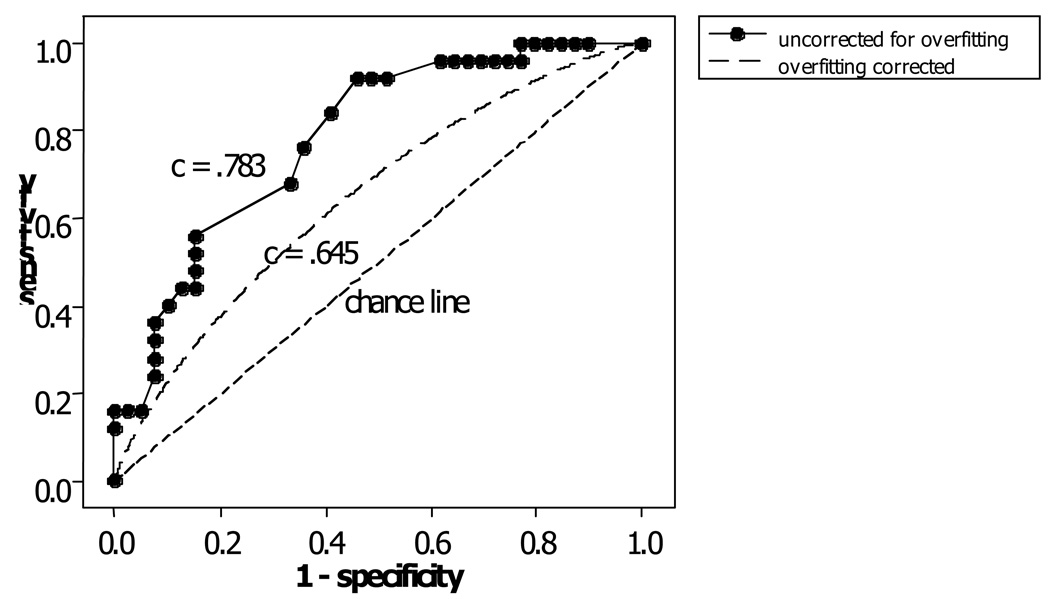
The composite predictor had c = .783 for the full sample and from bootstrapping coverfitting corrected = .645 (i.e., concordance of the composite predictor is .645 when applied to an independently selected sample). SE = .0438 and a 95% confidence interval of .559, .732. Figure 3 presents the ROC curve composite predictor for the full sample as well as the corrected AUC of .645.
Figure 3.
Composite Predictor ROC Curves.
The upper line (uncorrected for overfitting) is the empirical ROC curve for the full sample. The middle line (overfitting corrected) shows the theoretical ROC curve, assuming a binormal model, that corresponds to the overfitting-corrected c index. The chance line corresponds to a c index value of .5.
Discussion
Similar to the distribution of diabetes in the general adult population, most subjects had Type 2 rather than Type 1 diabetes. HbgA1c levels were higher than recommended for optimal blood glucose control. Blood values indicative of nutritional status (i.e., albumin and white blood cell count) were normal. Twenty-five (39%) of the subjects had high microbial loads in their DFUs. To our knowledge, no other studies have examined microbial load in DFUs so comparison was not possible.
Though not statistically significant, 46% of the low microbial group was on systemic antibiotics at the time of the data collection, as compared to 26% of the high microbial group. This distribution is expected since the goal of antibiotic therapy is to decrease wound bioburden, including microbial load. Because this study examined the relationship between clinical signs of infection and microbial load, it was not necessary to control for systemic antibiotic therapy. It would be expected that signs of infection would be associated with antibiotic therapy if antibiotic therapy impacts microbial load.
It is also noteworthy that the duration of the ulcer was substantially higher in the high microbial load group than in the low microbial load group although, again, not statistically significant. Duration of ulcer may have more impact on the expression of signs specific to secondary wounds than the classic signs of infection. Signs specific to secondary wounds are more consistent with chronic inflammation, while the classic signs are consistent with acute inflammation. It may be the duration of ulcer may explain differences in the expression of various clinical signs.
None of the individual signs of infection was able to discriminate between DFUs with high and low microbial load. Interestingly, the best performing sign was increasing pain, which is actually a symptom. Although DFUs are associated with loss of vibration and pressure perception, both carried by A-beta neurons, neuropathic changes may not impact nocioreceptor neurons, such as c-fiber and A-delta neurons. Finally, more than half of the signs performed worse than chance. No other studies on clinical signs of infection in DFUs were found for comparison.
The IDSA combination of signs is based on “classic” signs of infection. This particular combination of signs of infection performed poorly in identifying DFUs with high microbial load. Although this combination is recommended by the IDSA (Lipsky, et al., 2004), the IDSA acknowledged this recommendation was based on expert opinion, anecdotal evidence, and/or descriptive study alone. Further, strength of the IDSA recommendation not to culture “clinically uninfected lesions” was categorized as “optional”. Unfortunately, the sample size of this study would not support investigating the veracity of more optimal combinations.
The composite predictor, based on the classic and signs specific to secondary wounds, provided a moderate level of discrimination, suggesting that a linear composite may be clinically useful. Unfortunately, the sample size precluded the identification of the coefficients associated with each sign.
Finally, measures of wound bioburden, including microbial load, remain controversial as criteria for diagnosing infection in DFUs. While some believe wound bioburden measures, including microbial load, are not indicative of infection given the variation in wound culturing and processing, most agree the most reliable and valid wound cultures are those based on wound tissue specimens that are quantitatively processed (Robson, 1999). Many also believe this methodology the only valid measure of infection status available since DFUs may not express classic signs of infection due to dampened inflammatory responses. Guidelines published by the Wound Healing Society (Steed, et al., 2006) state DFUs with “suspected” infection, or those not healing in a two week time period, should be cultured to determine microbial load. The WHS guideline advises treatment of ulcers with a microbial load greater than 106 organisms per gram of tissue or beta hemolytic strep at any level. This recommendation implies infection be based on wound cultures, not clinical signs of infection. For this study, we chose to use the term microbial load, rather than infection, because the appropriate definition of wound infection remains equivocal.
Limitations
Although the sample of this study was adequate to address the validity of individual signs of infection and the IDSA combination of signs, studies of DFUs with larger samples are clearly needed to identify more optimal combinations of signs of infection or to delineate a clinical prediction rule.
Conclusions
After conducting a systematic review of the evidence, O’Meara and colleagues [19] concluded there was not enough evidence to identify the optimal method to diagnose DFU infections. The findings of this study confirm that little evidence exists to inform clinical practice with respect to identifying infection in DFUs. We believe this knowledge gap can only be addressed through further study of relationships between clinical signs of infection, microbial load, and DFU infections using larger samples and alternative reference standards, such as the development of infection-related complications.
Acknowledgements
Funding was provided by The Department of Veteran’s Affairs, HSR&D, Nursing Research Initiative (NRI-01-005-1), the NIH, National Institute of Nursing Research (NINR RO1 NRO7721), the Gerontological Nursing Interventions Research Center NIH #P30 NR03979 [PI: Toni Tripp-Reimer, The University of Iowa (UI) College of Nursing], and the Hartford Center for Geriatric Nursing Excellence The John A. Hartford Foundation [PI: Kathleen Buckwalter, The UI College of Nursing]. Views expressed are those of the authors and do not necessarily represent views of the Department of Veteran’s Affairs or the NINR.
Declaration of Competing Interests: None to declare.
List of abbreviations
- DFU
diabetic foot ulcer
- IDSA
Infectious Disease Society of America
References
- American Diabetes Association. Consensus Development Conference on Diabetic Foot Wound Care. Diabetes Care. 1999;22(8):1354–1360. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- Bamber D. The area above the ordinal dominance graph and the area below the receiver operating graph. Journal of Mathematical Psychology. 1975;12:387–415. [Google Scholar]
- Bates-Jensen B. The Pressure Sore Status Tool: A few thousand assessments later. Advances in Wound Care. 1997;10(5):65–73. [PubMed] [Google Scholar]
- Cutting KF, Harding KG. Criteria for identifying wound infection. Wound Care. 1994;3(4):198–201. doi: 10.12968/jowc.1994.3.4.198. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9(3):178–186. doi: 10.1046/j.1524-475x.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Park H, Scherubel M. The inter-rater reliability of the Clinical Signs and Symptoms Checklist in diabetic foot ulcers. Ostomy Wound Management. 2006;53(1):46–51. [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL. Diabetes and inflammation in infected chronic wounds. Wounds. 2005;17(8):203–205. [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques in identifying chronic wound infection. Wound Repair Regen. 2006;4(5):548–557. doi: 10.1111/j.1743-6109.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Troia C, Eastman S, MacDonald M, Buresh K, et al. A tool to assess the clinical signs and symptoms of localized infection in chronic wounds: Development and reliability. Ostomy Wound Management. 2001;47(1):40–47. [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Harrell FE., Jr . Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- Heggers JP. Variations of a theme. In: Heggers JP, Robson MC, editors. Quantitative bacteriology: Its role in the armamentarium of the surgeon. Boca Raton, FL: CRC Press; 1991. pp. 15–23. [Google Scholar]
- Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. American journal of surgery. 1975;130:579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clinical Infectious Diseases. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- O'Meara S, Nelson EA, Golder S, Dalton JE, Craig D, Iglesias C, et al. Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabetic Medicine. 2006;23:341–347. doi: 10.1111/j.1464-5491.2006.01830.x. [DOI] [PubMed] [Google Scholar]
- Robson MC. Lessons gleaned from the sport of wound watching. Wound Repair Regen. 1995;7(1):2–6. doi: 10.1046/j.1524-475x.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- Shao J, Tu D. The jackknife and bootstrap. New York: Springer; 1995. [Google Scholar]
- Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Williams DT, Hilton JR, Harding KG. Diagnosing foot infections in diabetes. Clinical Infectious Diseases. 2004;39(2):83–86. doi: 10.1086/383267. [DOI] [PubMed] [Google Scholar]