Abstract
Sonoporation uses ultrasound (US) to generate transient non-selective pores on the cell membrane and has been exploited as a non-viral intracellular drug and gene delivery strategy. The pore size determines the size of agents that can be delivered into the cytoplasm using the technique. However, measurements of the dynamic, submicron-scale pores have not been readily available. Electron microscopy or atomic force microscopy has been used to gauge pore size but such techniques are intrinsically limited to post US measurements that may not accurately reveal the relevant information. As previously demonstrated, changes of the transmembrane current (TMC) of a single cell under voltage clamp can be used for monitoring sonoporation in real time. Because the TMC is related to the diffusion of ions through the pores on the membrane, it can potentially provide information of the pore size generated in sonoporation. Using Xenopus laevis oocytes as the model system, the TMC of single cells under voltage clamp was measured in real time to assess formation of pores on the membrane in sonoporation. The cells were exposed to US (0.2 s, 0.3 MPa, 1.075 MHz) in the presence of Definity™ microbubbles. Experiments were designed to obtain the TMC corresponding to a single pore on the membrane. The size of the pores was estimated from an electro-diffusion model that relates the TMC with pore size from the ion transport through the pores on the membrane. The mean radius of single pores was determined to be 110 nm with standard deviation of 40 nm. This study reports the first results of pore size from the TMC measured using the voltage clamp technique.
Keywords: sonoporation, ultrasound, non-selective pores, voltage clamp, Xenopus laevis oocyte, microbubbles, Definity, electro-diffusion model, drug delivery, gene delivery
INTRODUCTION
Ultrasound (US)-induced cell membrane porosity, or sonoporation, has shown its unique potential in intracellular drug and gene delivery via generation of non-specific openings or pores on the cell membrane. It is important to have accurate measurements of the spatial and temporal scales of these pores because they determine the size of molecules or agents that can be efficiently delivered into the cytoplasm using the technique. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) have been employed to obtain information about the pores (Mehier-Humbert et al. 2005; Ross et al. 2002; Schlicher et al. 2006; Zhao et al. 2008). However, such techniques have intrinsic limitations in sonoporation study. Due to the rapid resealing of the pores (within seconds) in the cell membrane, pores recognized by SEM or other post US assays may not accurately represent practically relevant pore information for delivery. In addition, these techniques are usually time consuming and labor-intensive.
We have previously demonstrated the feasibility of using the voltage clamp technique to monitor the dynamic sonoporation process in real-time (Deng et al. 2004; Zhou et al. 2008a). The inward transmembrane current (TMC) of a single cell under voltage clamp exhibits a rapid increase in amplitude due to US application before recovering to pre-US level, indicating pore formation and resealing of the pores generated by US exposure (Deng et al. 2004). As the change of TMC is the result of ions flowing through all the transient pores generated on the membrane and thus related to the total area of all the pores, the TMC can be used to estimate the size of the pores. The number of pores and the size distribution of pores have to be known to obtain the size of an individual pore. It is hypothesized that with an appropriate model relating the TMC with the radius of pores, a single, reparable pore in the oocyte membranes produced by acoustic cavitation of microbubbles can be inferred with reasonable accuracy.
In order to obtain the size of single pore, sonoporation experiments were conducted under conditions designed to ensure generation of single pore in the cell membrane with high probability in this study. An electro-diffusion model was developed to relate the radius of a single pore with the TMC so that the size of a single pore was estimated from the recorded TMC. Applicable for quasi-steady state situations, we only consider the maximum pore size from the maximum TMC in this study, while the radius as a function of time can be derived from the time-dependent TMC values.
MATERIALS AND METHODS
Cell preparation
Collegenase-treated and defolliculated Xenopus laevis oocytes were used in this study. They were prepared following a protocol approved by our Institutional Animal Care and Use Committee (Deng et al. 2004; Zhou et al. 2008b). The oocytes were used immediately in experiments or stored in ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5mM HEPES, pH = 7.6) at 18°C for one or two days before use.
Sonoporation
During each experiment, a single oocyte (~1.1 mm diameter) was housed in a 35 mm polystyrene Petri dish (BD Biosciences, San Jose, CA) containing 4 mL ND96 solution with Definity™ microbubbles (Lantheus Medical Imaging, N. Billerica, MA) at a concentration of 6 × 103/mL to facilitate sonoporation (Deng et al. 2004; Zhou et al. 2008b). The actual microbubble concentration was confirmed by counting the bubbles with a hemocytometer. Tone-burst US exposures (center frequency 1.075 MHz, duration 0.2 s, peak negative pressure 0.3 MPa) were generated using an planar, circular piezoelectric US transducer (Piezo Technologies, Indianapolis, IN) driven by a waveform generator (33250A, Agilent Technologies, Palo Alto, CA) and a RF power amplifier (75A250, Amplifier Research, Souderton, PA). The Petri dish was tested to have minimal effect (>95% transmission) on the transmitting US. Fresh solution with Definity™ microbubbles was used after each US application (with or without TMC change) and each oocyte was used for only one detected TMC change.
Electrophysiological measurements
Microelectrodes connected to a voltage clamp amplifier (Dagan CA-1B, Dagan Corp., Minneapolis, MN) (Stuhmer 1998) were inserted into the oocyte membrane to measure the TMC when the membrane potential was clamped at −50 mV during recordings. Recording of TMC was synchronized with US application using the voltage clamp trigger signals to allow continuous measurement before, during, and after US (Deng et al. 2004) at a sampling rate of 1.0 kHz. The whole cell clamp configuration measures the ion movement through all of the pores generated on the cell membrane.
Experimental design for single-pore generation
We assume that cavitation (i.e., US induced bubble oscillation and collapse) (Zhou et al. 2008a) provides the primary mechanism for sonoporation in this study. Previous work has suggested that the microbubbles which facilitate cavitation must be close to the cell membrane to be effective in generating pores (Guzman et al. 2003). Below we describe the design of experimental condition to ensure generation of a single pore in our study.
The number of homogeneously distributed microbubbles within a given volume surrounding the cell follows a Poisson distribution. Assuming that each pore is generated by one bubble, the probability of having N pores (by N porating bubbles) on the membrane is described by a Poisson distribution (Walpole et al. 2007)
| (1) |
where λ is the mean value of the number of pores. The probability of having no pores is described by p0 = f(0;λ) and p1 = f(1;λ) describes the probability of having one pore. Thus the probability of having at least one pore (N ≥ 1) is
| (2) |
Furthermore, the conditional probability of having one pore whenever poration occurs is
| (3) |
Therefore generation of a single pore can be ensured in experiment if a high level (e.g., >95%) of p1|pore is maintained.
On the other hand, ppore can be calculated as the ratio of the number of the experimental tests in which a change of TMC is measured (ncurr) over the total number of tests ntot, or ncurr/ntot. Eqn. (2) allows an experimental determination of λ, or the mean of the Poisson distribution in Eqn. (1), and p1|pore is then determined from Eqn. (3). Obviously, decreasing the microbubble concentration reduces ppore and the likelihood of generating only one pore (p1|pore) increases consequently. Single pore sonoporation can be achieved by using sufficiently low bubble concentration to ensure an appropriately low value of ppore, thus high value of p1|pore.
Model
The change of the TMC during sonoporation results from ion transport through the non-specific pore(s), resulted from ion concentration and electrical potential gradient across the membrane. Of all the ions present, significant contribution to the TMC only comes from Na+, K+, and Cl− (Costa et al. 1989). The current density (current per unit area) of the kth ion is calculated via the GHK current equation (Hille 2001):
| (4) |
where Uk = zkFV/RT; zk are the ion valencies; F, the Faraday constant; V, the membrane potential in relation to the exterior surface of the membrane; R, the ideal gas constant, T, the temperature; Pk = DkKk/h, the ion permeabilities; Dk, the ion diffusion coefficients; Kk, the ion partition coefficients; ckin and ckex, the intra- and extracellular ion concentrations; and h, the membrane thickness. If itot is the total current density, then the total TMC is I = itot(πr2) where r is the (effective) radius of the pore, and hence
| (5) |
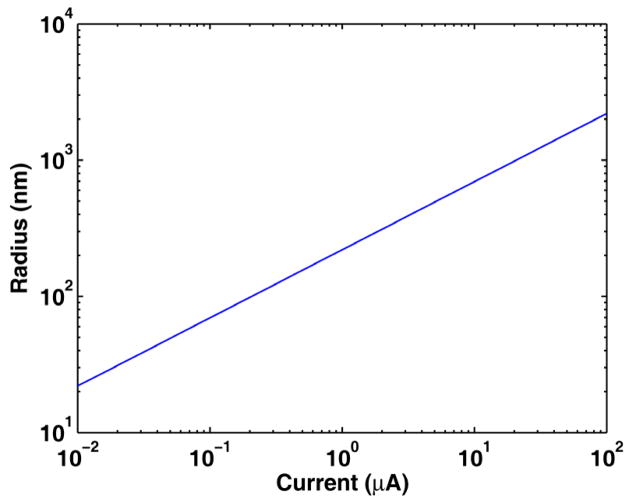
Figure 1 shows the pore radius as a function of pore TMC using Eqs. (4)–(5) with Pkpore. Table 1 lists the parameters used in calculation in the model. In addition, V = −50 mV, h = 5 nm (DeBruin and Krassowska 1999), and T = 293 K. In the case of poration, it is assumed Kk = 1 (no membrane in the pore), and hence Pkpore = Dk/h.
Figure 1.
Maximum pore radius as a function of the maximum transmembrane current amplitude computed using Eqs. (4)–(5) with Pkpore.
Table 1.
Model Parameters
| Ion | ckex [mM]* | ckin [mM]† | Dk [m2/s]‡ | Pk [m/s]† | Pkpore [m/s]§¶ |
|---|---|---|---|---|---|
| Na+ | 96 | 10.1 | 1.33×10−9 | 3.75×10−10 | 0.266 |
| K+ | 2 | 109.5 | 1.96×10−9 | 9.55×10−10 | 0.392 |
| Cl | 100 | 37.7 | 2.03×10−9 | 3.40×10−10 | 0.406 |
For ND96;
From Costa et al (1989);
From Cussler (1997);
Using Dk from Cussler (1997) with h = 5 nm
RESULTS AND DISCUSSION
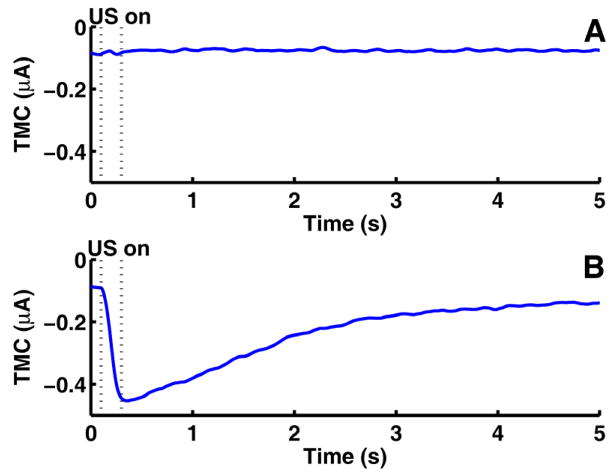
Typical TMC measurements from control and test group experiments are shown in Figure 2. The start and end of the ultrasound application is indicated by the vertical dotted lines. Figure 2A shows a typical control data with ultrasound application without microbubbles in the extracellular solution. Similarly, control experiments with no ultrasound (with and without microbubbles) exhibited no TMC change (data not shown). Figure 2B shows the result of a typical test experiment with ultrasound applied in the presence of microbubbles. The change of TMC was defined as the difference between the mean TMC before ultrasound was applied and the maximum amplitude of (inward) TMC measured. The TMC usually reaches its maximum value during or shortly after the end of the ultrasound pulse and then gradually recovers to its pre-ultrasound value over 5 to 20 s. For this study, the maximum TMC change was of primary interest as it corresponded to the maximum pore size.
Figure 2.
(A) Typical transmembrane current (TMC) for control experiment with ultrasound application but no microbubbles introduced. (B) Typical TMC for test experiment with ultrasound application with microbubbles introduced.
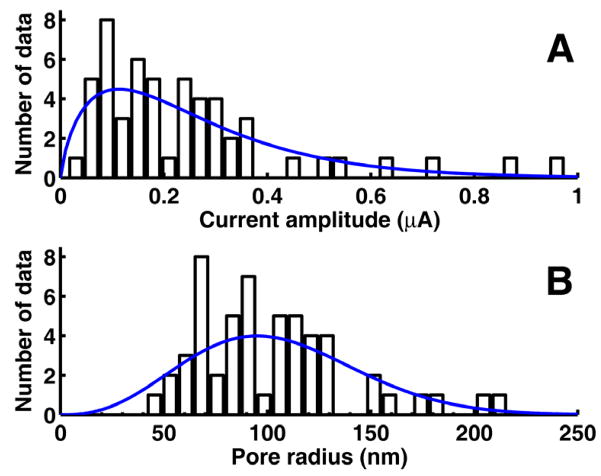
A total of 579 (ntot) tests were conducted under identical conditions for measuring TMC in sonoporation. Of these tests, TMC change was observed in 54 (ncurr) tests. Therefore, ppore = 54/579 = 0.0933, λ = 0.0979 from Eqn. (2), and p1|pore = 95.2% from Eqn. (3). Thus the measured TMC in these experiments very likely resulted from only a single pore on the membrane during sonoporation. Figure 3A shows the histogram of the maximum amplitude of the TMC measured from the set of experimental tests described above. The mean TMC is 261.4 nA (median 199.0 nA) with a standard deviation (SD) of 196.9 nA. The histogram is shown with a gamma distribution (shape = 1.76, scaling = 0.148) with the same mean, variance, and area as the measured histogram. Figure 3B shows the corresponding distribution of pore sizes computing via Eqs. (4)–(5) (cf. Fig. 1). The mean radius of single pores is 110 nm (median 98 nm) with SD of 40 nm. The curve is the size distribution corresponding to the gamma distribution in Fig. 3A transformed using Eq. (5) by the standard method (Walpole et al. 2007).
Figure 3.
(A) Measured histogram of the maximum change in TMC from single pore data along with fitted curve. Bin size = 0.03 μA, sample size n = 54. (B) Corresponding calculated histogram of maximum pore radii based on Fig. 1. Bin size = 7.5 nm.
Our estimates of pore size are in the same range as the previous studies obtained by SEM, which was reported to be ~50–75 nm (Mehier-Humbert et al. 2005), ~500 nm (Schlicher et al. 2006), and 500–2500 nm (Zhao et al. 2008) in radius. One advantage of our approach is the ability to estimate more readily the size of rapidly-closing or completely-closed pores without necessarily performing post-US assays. The effective range for a microbubble to induce sonoporation can also be estimated. With a spherical oocyte of diameter 1.1 mm, there is an average of only one bubble in a shell volume of a thickness Δhsb = 44 μm around the oocyte at bubble concentration of 6 × 103/mL. Pores occur only 9.33% of the time in our experiments, suggesting an effective range Δhpore = 4.1 μm.
Sonoporation is believed to create on the cell membrane non-selective pores, wherein the potential gradient and ion concentration are generally unknown. It is thus difficult to assess the strict applicability of the GHK current equation, which assumes a linear potential gradient across the membrane, no interaction between ions, no convection, and steady state conditions. Particularly if a large pore is created (diameter ≫ membrane thickness), it is difficult to know if the potential gradient is linear without detailed numerical simulations. Nevertheless, the GHK current equation should be applicable on a quasi-static basis because the characteristic ion diffusion time between the intra- to extracellular spaces is typically very fast (~μs), while the TMC in sonoporation typically recovers in ~10–20 s (Deng et al. 2004; Zhou et al. 2008b). Although convection due to bubble collapse may occur, its effect will also be very fast (~μs), after which electro-diffusion should be the dominant transport mechanism.
It is possible that US could enhance permeation of the membrane without creating large physical pores. In this case, the effect of sonoporation could be modeled by using an effective or equivalent permeability for the whole cell membrane. This effective permeability will have a value between the normal, pre-US permeabilities Pk and the pore permeabilities Pkpore (where Kk = 1, indicating no membrane) listed in Table I, as it is essentially the average of the pore permeability over the whole membrane. Even without precise knowledge of the physical picture or mechanisms responsible for the enhanced permeability resulted from US exposure, the estimation of pore size obtained using our method will be the upper bound, if consideration is given to the possibility of other sources of contribution to the measured TMC change.
In conclusion, this study reports results of pore size obtained from the TMC measured using the voltage clamp technique in single-pore sonoporation experiments. The TMC measurements were related to pore size using the GHK current equation, providing a simple approach to estimate pore size in sonoporation. The estimates of pore size computed from the TMC measurements are consistent with the proposed hypothesis and in the same range as previous studies using SEM (Mehier-Humbert et al. 2005; Schlicher et al. 2006; Zhao et al. 2008).
Acknowledgments
This work was supported in part by the National Institutes of Health (R01CA116592 to C. X. Deng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Costa PF, Emilio MG, Fernandes PL, Ferreira HG, Ferreira KG. Determination of ionic permeability coefficients of the plasma membrane of xenopus laevis oocytes under voltage clamp. J Physiol. 1989;413:199–211. doi: 10.1113/jphysiol.1989.sp017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussler EL. Diffusion: Mass transfer in fluid systems. New York, NY: Cambridge University Press; 1997. [Google Scholar]
- DeBruin KA, Krassowska W. Modeling electroporation in a single cell. I. Effects of field strength and rest potential. Biophys J. 1999;77(3):1213–1224. doi: 10.1016/S0006-3495(99)76973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30(4):519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Guzman HR, McNamara AJ, Nguyen DX, Prausnitz MR. Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: A unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound Med Biol. 2003;29(8):1211–1222. doi: 10.1016/s0301-5629(03)00899-8. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sunderland, MA: Sinauer Associates, Inc; 2001. [Google Scholar]
- Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J Control Release. 2005;104(1):213–222. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Ross JP, Cai X, Chiu JF, Yang J, Wu J. Optical and atomic force microscopic studies on sonoporation. J Acoust Soc Am. 2002;111(3):1161–1164. doi: 10.1121/1.1448340. [DOI] [PubMed] [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol. 2006;32(6):915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Stuhmer W. Electrophysiologic recordings from xenopus oocytes. Methods Enzymol. 1998;293:280–300. doi: 10.1016/s0076-6879(98)93019-1. [DOI] [PubMed] [Google Scholar]
- Walpole RE, Myers RH, Myers SL, Ye K. Probability & statistics for engineers & scientists. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- Zhao YZ, Luo YK, Lu CT, Xu JF, Tang J, Zhang M, Zhang Y, Liang HD. Phospholipids-based microbubbles sonoporation pore size and reseal of cell membrane cultured in vitro. J Drug Target. 2008;16(1):18–25. doi: 10.1080/10611860701637792. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui J, Deng CX. Dynamics of sonoporation correlated with acoustic cavitation activities. Biophys J. 2008a;94(7):L51–53. doi: 10.1529/biophysj.107.125617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shi J, Cui J, Deng CX. Effects of extracellular calcium on cell membrane resealing in sonoporation. J Control Release. 2008b;126(1):34–43. doi: 10.1016/j.jconrel.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]