Summary
Calorie restriction (CR) increases lifespan in organisms ranging from budding yeast through mammals. Mitochondrial adaptation represents a key component of the response to CR. Molecular mechanisms underlying this adaptation are largely unknown. Here we show that lysine acetylation of mitochondrial proteins is altered during CR in a tissue-specific fashion. Via large-scale mass spectrometry screening, we identify 72 candidate proteins involved in a variety of metabolic pathways with altered acetylation during CR. Mitochondrial acetylation changes may play an important role in the pro-longevity CR response.
Keywords: calorie restriction, longevity, sirtuins, mitochondria, metabolism, mass spectrometry
CR is a robust means of extending lifespan across a wide variety of species (Anderson & Weindruch 2007). Pharmacomimetics of CR could have far-reaching health benefits in humans. The molecular basis of the CR response remains incompletely understood. Metabolic alterations occurring as part of the adaptation to CR likely underlie the beneficial effects of this intervention (Anderson & Weindruch 2007). These changes implicate alterations in mitochondrial function in the CR response, since many essential metabolic processes occur in this organelle. Here we report that lysine acetylation of mitochondrial proteins is altered during CR in a tissue-specific manner. Since reversible acetylation is a well-characterized post-translational modification impacting protein biology, this finding offers one potential mechanism of how mitochondrial function may be regulated during CR.
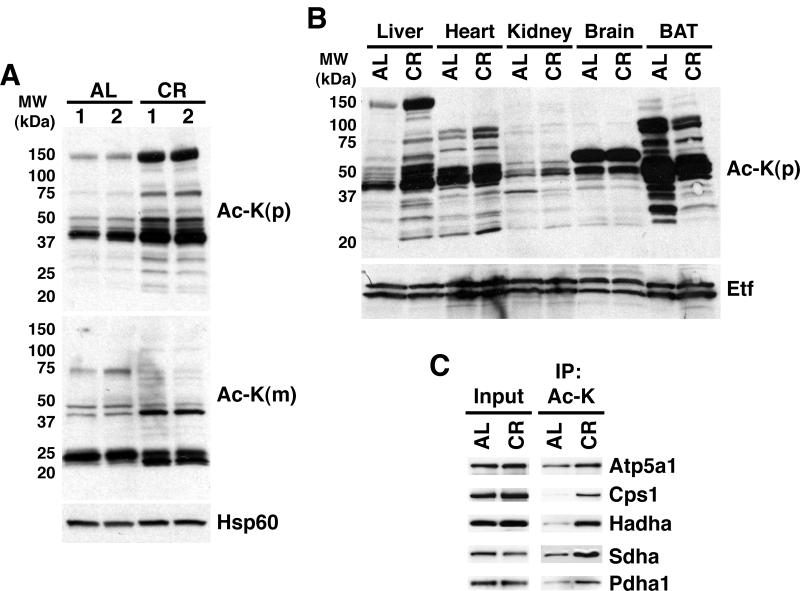
We assessed protein acetylation in purified liver mitochondria from 8-month-old C57BL/6 mice subjected to stepwise CR - 10% restriction initiated at 14 weeks of age, followed by 25% restriction at 15 weeks, and then 40% restriction at 16 weeks and thereafter - as well as from age-matched ad lib fed (AL) isogenic controls. This CR regimen provides robust lifespan extension in C57BL/6 mice (Turturro et al. 1999). CR was associated with dramatic changes in acetylation in liver mitochondria (Fig. 1A), with the majority of changes consisting of increases in acetylation. We then screened mitochondria from a panel of tissues for acetylation (Fig. 1B). In heart, kidney, and brain, only subtle changes in mitochondrial protein acetylation occurred during CR. By contrast, in brown adipose tissue (BAT), CR led to markedly decreased mitochondrial acetylation. Thus, mitochondrial acetylation is regulated in liver and BAT during CR.
Fig. 1.
Calorie restriction alters mitochondrial acetylation. (A) Liver mitochondrial acetylation in CR. Mitochondrial extracts were generated from either CR or AL mice, fractionated by SDS-PAGE, and probed with the indicated antibodies. Ac-K, anti-acetyl-lysine. p=polyclonal, m=monoclonal. (B) Tissue-specific changes in mitochondrial acetylation. Mitochondria were prepared from indicated tissues of CR and AL mice and probed as in panel A. (C) Identification of proteins hyperacetylated in CR. Total acetylated proteins were immunopurified from AL and CR mitochondrial extracts and probed for the indicated proteins. Each panel represents mitochondrial extracts derived from a single AL/CR pair and is representative of at least three independent experiments.
To identify mitochondrial proteins changing in acetylation during CR, a large-scale proteomics survey was performed. Liver mitochondrial extracts from AL and CR animals were digested with trypsin and immunopurified with acetyl-lysine affinity matrix. Acetylated peptides were subsequently analyzed by mass spectrometry and label-free quantitation (LFQ) (Rush et al. 2005). We identified a total of 287 unique acetylated proteins, of which at least 165 are mitochondrial (see Methods section for further details) (Supplemental Table 1). Among this latter group, LFQ predicted 72 candidates as changing in acetylation during CR by at least 2.5-fold (Supplemental Table 2). We also identified a number of potentially significant acetylation changes that did not meet these strict criteria; i.e., acetylation changes from 2.0 up to 2.5-fold (Supplemental Table 3).
To validate the LFQ findings, we performed immunoprecipitation (IP) with acetyl-lysine affinity matrix followed by immunoblot (IB) using commercially available antibodies against candidate proteins. We confirmed hyperacetylation of five of these proteins, which are involved in a variety of biochemical pathways, namely ATP generation (ATP5a1), urea cycle (CPS1), lipid metabolism (HADHA), glycolysis/Krebs cycle (PDHA1), and Krebs cycle/electron transport (SDHA) (Fig. 1C). For 21 other proteins tested, we were unable to obtain immunologic reagents of sufficient quality to definitively assess acetylation.
Of note, in contrast to our findings, a recent study reported that CPS1 acetylation is decreased in mice during CR (Nakagawa et al. 2009). In support of our LFQ and IP results, acetyl-lysine IB of CPS1 purified by IP also revealed hyperacetylation of this enzyme in CR (Fig. S1). The cause of the discrepancy between our findings and those of Nakagawa et al. is unclear.
Sirtuin family deacetylases are required for increased longevity in response to some CR regimens in invertebrates (Schwer & Verdin 2008). The three mammalian mitochondrial sirtuins -- SIRT3, SIRT4, and SIRT5 -- are logical candidates to mediate acetylation changes we observe in liver mitochondria in response to CR (Schwer & Verdin 2008). We previously showed that SIRT3 plays an important role in deacetylating many mitochondrial proteins (Lombard et al. 2007). Expression levels of mitochondrial sirtuins were compared in liver mitochondria from mice on AL or CR diets (Fig. S1). CR elicited a moderate increase in SIRT3 protein levels, consistent with mRNA expression profiling results (Han et al. 2000). SIRT4 protein levels were slightly decreased, whereas SIRT5 protein levels did not change during CR, in agreement with previous reports (Fig. S1) (Haigis et al. 2006; Nakagawa et al. 2009). It is unlikely that this reduction in SIRT4 levels plays a role in mediating the acetylation increases we observe during CR, since SIRT4-deficient mice show no gross alterations in hepatic mitochondrial acetylation (Lombard et al. 2007). Despite increased SIRT3 expression, SIRT3 activity could be modulated in the context of CR by means other than changes in protein levels: i.e., mitochondrial NAD+ content, post-translational modifications, and/or interacting partners. Further studies will be required to identify enzymes (deacetylases and acetyltransferases), or putative non-enzymatic processes, that modulate lysine acetylation of mitochondrial proteins during CR.
Here we show that mitochondrial protein acetylation is altered dramatically in a tissue-specific fashion during CR. These acetylation changes may have important functional consequences for activity, complex formation, turnover, or other aspects of protein biology. Recent work indicates that lysine acetylation is present in E. coli, and is altered in response to diverse environmental stresses, including starvation (Zhang et al. 2009). In mammals, acute fasting and chronic ethanol ingestion also alter hepatic mitochondrial protein acetylation (Kim et al. 2006; Picklo 2008), suggesting that changes in acetylation represents an ancient, evolutionarily conserved mechanism for adaptation to metabolic/nutritional stress. Activities of a wide variety of mitochondrial enzymes have been shown to change during CR (Tillman et al. 1996; Dhahbi et al. 2001; Hagopian et al. 2003; Hagopian et al. 2004; Hagopian et al. 2005). The targets we validated are involved in a wide range of biological activities. This suggests that lysine acetylation changes may have far-reaching effects on mitochondrial function.
Supplementary Material
Acknowledgments
F.W.A. is on the scientific advisory board of Sirtris Pharmaceuticals and an Investigator of the Howard Hughes Medical Institute. This work was supported by an Ellison Medical Foundation/American Federation for Aging Research Senior Postdoctoral Fellow Research Grant (B.S.); an Ellison Foundation Senior Scholar Award (F.W.A); and an NIA/NIH K08 award (AG022325), a Hartford Foundation grant, and startup funds from the University of Michigan (D.B.L). The authors would like to thank the following individuals for comments on previous versions of this manuscript: Andrezj Bartke, Anne Brunet, Matt Kaeberlein, Brian Kennedy, Richard Miller, Raul Mostoslavsky, and Heidi Tissenbaum.
References
- Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Wingo J, Rowley BC, Cao SX, Walford RL, Spindler SR. Caloric restriction alters the feeding response of key metabolic enzyme genes. Mech Ageing Dev. 2001;122:1033–1048. doi: 10.1016/s0047-6374(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Harper ME, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp Gerontol. 2003;38:267–278. doi: 10.1016/s0531-5565(02)00202-4. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Krebs cycle enzymes from livers of old mice are differentially regulated by caloric restriction. Exp Gerontol. 2004;39:1145–1154. doi: 10.1016/j.exger.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Han E, Hilsenbeck SG, Richardson A, Nelson JF. cDNA expression arrays reveal incomplete reversal of age-related changes in gene expression by calorie restriction. Mech Ageing Dev. 2000;115:157–174. doi: 10.1016/s0047-6374(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picklo MJ., Sr. Ethanol intoxication increases hepatic N-lysyl protein acetylation. Biochem Biophys Res Commun. 2008;376:615–619. doi: 10.1016/j.bbrc.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Tillman JB, Dhahbi JM, Mote PL, Walford RL, Spindler SR. Dietary calorie restriction in mice induces carbamyl phosphate synthetase I gene transcription tissue specifically. J Biol Chem. 1996;271:3500–3506. doi: 10.1074/jbc.271.7.3500. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, Zhao Y. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.