Abstract
Introduction
Approximately 23% of melanoma patients will eventually develop pulmonary metastases and have a median survival of only about 7-11 months. Because pulmonary metastasectomy can improve this statistic, we investigated clinicopathologic features and biological correlates that might be used to identify surgical candidates.
Methods
Archived operative specimens and clinical records were retrieved for 20 melanoma patients who underwent resection of isolated pulmonary metastases at the John Wayne Cancer Institute, Saint John’s Health Center. Five-year post-metastasectomy survival (PMS) rate was correlated with age, number of pulmonary metastases, tumor doubling time (TDT), tumor necrosis, and immunohistochemical expressions of four biological markers: Ki-67, glucose transporter-1 (Glut-1), caspase-3, and CD31.
Results
The median TDT was 61 days. By multivariate analyses, TDT (P=0.008), Glut-1 intensity (P=0.04) and CD31 expression (P=0.004) were the significant predictors of PMS. Age, number of pulmonary metastases, tumor necrosis, and expression of Ki-67 or caspase-3 did not significantly impact survival. Median TDT was 56 days with Glut-1 expression versus 165 days without Glut-1 expression (P=0.002), and Glut-1 staining intensity independently affected TDT (P=0.012).
Conclusions
Surgical resection may be preferable to toxic systemic therapies in melanoma patients whose isolated pulmonary metastases have a long TDT (≥61 days) and no biopsy evidence of Glut-1 expression.
SYNOPSIS
Surgical resection can be useful in a subset of melanoma patients with pulmonary metastasis. Data from this preliminary study suggest that patients whose tumors have longer doubling time and/or lower glucose utilization may benefit the most from surgery.
Keywords: Melanoma, pulmonary metastasis, metastasectomy, biological factors
INTRODUCTION
Both incidence and mortality of melanoma continue to rise rapidly in the United States. In 1997, the projected annual incidence and mortality from melanoma were 40300 and 7300, respectively.1 By 2008, these numbers had jumped to 62480 and 8420, respectively.2 Although early-stage melanoma can be cured by surgery alone, there is no effective treatment for metastatic melanoma. Despite anecdotal reports of success, adjuvant treatments such as chemotherapy or radiation therapy have not been significantly effective for advanced disease. Recent advances in immunotherapies, such as interferon-alfa (IFNα-2b) and interleukin-2 (IL-2), have shown some encouraging results in small cohorts of patients, resulting in approval of these agents by the United States Food and Drug Administration.3, 4 However, despite such advances and numerous other clinical trials, the prognosis for advanced-stage melanoma patients remains poor. The presence of distant metastasis marks the beginning of stage IV melanoma, resulting in median survival of 6-8 months.5-8 Although 5-year overall survival (OS) rate for stage IV melanoma patients is approximately 5-8%, evidence suggests that successful surgical excision of isolated metastatic lesions is associated with higher 5-year OS.6-11 Data from a multicenter phase III trial of postoperative adjuvant immunotherapy for patients with stage IV melanoma demonstrated 5-year survival rates of approximately 40-45% after complete resection.12 Given the lack of uniformly effective systemic therapy for stage IV melanoma, many have advocated surgery for selected patients who can undergo complete, potentially curative resection.8-13
The lung is one of the most common sites of initial recurrence, accounting for approximately 36-42% of initial metastases.9, 14 Approximately 23% of all melanoma patients will eventually develop pulmonary metastasis.8 While median survival is reportedly about 7-11 months after detection of pulmonary metastasis, significant improvement in survival has been noted after successful pulmonary metastasectomy.8-10, 15, 16 For stage IV melanoma, surgery for palliative intent has relatively clear indications, however indications for surgery in asymptomatic patients remain uncertain. Studies have identified several factors that are associated with improved prognosis after surgical resection. Investigators noted that the lung is a relatively favorable low-risk site of metastasis,9, 15, 17 and that number of metastatic lesions and organ sites, and disease-free interval are significant prognostic factors after surgical resection.8, 9, 11 Petersen et al. also showed that complete pulmonary metastasectomy is an independent prognostic factor, with hazard ratio (HR) of 0.5.8 These findings suggest that the biology of metastatic disease, such as tumor growth rate, may be the underlying factor in determining prognosis, and that resection of biologically favorable disease may improve survival. Indeed, Ollila et al. proposed a tumor doubling time (TDT) of 60 days as a cutoff point for pulmonary metastasectomy.16
There has been an increasing emphasis on developing in-vitro and in-vivo molecular markers to identify patients with prognostically favorable metastatic disease, who could benefit from more aggressive surgical interventions. The primary goal of this study was to identify some of the biological factors of metastatic disease that can be used to determine which melanoma patients with pulmonary metastasis could benefit from surgical resection. We also evaluated tumor proliferative and glycolytic activities, apoptosis, microvessel density (MVD) and TDT as they relate to each other, and correlated these factors with survival.
MATERIAL AND METHODS
Patient Population, Clinicopathologic Features, and Molecular Profiling
The John Wayne Cancer Institute (JWCI) database was queried to identify patients with stage IV melanoma confined to the lung. Resected metastatic lesions from 20 of these patients (1986-1996) were retrieved from archival storage. TDT of the pulmonary lesions was calculated from chest x-rays, as described elsewhere.18 Five-yr post-metastasectomy survival (PMS) was measured and correlated with clinicopathologic features (age, number of metastases, and TDT) and with the following biological correlates: immunohistochemical (IHC) detection of Ki-67 (proliferative activity), Glut-1 (glycolytic activity), caspase-3 (apoptotic activity), and/or CD31 (MVD) in pulmonary metastases; and hematoxylin and eosin (H&E) assessment of tumor necrosis. Use of specimens and clinical data in this study was approved by the institutional review board.
Immunohistochemical Techniques (Table 1)
TABLE 1.
Immunohistochemical staining methods
| Antibody | Antibody Clone | Antibody Titer | Primary Antibody Incubation Time | Staining Platform | Antigen Retrieval Method | Detection System |
|---|---|---|---|---|---|---|
| CD31 | 1A10 | Pre-diluted | 32 Minutes | Benchmark / Ventana | CC1 Standard Conditioning | iView -HRP( Avidin-Streptavidin) - Ventana |
| Glut-1 | Polyclonal | 1:50 | 32 Minutes | Ventana | CC1 Standard Conditioning | iView -ALK( Avidin-Streptavidin) - Ventana |
| Ki-67 | Mib 1 | 1:50 | 30 Minutes | Dako Autostainer | Citrate Decloaker, 20 min | EnVision+ System-HRP - Labeled Polymer - Dako |
| Caspase-3 | Polyclonal | Pre-diluted | 30 Minutes | Dako Autostainer | BORG Decloaker, 20 min | MACH 3 -AP - BioCare |
IHC detection of Glut-1, caspase-3, Ki-67, and microvessel counts was performed by using DakoCytomation and Ventana (Benchmark) Auto-stainers. IHC was performed on 4-μm thick sections cut from formalin-fixed, paraffin-embedded tissue blocks. Sections were mounted on slides, deparaffinized in xylene, and washed in graded ethanols. Antigen retrieval included 20 minutes of boiling in a citrate buffer (pH 6.0) for Ki-67, a Borg solution for caspase-3, and EDTA Buffer for CD31 and Glut-1. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 5 minutes followed by rinsing in PBS buffer. Non-specific staining was blocked by incubation with normal goat serum for 5 minutes. Primary antibody incubation was performed using a pre-diluted monoclonal CD31 antibody (clone 1A10; Ventana, Tucson, AZ), Ki-67 antibody (clone Mib1; DakoCytomation, Carpinteria, CA) at 1/50 dilution, a polyclonal Glut-1 antibody (Lab Vision, Fremont, CA) at 1/50 dilution, and a pre-diluted polyclonal caspase-3 antibody (BioCare, Walnut Creek, CA). CD31 and Glut-1 sections were then incubated sequentially with an iView-HRP (Avidin-Streptavidin Complex) and iView -ALK Detection Systems (Ventana, Tucson, AZ) for 16 minutes. Mouse EnVision+ System-HRP (Labeled Polymer) and Rabbit EnVision+ System-AP (DakoCytomation, Carpinteria, CA) were used as a detection system for Ki-67 and caspase-3 stains, sequentially. Between incubations, the slides were rinsed with TBS buffer. A DAB or Vulcan Red was used as a chromogen and slides were counterstained with hematoxylin. Positive and negative controls were run in parallel with each series of slides to ensure appropriate staining. The negative control consisted of non-immune mouse IgG substituted for the primary antibody.
Staining Assessment
Light microscopy was used to identify three regions within or immediately adjacent to the tumor that contained the greatest MVD (“hotspots”). Microvessel counts were then performed using a 200X field within the designated hotspot. Of the three areas with the highest number of discrete microvessels, the area of greatest counts was chosen for scoring. Any immunoreactive endothelial cell that was separate from adjacent microvessels was considered a “countable” vessel. Glut-1 expression was reported by staining intensity in a following manner: 0, cytoplasmic staining not detectable; 1+, cytoplasmic staining translucent; 2+, cytoplasmic staining opaque; and 3+, cytoplasmic staining solid. Ki-67 and caspase-3 staining results were scored as a percentage of proliferating and apoptotic tumor cells, respectively. Necrosis was estimated as a percentage of necrotic tumor area.
Statistical Analyses
Univariate analyses were done by using Spearman’s rank correlation for ordered categorical variables, and Wilcoxon rank sum test for comparing continuous variables. Univariate and multivariate analyses of linear regression and logistic regression were performed to identify the biological factors that affect TDT. Univariate and multivariate survival analyses were done by using Cox proportional hazard regression models. Statistical analyses were performed by using SAS 8.2 (Cary, NC).
RESULTS
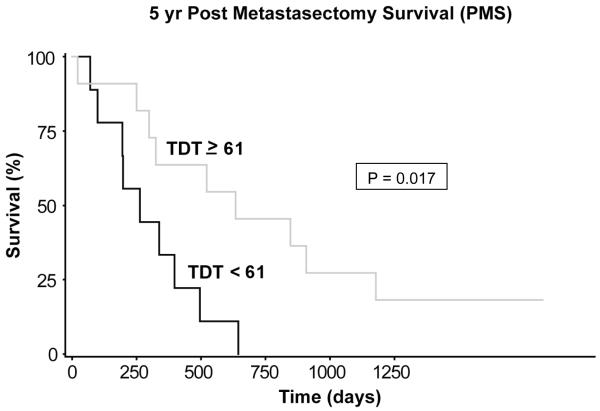
Of the 20 tissues analyzed, 15 were from males, and 5 were from females. The median age of the patients was 58.5 years (range, 36 - 75 years). Median number of pulmonary lesions was 1 (range, 1 - 6; mean, 2.0 ± 1.7). The median TDT was 61 days (range, 21 - 287 days). The follow-up period ranged from 8 to 26 years. By univariate Cox regression analysis using the median values, among the three clinicopathologic features (age, number of metastases, and TDT), only TDT was significantly associated with differential survival (P = 0.008). Using the median TDT value as the cutoff point (< 61 vs. ≥ 61), TDT ≥ 61 days was significantly associated with better 5-yr PMS (hazard ratio [HR], 0.36; 95% confidence interval [CI], 0.08 - 0.78; P = 0.017; Figure 1). Median PMS time was 8.7 months for TDT < 61 vs. 20.9 months for TDT ≥ 61. The median DFS (disease free survival) times for the two groups were 5 months and 6.5 months, respectively (P = 0.19). Among the biological correlates (Ki-67, Glut-1, caspase-3, CD31, and necrosis), expressions of Glut-1 and CD31 significantly correlated with differential survival.
Fgure 1.
The impact of tumor doubling time (TDT) on 5-year post-metastasectomy survival (PMS). Using the median TDT value as the cutoff point (< 61 vs. ≥ 61), TDT ≥ 61 days was significantly associated with better 5-yr PMS (HR = 0.36; 95% CI = 0.08 - 0.78; P = 0.017). Median PMS was 8.7 months for TDT < 61, vs. 20.9 months for TDT ≥ 61.
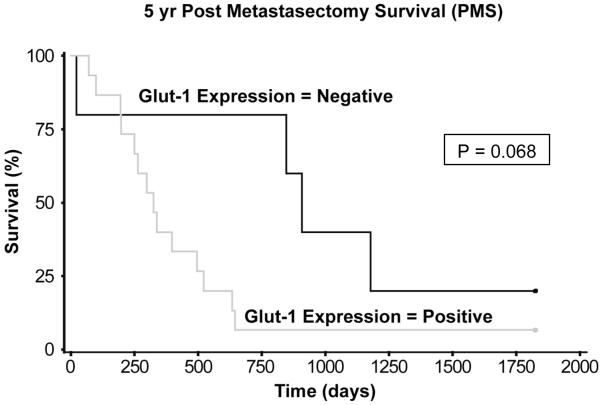
Multivariate survival analysis used median values of four covariates: TDT, Glut-1 staining intensity, CD31 expression, and percent necrosis. Stepwise procedures of multivariate Cox regression analysis including interaction terms were performed to select the best model. The results show that TDT, Glut-1 intensity, and CD31 expression were the best predictors of 5-yr PMS (Table 2). Other studies16, 19 have also shown that TDT is an important factor in determining prognosis.17 Given that TDT is a clinicopathologic feature that may depend on the biology of the disease, we examined the relationship between Glut-1 staining intensity and TDT. Glut-1 staining intensity correlated inversely with TDT (correlation coefficient, r = -0.54; P = 0.012). Additionally, multivariate analysis showed that Glut-1 staining intensity independently affected TDT (P= 0.001). Because Glut-1 expression was associated significantly with longer TDT (median, 56 vs. 165 days; P = 0.002), we examined PMS in the absence of Glut-1 expression. For qualitative analysis of Glut-1, absence of Glut-1 expression showed a trend toward improved PMS (P = 0.068; Figure 2).
TABLE 2.
Multivariate Cox proportional hazard regression analysis: factors significant for 5-year survival after metastasectomy
| Factor | Median Value* | Range | Hazard Ratio | P value |
|---|---|---|---|---|
| Tumor Doubling Time | 61 days | 21 - 287 days | 0.165 | 0.008 |
| Glut-1 Intensity | 1.5+ | 0 - 2.5+ | 3.558 | 0.046 |
| Max CD31 count | 76/field | 32 - 146/field | 0.147 | 0.004 |
Used for regression analysis
Figure 2.
The impact of Glut-1 expression on 5-year post-metastasectomy survival (PMS). Qualitative analysis show that absence of Glut-1 expression showed a trend towards better 5-yr PMS (Hazard ratio, 0.39; 95% C.I., 0.14 - 1.07; P = 0.068). Median PMS was 10.8 months for positive Glut-1 expression vs. 30.0 months for negative Glut-1 expression.
DISCUSSION
Although only approximately 6% of melanoma patients initially present with stage IV disease, approximately 30-35% of all melanoma patients will eventually develop distant metastatic disease.6 The current NCCN (National Comprehensive Cancer Network) guideline lists “Clinical Trial” as the preferred mode of therapy for metastatic melanoma, reflecting lack of a uniformly effective therapeutic regimen for these patients and no consensus on optimal management.20 Surgery has been advocated for patients with slow-growing tumors, and there is evidence that the degree of metastatic spread and the interval between initial diagnosis and detection of stage IV disease are significantly associated with differential PMS.8-13 While the relationship between biology of disease and extent of metastatic spread might be intuitively obvious, how biology of disease affects interval duration remains somewhat enigmatic.
In patients with pulmonary lesions, one of the most well studied indicators of tumor biology after surgical resection is TDT.18 Ollila et al. demonstrated that median survival after pulmonary metastasectomy correlated positively with TDT.16, 21 Our results confirm the prognostic significance of TDT in patients with pulmonary metastases (Table 2). A cutoff based on our median TDT of 61 days is in agreement with a previously reported cutoff of 60 days.16 However, although we found that a TDT < 61 days was significantly associated with poor 5-yr PMS (Figure 1), measurement of TDT is not always practical because an interval of 2 to 3 months may be necessary to obtain the serial scans used for accurate calculation of the growth rate of metastatic lesions. In an effort to identify characteristics that can be measured without delaying surgical intervention, we examined several biological factors that can be assessed when stage IV disease is diagnosed and can impact prognosis. We hypothesized that consideration of these factors might be a fast and accurate means of determining the operability of metastases.
We examined the individual components of tumor growth kinetics that might determine clinical TDT, which is mainly a net result of tumor cell division minus tumor cell loss. The factors that can contribute to tumor growth include cellular proliferative rate and vascularity of the tumor, which enables the delivery of adequate nutrients. Therefore we examined the expression of Ki-67 and CD31 (as a measure of MVD) within the tumor. Ki-67 is a nuclear antigen present only in the nuclei of proliferating cells, and there are good correlations between Ki-67 and S-phase indices by flow cytometry.22, 23 Conversely, the factors that can contribute to tumor shrinkage include apoptotic and necrotic cell death. Caspase-3 is one of the effector caspases and is responsible for most of the cleavages that disassemble the cell in the process of apoptosis.24 We found no significant relationship between necrosis, Ki-67 or caspase-3 expression and either TDT (data not shown) or survival. A larger sample size might be required to determine the relationships between these factors and tumor growth kinetics.
In our analyses of the biological correlates, a strong negative relationship was found between Glut-1 expression level and PMS. Since we were interested in the biological factors that might impact TDT and/or PMS, instead of just looking at the median numbers as the cutoff points (which is somewhat arbitrary), we wanted to see if the qualitative expression (Positive vs. Negative) of Glut-1 was important. The qualitative analysis showed a difference with P-value of 0.068 (Figure 2). A larger sample size may have been needed to show the statistical significance when analyzed this way. Glut-1 is one of the glucose transporter proteins that account for increased glucose influx in malignant tissues.25 Malignant cells exhibit altered metabolic patterns characterized by increased reliance on anaerobic metabolism of glucose to lactic acid even in the presence of abundant oxygen. Inherent inefficiency of the anaerobic metabolic pathway is compensated for by increased glucose flux, a phenomenon first noted by Otto Warburg approximately 80 years ago.26 The potential role of glycolytic phenotype in facilitating tumor invasion has been investigated through mathematical models of the tumor-host interface.27, 28 Interestingly, although we initially expected that increased angiogenic milieu would favor tumor growth and therefore result in worse outcome, our data show that CD31 expression, a measure of MVD within the tumor microenvironment, favorably influenced PMS. It is possible that tumor cells are so dependent on glycolytic pathway, that additional oxygen may not necessarily benefit their survival. By using the median value as the cutoff point, CD31 expression was associated with an HR of 0.147 (Table 2), arguing against anti-angiogenic therapy for melanoma pulmonary metastases. What is evident from our data is that Glut-1 expression level may be the driving force behind tumor proliferation and subsequently results in decreased TDT. Various associations between Glut-1 expression and tumor aggressiveness have been demonstrated in a number of other malignancies.29 Although many studies have shown that the number of pulmonary metastases adversely impacts outcome, our data could not confirm those findings, most likely due to a small sample size.
Our data show that patients with TDT < 61 days or positive Glut-1 expression had median PMS times of 8.7 months and 10.7 months, respectively. Given the reported median survival of 7.3 months for patients who develop pulmonary metastasis,8 performing pulmonary metastasectomy on patients with TDT < 61 days and/or high Glut-1 expression does not seem to significantly increase survival. On the other hand, patients with TDT ≥ 61 days or low Glut-1 expression showed significantly higher median PMS. Given the significant toxicities of currently available systemic therapies, surgical resection may represent a better option for these patients. Not surprisingly, the median TDTs for the “TDT < 61 Group” and the “TDT ≥ 61 Group” were 47 days and 79 days, respectively (P < 0.001). It is probable that the difference in survival may be partly due to difference in TDTs. However, assuming exponential growths of the tumors, the biggest clinical impact of any given doubling cycle will be seen when the lesion is macroscopically detectable. For example, when a tumor is 0.1 mm in size, one or two tumor doubling cycles will most likely not have any significant clinical impact. However, if the tumor is 3 cm in size, then one or two doubling cycles can have a significant clinical impact. Therefore, even if metastasectomy does not impact directly the biology of the disease, by removing these larger lesions, we can provide the patients with a significant clinical benefit. TDT can be calculated by using measurements obtained from serial chest x-rays or computed tomographic scans. Although Glut-1 expression cannot be measured non-invasively, perhaps tissue from a diagnostic biopsy can be analyzed for Glut-1 expression. If biopsied tissue is not available, then as a radiographic surrogate for Glut-1 expression, F-18fluorodeoxyglucose-positron emission tomography (FDG-PET) can be used for clinical evaluation and quantitation of glycolytic phenotype. Minn et. al. have already shown that standardized uptake value (SUV), a quantitative index for FDG uptake in PET studies, can be predictive of differential prognosis in patients with head and neck squamous cell carcinoma.30 In addition, Burt et. al., using an animal model, have shown that PET SUVs correlated directly (r = 0.82) with the tumor volume doubling time.31 A recent study by Reidl et. al. demonstrates that SUV can correlate with Glut-1 levels and with tumor aggressive behavior.32 Perhaps TDT and Glut-1 expression might help determine a patient’s candidacy for postoperative adjuvant therapy as well. While the role of quantitative FDG-PET in determining operability of the metastatic melanoma is yet to be determined, our data provide reasonable evidence to pursue such study. In addition, our data provide evidence to pursue a larger surgical therapeutic trial for melanoma patients with resectable pulmonary metastasis.
Acknowledgments
Supported by grants P01 CA29605 and P01 CA12582 from the National Cancer Institute and by funding from the Wayne and Gladys Valley Foundation (Oakland, CA), the Amyx Foundation, Inc. (Boise, ID), Berton M. Kirshner (Los Angeles, CA), Todd Kirshner (Los Angeles, CA), Mr. and Mrs. Louis Johnson (Stanfield, AZ), Heather and Jim Murren (Las Vegas, NV), Mrs. Marianne Reis (Lake Forest, CA), the Wallis Foundation (Los Angeles, CA), Dr. Miriam & Sheldon G. Adelson Medical Research Foundation (Santa Monica, CA), and the Lincy Foundation (Beverly Hills, CA).
Footnotes
Presented in part at the annual meeting of the Society of Surgical Oncology,March 3-6, 2005, Atlanta, GA.
References
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 5.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 7.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 8.Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 2007;133(1):104–10. doi: 10.1016/j.jtcvs.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 9.Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139(9):961–6. doi: 10.1001/archsurg.139.9.961. discussion 966-7. [DOI] [PubMed] [Google Scholar]
- 10.Wong JH, Euhus DM, Morton DL. Surgical resection for metastatic melanoma to the lung. Arch Surg. 1988;123(9):1091–5. doi: 10.1001/archsurg.1988.01400330067010. [DOI] [PubMed] [Google Scholar]
- 11.Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol. 1999;71(4):209–13. doi: 10.1002/(sici)1096-9098(199908)71:4<209::aid-jso1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Morton D, Mozzillo N, Thompson J, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. Journal of Clinical Oncology. 2007;25(18S) Abstract 8508. [Google Scholar]
- 13.Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20(23):4549–54. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 14.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma. IV. Prognostic factors in 200 melanoma patients with distant metastases (stage III) J Clin Oncol. 1983;1(2):126–34. doi: 10.1200/JCO.1983.1.2.126. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II) Ann Surg. 1981;193(3):377–88. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ollila DW, Stern SL, Morton DL. Tumor doubling time: a selection factor for pulmonary resection of metastatic melanoma. J Surg Oncol. 1998;69(4):206–11. doi: 10.1002/(sici)1096-9098(199812)69:4<206::aid-jso3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Tafra L, Dale PS, Wanek LA, et al. Resection and adjuvant immunotherapy for melanoma metastatic to the lung and thorax. J Thorac Cardiovasc Surg. 1995;110(1):119–28. doi: 10.1016/S0022-5223(05)80017-0. discussion 129. [DOI] [PubMed] [Google Scholar]
- 18.Morton DL, Joseph WL, Ketcham AS, et al. Surgical resection and adjunctive immunotherapy for selected patients with multiple pulmonary metastases. Ann Surg. 1973;178(3):360–6. doi: 10.1097/00000658-197309000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ollila DW, Morton DL. Tumor doubling time and survival. J Surg Oncol. 1999;71(4):249. doi: 10.1002/(sici)1096-9098(199908)71:4<249::aid-jso10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.NCCN Clinical Practice Guidelines in Oncology. 2007 Melanoma. V.2.2007. [Google Scholar]
- 21.Ollila DW, Morton DL. Surgical resection as the treatment of choice for melanoma metastatic to the lung. Chest Surg Clin N Am. 1998;8(1):183–96. [PubMed] [Google Scholar]
- 22.Pich A, Ponti R, Valente G, et al. MIB-1, Ki67, and PCNA scores and DNA flow cytometry in intermediate grade malignant lymphomas. J Clin Pathol. 1994;47(1):18–22. doi: 10.1136/jcp.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt F, Tani E, Tribukait B, Skoog L. Assessment of cell proliferation by Ki-67 staining and flow cytometry in fine needle aspirates (FNAs) of reactive lymphadenitis and non-Hodgkin’s lymphomas. Cytopathology. 1999;10(2):87–96. doi: 10.1046/j.1365-2303.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- 24.Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278(5336):294–8. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 25.Brown RS, Leung JY, Fisher SJ, et al. Intratumoral distribution of tritiated-FDG in breast carcinoma: correlation between Glut-1 expression and FDG uptake. J Nucl Med. 1996;37(6):1042–7. [PubMed] [Google Scholar]
- 26.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 27.Gatenby RA, Vincent TL. Application of quantitative models from population biology and evolutionary game theory to tumor therapeutic strategies. Mol Cancer Ther. 2003;2(9):919–27. [PubMed] [Google Scholar]
- 28.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 2003;63(14):3847–54. [PubMed] [Google Scholar]
- 29.Gulec SA. A surgical perspective on positron emission tomography. J Surg Oncol. 2007;95(6):443–6. doi: 10.1002/jso.20667. [DOI] [PubMed] [Google Scholar]
- 30.Minn H, Lapela M, Klemi PJ, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38(12):1907–11. [PubMed] [Google Scholar]
- 31.Burt BM, Humm JL, Kooby DA, et al. Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia. 2001;3(3):189–95. doi: 10.1038/sj.neo.7900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedl CC, Akhurst T, Larson S, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48(5):771–5. doi: 10.2967/jnumed.106.037291. [DOI] [PubMed] [Google Scholar]