Abstract
Purpose
To describe fundus autofluorescence (FAF) finding in a case of cone dystrophy.
Methods
Interventional case report.
Results
A 23-year-old woman presented with increasing photophobia and decreasing vision in both eyes for 2 years. Fundus examination showed several drusen-like dots. FAF revealed hyper-autofluorescence in the foveola. Electroretinogram (ERG) demonstrated a pure “cone” dystrophy.
Conclusion
Hyper-autofluorescence in the foveola is a non-specific manifestation of photoreceptor-retinal pigment epithelium dysfunction. ERG studies are essential for accurate diagnosis.
Keywords: Cone dystrophy, Electroretinogram, Fundus autofluorescence, Optical coherent tomography
Text pages
Cone dystrophies are characterized by vision loss, sensitivity to bright light, poor color vision, and electrophysiological or psychophysical evidence of abnormal cone function. In some patients with cone dystrophy, the electroretinogram photopic cone reponses are progressively reduced in the presence of normal rod function.
Cone dystrophies frequently present as bull’s-eye maculopathy, and a doughnut-like zone of atrophic pigment epithelium surrounding a central darker area is commonly observed. Other forms of cone dystrophy show diffuse atrophy of the posterior pole with spotty pigment clumping in the macular area. Due to its genetic heterogeneity, phenocopies from chloroquine/hydroxychloroquine toxicity, and the wide spectrum of fundus changes, ERG testing is essential to the best test for making cone dystrophy diagnosis [1]. Herein, we report fundus autofluorescence imaging (FAF) in a case of cone dystrophy.
Case report
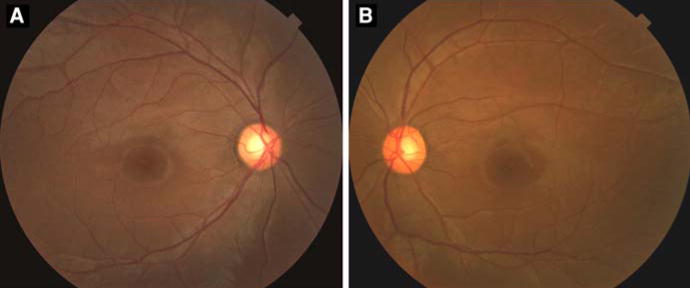
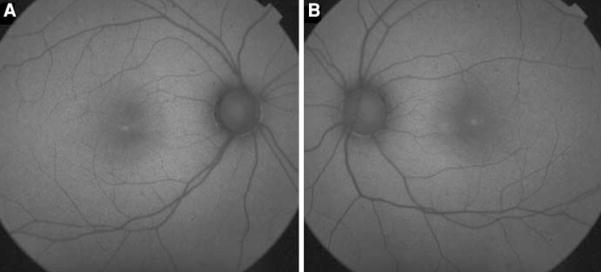
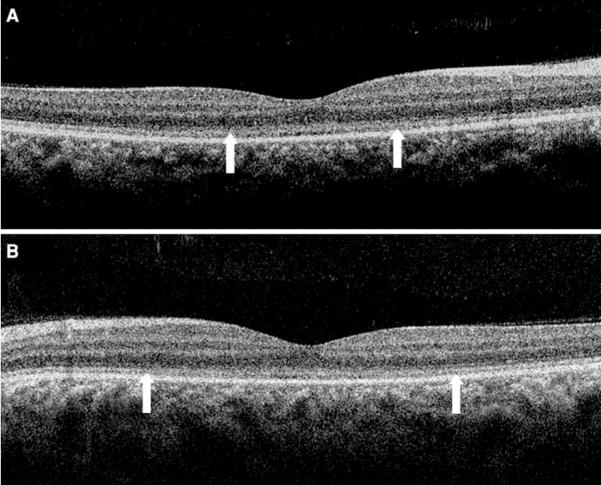
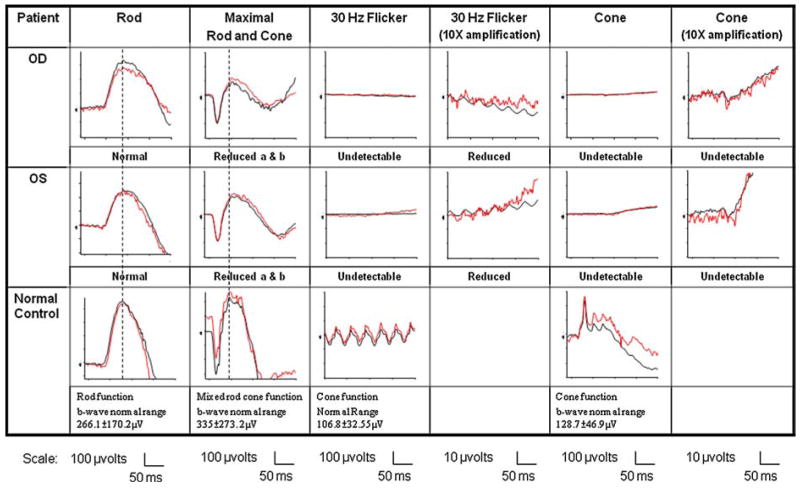
A 23-year-old female with no significant medical history was examined because of increasing photophobia and decreasing vision in both eyes over a period of 2 years. Best-corrected visual acuity was 20/80 in both eyes. Fundoscopic examination revealed several small drusen-like dots around the foveal region (OU) (Fig. 1). Fundus autofluorescence (FAF) imaging revealed a ring of increased autofluorescence and central spots in the foveola (OU) (Fig. 2). Optical coherent tomography (OCT) showed segmentation of the photoreceptor inner segment/outer segment junction in the fovea and no cystic changes (Fig. 3). Electroretinogram revealed generalized depression of cone function and normal scotopic rod response (Fig. 4).
Fig. 1.
Fundus photography (a right eye, b left eye) revealed some small drusen-like dots around the foveal region
Fig. 2.
Fundus autofluorescence imaging (a right eye, b left eye) demonstrated increased autofluorescence ring and central spots in the foveola
Fig. 3.
Optical coherent tomography (a right eye, b left eye) showed segmentation of photoreceptor inner segment/outer segment junction between the two arrows in the fovea and no cystic changes
Fig. 4.
Electroretinogram revealed nearly no detectable cone responses and normal rod responses in both eyes. Black line represents the average of all tracings, and red line denotes values of a single trace
Discussion
The patient had nearly undetectable cone responses and rod monochromatism is a potential differential diagnosis. However, based on the history of the progressive nature of symptoms, absence of nystagmus, and ERG waveforms, the diagnosis is more consistent with autosomal recessive cone dystrophy.
The increased central foveolar autofluorescence in our case mimics Type-1 idiopathic macular telangiectasia, which may be a consequence of reduced macular pigment optical density due to retinal tissue defects in the area with cystoid changes [2]. However, no cystic formations were noted in OCT, and the absence of dilated capillary aneurysms in the foveal area excluded this possibility. The small drusen-like dots in our patient is likely a sign of early foveal atrophy.
Bull’s-eye maculopathy is commonly reported in cone dystrophies. Previous publications acknowledged that some cases showed rod system involvement as the disease progresses [3–5]. Kurz-Levin et al. categorized 47 patients with bull’s-eye maculopathy into three groups based on FAF imaging, and they found that clinical appearance was not helpful in assessing the extent of retinal dysfunction [1]. Moreover, the presence of a perifoveal ring or a central macular area of relatively increased autofluorescence has been noted in FAF imaging for patients with bull’s-eye maculopathy, cone-rod dystrophy, cone dystrophy with supernormal rod response, and rod-cone dystrophy [6, 7]. Increased autofluorescence either in the perifoveal area or central macular area is a non-specific manifestation of cone dystrophy that can occur in other forms of retinal degenerations. Therefore, electrophysiology testing is an indispensable tool for accurate diagnosis of cone dystrophy.
Acknowledgments
Burroughs-Wellcome Program in Biomedical Sciences Fellow, Charles Culpeper Scholarship, Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Joel Hoffmann Foundation, Jonas Family Fund, Crowley Research Fund, Jahnigen/Hartford/American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), and EY018213.
References
- 1.Kurz-Levin MM, Halfyard AS, Bunce C, Bird AC, Holder GE. Clinical variations in assessment of bull’s-eye maculopathy. Arch Ophthalmol. 2002;120:567–575. doi: 10.1001/archopht.120.5.567. [DOI] [PubMed] [Google Scholar]
- 2.Holz FG, Spaide RF, Bird AC. Atlas of fundus autofluorescence imaging. 1. Springer; New York: 2007. pp. 199–205. [Google Scholar]
- 3.Krill AE, Deutman AF. Dominant macular degenerations. The cone dystrophies. Am J Ophthalmol. 1972;73:352–369. doi: 10.1016/0002-9394(72)90064-5. [DOI] [PubMed] [Google Scholar]
- 4.Deutman AF. Benign concentric annular macular dystrophy. Am J Ophthalmol. 1974;78:384–396. doi: 10.1016/0002-9394(74)90225-6. [DOI] [PubMed] [Google Scholar]
- 5.Krill AE, Deutman AF, Fishman M. Doc Ophthalmol. Vol. 35. 1973. The cone degenerations; pp. 1–80. [DOI] [PubMed] [Google Scholar]
- 6.Michaelides M, Holder GE, Webster AR, et al. A detailed phenotypic study of cone dystrophy with supernormal rod ERG. Br J Ophthalmol. 2005;89:332–339. doi: 10.1136/bjo.2004.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson AG, Michaelides M, Luong VA, et al. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br J Ophthalmol. 2008;92:95–102. doi: 10.1136/bjo.2007.124008. [DOI] [PubMed] [Google Scholar]