Summary
Background
There are two O-linked and two N-linked glycosylation sites on the activation peptide of factor X (FX) involving residues Thr-17, Thr-29, Asn-39 and Asn-49.
Objectives
The purpose of this study was to explore the contribution of carbohydrates of the FX activation peptide to zymogen recognition by physiological activators.
Methods
The putative glycosylation sites were substituted individually or in combinations with Ala and mutants were expressed in mammalian cells. The entire activation peptide up to the P3 residue was deleted in another construct.
Results
It was discovered that activation of zymogen mutants by both FVIIa and FIXa on negatively charged phospholipid vesicles has been improved 2-40-fold independent of a cofactor. These mutants were activated with slightly lower catalytic efficiency (kcat/Km) by FVIIa in the extrinsic Xase complex, though both Km and kcat constants for mutants were elevated. With the exception of ∼3-fold improvement in the activation of N49A, the catalytic efficiency of FIXa toward mutants was decreased 2-5-fold in the intrinsic Xase complex.
Conclusions
The carbohydrate chains of the FX activation peptide play an important role in restricting the specificity of zymogen recognition by both FVIIa and FIXa, thereby preventing the cofactor-independent activation of FX by these proteases. On the other hand, the carbohydrates contribute to the cofactor-dependent recognition of the zymogen by both extrinsic and intrinsic Xase complexes.
Keywords: FX, FVIIa, FIXa, extrinsic Xase, intrinsic Xase, glycosylation
Introduction
Factor X (FX) is a vitamin K-dependent coagulation serine protease zymogen in plasma that upon activation to factor Xa (FXa) forms a high affinity complex with factor Va on injured vessels and/or activated platelets to activate prothrombin to thrombin in final stage of the blood clotting cascade [1-3]. The activation of FX by physiological activators, factor VIla (FVIIa) in complex with tissue factor (extrinsic Xase) and factor IXa (FIXa) in complex with factor VIlla (intrinsic Xase) involves proteolytic cleavage of a single peptide bond that releases a heavily glycosylated 52-residue activation peptide [1,4]. An efficient cleavage of this peptide bond by either one of the physiological activators occurs only in the presence of cofactors since neither FVIIa nor FIXa alone can activate FX at a significant rate. The molecular basis for the slow reactivity of these coagulation proteases with FX in the absence of cofactors is not completely understood. There is some evidence in the literature which suggests that neither FVIIa nor FIXa has a fully developed active-site pocket in order to effectively catalyze cleavage of the scissile bond in its substrate. Thus, one hypothesis is that both tissue factor (TF) and factor VIlla (FVIIIa) induce conformational changes in the active-site pocket of these proteases that lead to optimal catalysis of the substrate [5-10]. Several studies, monitoring the cleavage of chromogenic and synthetic peptide substrates by FVIIa and FIXa in free forms or in complex with their respective cofactors have provided strong support for this hypothesis [11,12]. Another mechanism through which coagulation cofactors may improve the catalytic efficiency of their target proteases is to interact with specific sites (exosites) remote from the catalytic pocket of either the protease and/or the scissile bond of the macromolecular substrate to facilitate the formation of a ternary complex on the membrane surface. There are numerous studies in support of a dominant role for this mechanism of cofactor function in essentially all coagulation reactions [13,14]. There are also examples where cofactor-interactive exosites contain patches of similarly charged residues on both the protease and the substrate so that they repulse each other and thus cofactors not only facilitate assembly of a ternary complex, but also eliminate inhibitory interaction of the protease with its substrate. This mechanism of cofactor function has been observed in protein C activation by thrombin where the cofactor thrombomodulin by binding to similarly charged basic residues of exosite-1 of thrombin and 70-80-loop of protein C eliminates repulsive interactions of these exosites, thereby dramatically improving the rate of the catalytic reaction [15,16].
The activation peptide of FX has two O-linked and two N-linked glycosylation sites and previous results using deglycosylated derivatives of FX prepared by various glycanases have supported a role for carbohydrate chains of the activation peptide in zymogen activation by physiological activator complexes [17,18]. However, the exact role of each carbohydrate chain of the FX activation peptide to activation of the substrate by physiological activators has remained unknown. To determine whether the interaction of carbohydrate chains of the FX activation peptide with specific exosites of FVIIa and FIXa in the absence and presence of cofactors regulates the activation of the substrate, we took a mutagenesis-based approach and eliminated glycosylation sites of the activation peptide by substituting the related residues Thr-17, Thr-29, Asn-39 and Asn-49 on the heavy chain individually or in combinations with Ala and expressed the mutant constructs in mammalian cells. Furthermore, we constructed another mutant in which 49 residues of the activation peptide up to the P3 residue (Leu-50) was deleted. Characterization of the zymogenic properties of FX derivatives in appropriate kinetic assays revealed an unexpected critical role for carbohydrates of the activation peptide in restricting the specificity of both FVIIa and FIXa toward FX, thereby ensuring that the zymogen will only be activated when these proteases are in complex with their physiological cofactors. Interestingly, the interaction of intrinsic and extrinsic Xase complexes with the FX activation peptide carbohydrates not only eliminates inhibitory effects, but the energy of binding is also utilized to optimally catalyze the activation of the substrate.
Materials and Methods
Mutagenesis and expression of recombinant proteins
We previously constructed an expression and purification vector system for FX by replacing the first 12-residues of the activation peptide of FX with the 12-residues epitope for the Ca2+-dependent monoclonal antibody, HPC4 (Fig. 1) [19]. The Ala substitution mutants of FX including Thr-17 (T17A), Thr-29 (T29A), Asn-39 (N39A) and Asn-49 (N49A) corresponding to residues Thr-199, Thr-211, Asn-221 and Asn-231 in FX cDNA numbering [4] were prepared by PCR methods and expressed in HEK-293 cells using the same system. These residues in combinations of 2 (Thr-17/Thr-29 and Asn-39/Asn-49) and four (Thr-17/Thr-29/Asn-39/Asn-49) were also substituted with Ala and expressed using the same system. This vector was also used to express another construct, in which all residues of the activation peptide, with the exception of HPC4 epitope (Fig. 1) up to the P3 site (Leu-50) were deleted (FX-des-AP). Thus, this mutant contained 12-residues of the HPC4 epitope plus two Ala's for construction purposes followed by three native P3-P1 50Leu-Thr-Arg52 residues of FX and lacked the putative Thr or Asn residues required for carbohydrate attachments (Fig. 1). All constructs were expressed in HEK-293 cells and mutants were isolated from 20-40-L cell culture supernatants by a combination of immunoaffinity and ion exchange chromatography using HPC4 and a Mono Q column, respectively, as described [19,20].
Figure 1.
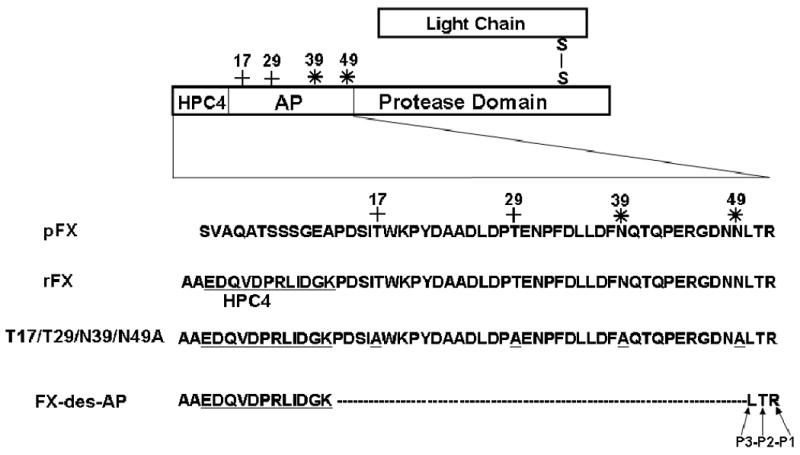
Schematic representation of modifications introduced into the activation peptide (AP) on the heavy chain of recombinant FX (rFX). The 12 residues of the HPC4 epitope have been underlined. The O-linked (+) and N-linked (*) glycosylation sites on residues Thr-17, Thr-29, Asn-39 and Asn-49 are marked.
Human plasma proteins including FVIIa, FIXa and FX and the factor X-activating enzyme from Russell's viper venom (RVV-X) were purchased from Haematologic Technologies Inc. (Essex Junction, VT). Human factor VIII was a generous gift from Dr. Philip Fay (University of Rochester, New York). Factor VIII (2 μM) was activated to FVIIIa by using 0.2 nM thrombin in the presence of Ca2+ for 10 min at 37°C and immediately used for FX activation studies. Phospholipid vesicles containing 80% phosphatidylcholine and 20% phosphatidylserine (PC/PS) were prepared as described [21]. TF lacking the cytoplasmic domain (dc-TF) was incorporated into PC/PS vesicles as described [22]. The chromogenic substrate Spectrozyme FXa (SpFXa) was purchased from American Diagnostica (Greenwich, CT).
Activation by the FVIla-TF complex
Initial rates of the concentration-dependence of FX activation by FVIIa were studied in both the absence and presence of dc-TF incorporated into PC/PS as described [22]. Briefly, the activation of increasing concentrations of FX derivatives (3.9–500 nM) by FVIIa (5-25 nM in the absence of TF and 0.025 nM in its presence) was monitored in the absence or presence of a saturating concentration of relipidated dc-TF (2.5 nM) in 0.1 M NaCl, 0.02 M Tris-HCI (pH 7.5) containing 0.1 mg/mL bovine serum albumin, 0.1% polyethylene glycol 8000 and 5 mM Ca2+ (TBS/Ca2+) for 5-30 min at room temperature in 30-μL reactions in 96-well assay plates. The activation reactions were terminated by an addition of 20 μL of EDTA to obtain a final concentration of 20 mM, and the rate of FXa generation was determined by an amidolytic activity assay using 50 uL of 0.4 mM SpFXa. It was ensured that less than 10% of FX was activated at all substrate concentrations. Km and kcat values in the presence of dc-TF were calculated from Michaelis-Menten equation.
Activation by the FIXa-VIIIa complex
The initial rate of activation of FX derivatives by FIXa was studied in both the absence and presence of FVIIIa on PC/PS vesicles as described [23]. Briefly, the activation of increasing concentrations of FX derivatives (3.9–500 nM) by FIXa (5-25 nM in the absence of FVIIIa and 0.025 nM in its presence) on PC/PS (50 μM) was monitored in the absence or presence of a saturating concentration of FVIIIa (30 nM) in TBS/Ca2+ for 2-30 min at room temperature in 30 μL reactions in 96-well assay plates. Activation reactions were terminated by an addition of 20 μL of EDTA and the concentration of FXa generated was determined from standard curves as described above. Km and kcat values were calculated from Michaelis-Menten equation.
Results
Expression and purification of recombinant proteins
Recombinant FX (rFX) derivatives were expressed in HEK-293 cells as described under “Materials and Methods”. Recombinant proteins were isolated from cell culture supernatants by immunoaffinity chromatography using HPC4 as described [19]. Fully γ-carboxylated zymogens were separated from partially modified proteins using a Mono Q column as described [20]. The protein recovery for two derivatives, N39A and its double substitution mutant N39A/N49A, were not sufficient for full characterization, thus these two mutants had to be excluded from the study. SDS-PAGE analysis of all other derivatives indicated that recombinant proteins have been purified to near homogeneity (Fig. 2). Both recombinant wild-type FX and T17A migrated with nearly identical and expected molecular masses of ∼65-70 kDa (Fig. 2, lanes 1 and 2). T29A, N49A and T17A/T29A mutants migrated slightly faster (lanes 3-5, respectively) which is expected since side chains of these mutants lack carbohydrates. The quadruple T17/T29/N39/N49A mutant (lane 6) migrated as three bands with molecular masses ranging from ∼40-55 kDa. Based on a high background amidolytic activity for this mutant, the ∼40 kDa band may represent the activated form of the quadruple mutant. Further analysis indicated that ∼10% of this mutant is converted to its activated form during and/or after secretion. Thus, it appears that elimination of carbohydrate chains of the activation peptide renders the zymogen susceptible to proteolysis during secretion. FX-des-AP migrated primarily as a doublet with molecular masses of ∼45-50 kDa (Fig. 2, lane 7). The background activity of this mutant was also high, though to a lesser extent than the quadruple mutant. We expressed 2-3 different batches of T17/T29/N39/N49A and FX-des-AP and obtained the same SDS-PAGE migration pattern with both mutants.
Figure 2.
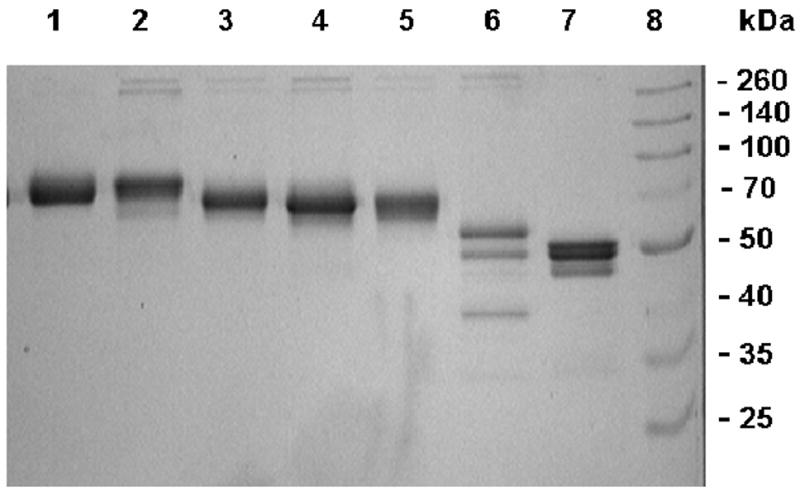
SDS-PAGE analysis of recombinant FX derivatives. Under non-reducing conditions: (lane 1) rFX, (lane 2) T17A, (lane 3) T29A, (lane 4) N49A, (lane 5) T17A/T29A, (lane 6) T17/T29/N39/N49A, (lane 7) FX-des-AP, and (lane 8) molecular mass standards in kDa.
We previously showed that rFX is activated by extrinsic Xase with identical kinetic parameters as the plasma-derived FX, however, rFX is activated 2-4-fold slower by intrinsic Xase [20]. All studies described below have been conducted with rFX.
Factor X activation by extrinsic Xase
The concentration-dependence of activation of FX derivatives by FVIIa alone or in complex with TF in extrinsic Xase is shown in Fig. 3. The kinetic parameters derived from Fig. 3 in the presence of TF are presented in Table 1. FVIIa activated the O-linked glycosylation site mutants (T17A and T29A) with normal rates, however, the activation of N49A was ∼20-fold faster than wild-type (Fig. 3, panel A). Both FX-des-AP and T17/T29/N39/N49A were activated by FVIIa alone 3-5-fold faster than wild-type FX. In contrast to improvements by FVIIa alone, activation of all mutants by FVIIa in complex with TF was associated with an elevation of Km ranging from less than 2-fold for T17A/T29A and ∼20-fold for the mutant lacking all four glycosylation sites (Table 1). FX-des-AP exhibited ∼5-fold elevation in Km. Interestingly, kcat for activation of all mutants were proportionately improved so that the catalytic efficiency (kcat/Km) of FVIIa toward mutants was not significantly altered in the presence of the cofactor (Table 1).
Fig. 3.
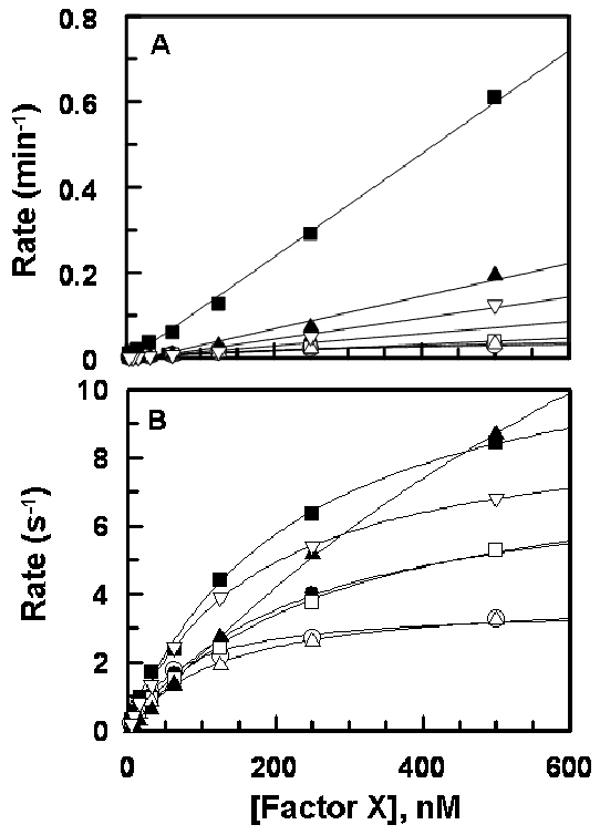
Activation of FX derivatives by FVIIa. (A) In the absence of TF, FVIIa (5-25 nM) was added to increasing concentrations of rFX (○), T17A (●), T29A (□), N49A (■), T17A/T29A (△), T17/T29/N39/N49A (▲), and FX-des-AP (▽) and PC/PS (50 μM) in TBS/Ca2+. After 30 min incubation, the reactions were terminated by EDTA and the rate of FXa generation was measured from the cleavage rate of SpFXa as described under “Materials and Methods”. (B) The same as (A) except that FVIIa (0.025 nM) in complex with the relipidated dcTF (2.5 nM) was incubated with FX derivatives for 5 min. Lines in panel B are nonlinear regression fits of kinetic data to Michaelis-Menten equation, and those in panel A are fits to a linear equation. Rates are normalized for the concentrations of enzymes in both panels.
Table1.
Kinetic constants for the activation of FX derivatives by extrinsic Xase
| Km (M) | kcat (s−1) | kcat/Km (M−1 s−1) | |
|---|---|---|---|
| rFX-WT | 0.71 ± 0.08 × 10-7 | 3.6 ± 0.22 | 5.1 × 107 |
| T17A | 2.7 ± 0.51 × 10-7 | 8.5 ± 0.81 | 3.1 × 107 |
| T29A | 3.0 ± 0.29 × 10-7 | 8.4 ± 0.82 | 2.8 × 107 |
| N49A | 2.3 ± 0.18 × 10-7 | 12.2 ± 0.66 | 5.3 × 107 |
| T17/T29A | 1.2 ± 0.16 × 10-7 | 4.0 ± 0.25 | 3.3 × 107 |
| T17/T29/N39/N49A | 13.4 ± 2.5 × 10-7 | 32.5 ± 3.06 | 2.4 × 107 |
| FX-des-AP | 3.2 ± 0.48 × 10-7 | 10.9 ± 1.53 | 3.4 × 107 |
Kinetic constants were determined from the concentration-dependence of the activation of FX derivatives by extrinsic Xase as described under “Materials and Methods”. All values are the average of at least three measurements ± S.E.
Factor X activation by intrinsic Xase
Similar to activations by FVIIa, FIXa by itself exhibited normal activity toward T17A, T29A and T17A/T29A mutants (Fig. 4A). However, elimination of the N-linked glycosylation site at position 49 was associated with a dramatic ∼40-fold improvement in activation of the mutant by FIXa independent of its cofactor (Fig. 4A). FIXa also activated both T17/T29/N39/N49A and FX-des-AP with ∼10-fold faster rate, suggesting that carbohydrates of the FX activation peptide prevent un-regulated rapid activation of FX by FIXa when the protease is not in complex with FVIIIa. FIXa in the intrinsic Xase complex activated the quadruple mutant with kinetic constants which were essentially similar to those observed for wild-type (Fig. 4B, Table 2). With the exception of N49A, intrinsic Xase activated all other mutants with ∼2-5-fold lower catalytic efficiency (Fig. 4B, Table 2). The basis for the lower activity of intrinsic Xase toward mutants was an elevation of Km in T17A and FX-des-AP and a decrease of kcat in both T29A and T17A/T29A (Table 2). Thus, both O-linked carbohydrates of the FX activation peptide make productive interactions with intrinsic Xase. Nevertheless, analysis of individual kinetic constants indicates that the carbohydrate chain attached to Thr-17 contributes to stabilization of the Michaelis complex in intrinsic Xase, as evidenced by the elevated Km of T17A (Table 2). On the other hand, the carbohydrate chain on Thr-29 contributes to stabilization of the FX transition-state intermediate, as evidenced by a decrease in kcat of T29A (Table 2). The elimination of the N-linked glycosylation site at Asn-49 resulted in ∼2-fold increase in Km, but ∼5-fold increase in kcat. Thus, the decrease in the rate of activation of O-linked glycosylation site mutants is likely compensated by the improvement in the activation of N49A in the quadruple mutant explaining the near normal activation of T17/T29/N39/N49 by intrinsic Xase (Table 2). The activation of FX-des-AP was impaired ∼4-fold, possibly suggesting that FIXa is making other interactions with the activation peptide and/or the conformation of the scissile bond of FX-des-AP has been slightly altered.
Fig. 4.
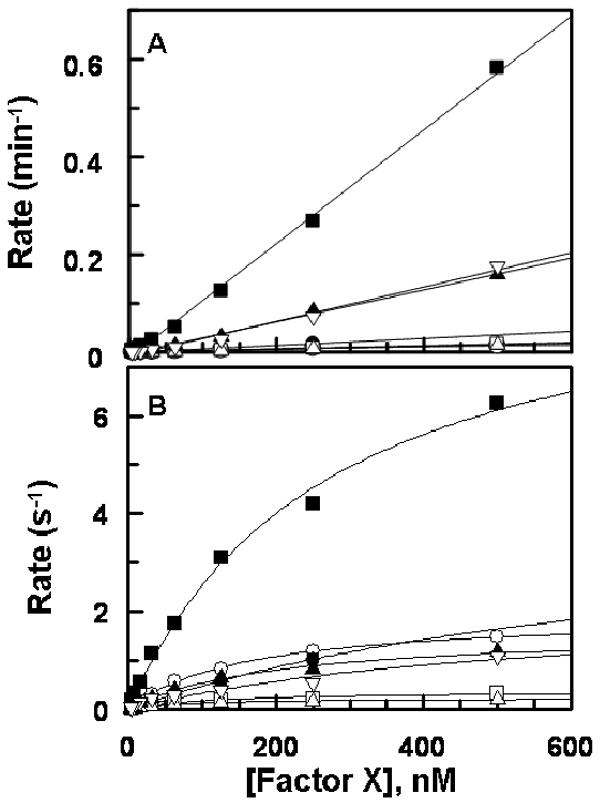
Activation of FX derivatives by FIXa. (A) In the absence of FVIIIa, FIXa (5-25 nM) was incubated with increasing concentrations of rFX (○), T17A (●), T29A (□), N49A (■), T17/T29A (△), T17/T29/N39/N49A (▲), and FX-des-AP (▽) on PC/PS (50 μM) in TBS/Ca2+. After 30 min of incubation, the reactions were terminated by EDTA, and the rate of FXa generation was measured as described under “Materials and Methods”. (B) The same as (A) except that FIXa (0.025 nM) in complex with FVIIIa (30 nM) was used for activation for 2-10 min. Lines in panel B are nonlinear regression fits of kinetic data to Michaelis-Menten equation, and those in panel B are fits to a linear equation. Rates are normalized for the concentrations of enzymes in both panels.
Table 2.
Kinetic constants for the activation of FX derivatives by intrinsic Xase
| Km (M) | kcat (s−1) | kcat/Km (M−1 s−1) | |
|---|---|---|---|
| rFX-WT | 1.4 ± 0.32 × 10-7 | 1.8 ± 0.06 | 1.3 × 107 |
| T17A | 8.0 ± 1.8 × 10-7 | 4.4 ± 4.9 | 0.55 × 107 |
| T29A | 1.3 ± 0.28 × 10-7 | 0.39 ± 0.0.3 | 0.30 × 107 |
| N49A | 2.7 ± 0.48 × 10-7 | 9.8 ± 1.9 | 3.6 × 107 |
| T17/T29A | 0.91 ± 0.18 × 10-7 | 0.24 ± 0.01 | 0.26 × 107 |
| T17/T29/N39/N49A | 2.0 ± 0.63 × 10-7 | 1.6 ± 0.30 | 0.8 × 107 |
| FX-des-AP | 11.7 ± 2.1 × 10-7 | 3.5 ± 1.0 | 0.3 × 107 |
Kinetic constants were determined from the concentration-dependence of activation of FX derivatives by intrinsic Xase as described under “Materials and Methods”. All values are the average of at least three measurements ± S.E.
Factor X activation by RVV-X
RVV-X activated all FX derivatives with a similar kcat of 65-70 nM/min/nM (data not shown). A Km value of 150-200 nM was observed for wild-type, N49A and FX-des-AP. However, this value was increased approximately 2-fold for other mutants, suggesting that the O-linked carbohydrates of the activation peptide make a minor contribution to the specificity of FX recognition by RVV-X.
Discussion
In this study, we investigated the role of O-linked and N-linked carbohydrates of the FX activation peptide by a mutagenesis approach and for the first time demonstrate that carbohydrates of the activation peptide play critical roles in restricting the recognition specificity of FX, thereby preventing rapid cofactor-independent activation of the zymogen by FVIIa and FIXa. This hypothesis is based on the observation that the activation of FX mutants was either normal or improved with both FVIIa and FIXa alone in the absence of cofactors. Furthermore, these carbohydrate chains appear to also protect the scissile bond from recognition and activation by non-specific proteases. This was evidenced by the observation that both quadruple and FX-des-AP mutants were expressed as partially activated proteins. Analysis of the activity of expressed proteins before and after full activation suggested that ∼5-10% of zymogens have been converted to active forms during and/or after secretion. In this context, the carbohydrate chain of Asn-49 was found to play the most critical role, since elimination of this glycosylation site resulted in 20- and 40-fold improvement in its activation by free forms of FVIIa and FIXa, respectively. Asn-49 constitutes the P4 residue of the activation peptide (Fig. 1), thus the carbohydrate attached to this residue confers protease resistance to FX by sterically hindering the scissile bond from docking into active-site pockets in the absence of cofactors.
In addition to their role in restricting the specificity of FX recognition by FVIIa and FIXa, carbohydrates of the FX activation peptide play a critical role in the cofactor-dependent recognition of the zymogen by both proteases when they are in complex with their physiological cofactors. In general, elimination of glycosylation sites of the activation peptide elevated Km for the activation of FX by extrinsic Xase. Extent of the Km effect ranged from 3-4-fold for single mutants and ∼20-fold for the quadruple mutant. Nevertheless, impairments in Km in all cases were proportionately associated with a similar extent of improvements in kcat, thus the catalytic efficiency (kcat/Km) of extrinsic Xase toward mutants was not significantly (within 2-fold) altered (Table 1). In the case of FX-des-AP, the 4.5-fold elevated Km was compensated by ∼3-fold improvement in kcat. The observation that the extent of increase in Km of the quadruple mutant is higher than that of FX-des-AP may suggest that the carbohydrate free form of FX somehow restricts the high affinity interaction of the mutant with the FVIIa-TF complex. These results suggest that, unlike the reaction with free FVIIa, carbohydrates of the FX activation peptide make productive interactions with the FVIIa-TF complex to reduce Km, though the same interactions appear to have negative impact on kcat. Noting the low concentration of FX in circulation, this kinetic mechanism may be advantageous since it ensures that the protease interaction with the substrate is not rate-limiting.
Characterization of the FX mutants in activation studies by FIXa in the presence of FVIIIa showed that when O-linked carbohydrate chains of the activation peptide were removed, activation rates of mutants were significantly impaired (3-5-fold). However, removal of the N-linked Asn-49 glycosylation site improved kcat ∼5-fold. The observation that removal of all glycosylation sites in the quadruple mutant resulted in a minimal decrease (<1.5-fold) in the catalytic efficiency of FIXa in the presence of FVIIIa suggests that, unlike O-linked carbohydrates, N-linked carbohydrates do not contribute positively to specificity of the intrinsic Xase reaction. Thus, the primary function of N-linked carbohydrates in the activation peptide of FX is to restrict the specificity of free FIXa toward the zymogen, thereby preventing the un-regulated activation of FX by the protease independent of its cofactor. This mechanism could act as a safe guard to ensure that the amplification of the clotting cascade during injury only occurs after a small amount of thrombin has been generated in order to activate cofactors V and VIII.
A previous study, employing a proteolytic fragment of porcine FX, which was cleaved by Agkistrodon rhodostoma snake venom and lacked all but three P3-P1 residues of the activation peptide, reported a critical role for the activation peptide of FX in reaction with intrinsic Xase based on ∼100-fold impaired activation for the cleaved zymogen [24]. The Km for activation of FX-des-AP by intrinsic Xase in this study was elevated greater than 8-fold, suggesting that the activation peptide of human FX also plays a crucial role in recognition of the zymogen by the activator complex, though to a lesser extent than that observed for porcine FX. Furthermore, using the same approach, a human FX derivative was previously prepared which lacked all residues of the activation peptide with the exception of P3-P1 residues [25]. Characterization of the snake venom-derived human FX did not reveal any significant role for the activation peptide of FX in reaction with extrinsic Xase [25]. Although, the catalytic efficiency of extrinsic Xase toward FX-des-AP was nearly normal (Table 1), nevertheless, both Km and kcat constants were elevated 3-4-fold. Whether the presence of an additional antibody epitope in the activation peptide of the recombinant FX mutant in our study accounts for differences between results of the two studies was not further investigated. Nevertheless, results with glycosylation site mutants clearly suggest that carbohydrate chains of the FX activation peptide play essential roles in regulation of the substrate by both intrinsic and extrinsic Xase complexes. Our results recapitulate other studies in the literature, which have used O- and N-glycanases to remove carbohydrate chains of FX, showing that they contribute to the recognition and activation of the zymogen by both physiological activators [17,18].
In summary, results of this study suggest that carbohydrates of the FX activation peptide (in particular Asn-49) play critical roles in restricting the specificity of zymogen recognition by target proteases in the absence of physiological cofactors. This role must be highly critical for the regulation of FX activation by both target proteases since elimination of the Asn-49 carbohydrate improved the activity of free FVIIa and FIXa toward the zymogen mutant 20- and 40-fold, respectively. Based on the observation that elimination of both O-linked glycosylation sites of the FX activation peptide is associated with ∼5-fold impairment in the rate of zymogen activation by intrinsic Xase, it is concluded that a differential glycosylation of these residues in recombinant FX expressed by mammalian cells accounts for its 2-4-fold slower activation rate when compared to plasma-derived FX (20). Based on the ∼20-fold elevated Km for activation of the quadruple mutant by extrinsic Xase, it is concluded that carbohydrates of the activation peptide make productive interactions with specific sites of the activation complex to ensure that the concentration of FX is not rate-limiting in circulation during the initiation of the clotting cascade. Finally, based on the observation that both quadruple and FX-des-AP mutants are expressed as partially activated proteins, it is concluded that oligosaccharides of the activation peptide protect the scissile bond of FX from non-specific proteolysis.
Acknowledgments
The research discussed herein was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL 62565 and HL 68571 to ARR).
References
- 1.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Ann Rev Biochem. 1988;57:915–56. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 2.Rosing J, Tans G, Govers-Riemslag JWP, Zwaal RFA, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980;255:274–83. [PubMed] [Google Scholar]
- 3.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: Initiation, maintenance and regulation. Biochemistry. 1991;30:10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 4.Leytus SP, Foster DC, Kurachi K, Davie EW. Gene for human factor X: A blood coagulation factor whose gene organization is essentially identical with that of factor IX and protein C. Biochemistry. 1986;25:5098–102. doi: 10.1021/bi00366a018. [DOI] [PubMed] [Google Scholar]
- 5.Higashi S, Matsumoto N, Iwanaga S. Molecular mechanism of tissue factor-mediated acceleration of factor VIIa activity. J Biol Chem. 1996;271:26569–74. doi: 10.1074/jbc.271.43.26569. [DOI] [PubMed] [Google Scholar]
- 6.McCallum CD, Su B, Neuenschwander PF, Morrissey JH, Johnson AE. Tissue factor positions and maintains the factor VIIa active site far above the membrane surface even in the absence of the factor VIIa Gla domain. J Biol Chem. 1997;272:30160–6. doi: 10.1074/jbc.272.48.30160. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson CD, Kelly CR, Ruf W. Identification of surface residues mediating tissue factor binding and catalytic function of the serine protease factor VIla. Proc Natl Acad Sci (USA) 1996;93:14379–84. doi: 10.1073/pnas.93.25.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopfner KP, Lang A, Karcher A, Sichler K, Kopetzki E, Brandstetter H, Huber R, Bode W, Engh RA. Coagulation factor IXa: the relaxed conformation of Tyr99 blocks substrate binding. Structure. 1999;7:989–96. doi: 10.1016/s0969-2126(99)80125-7. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AE, Bajaj SP. Structure-function relationships in factor IX and factor IXa. Trends Cardiovasc Med. 2003;13:39–45. doi: 10.1016/s1050-1738(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 10.Kolkman JA, Christophe OD, Lenting PJ, Mertens K. Surface loop 199-204 in blood coagulation factor IX is a cofactor-dependent site involved in macromolecular substrate interaction. J Biol Chem. 1999;274:29087–93. doi: 10.1074/jbc.274.41.29087. [DOI] [PubMed] [Google Scholar]
- 11.Morrissey JH, Neuenschwander PF, Huang Q, McCallum CD, Bixia S, Johnson AE. Factor VIla-tissue factor: Functional importance of protein-membrane interactions. Thromb Haemostas. 1997;78:112–6. [PubMed] [Google Scholar]
- 12.Sichler K, Kopetzki E, Huber R, Bode W, Hopfner KP, Brandstetter H. Physiological fIXa activation involves a cooperative conformational rearrangement of the 99-loop. J Biol Chem. 2003;278:4121–6. doi: 10.1074/jbc.M210722200. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaswamy S. Exosite-driven substrate specificity and function in coagulation. J Thromb Haemost. 2004;3:54–67. doi: 10.1111/j.1538-7836.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 14.Baugh RJ, Dickinson CD, Ruf W, Krishnaswamy S. Exosite Interactions determine the affinity of factor X for the extrinsic Xase complex. J Biol Chem. 2000;275:28826–33. doi: 10.1074/jbc.M005266200. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes-Prior P, Iwanaga Y, Huber R, Pagila R, Rumennik G, Seto M, Morser J, Light DR, Bode W. Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex. Nature. 2000;404:518–25. doi: 10.1038/35006683. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Manithody C, Rezaie AR. Activation of protein C by the thrombin-thrombomodulin complex: Cooperative roles of Arg-35 of thrombin and Arg-67 of protein C. Proc Natl Acad Sci (USA) 2006;103:879–84. doi: 10.1073/pnas.0507700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Morita T. Identification of O-linked oligosaccharide chains in the activation peptides of blood coagulation factor X: The role of the carbohydrate moieties in the activation of factor X. Eur J Biochem. 1993;218:153–63. doi: 10.1111/j.1432-1033.1993.tb18361.x. [DOI] [PubMed] [Google Scholar]
- 18.Sinha U, Wolf DL. Carbohydrate residues modulate the activation of coagulation factor X. J Biol Chem. 1993;268:3048–51. [PubMed] [Google Scholar]
- 19.Manithody C, Yang L, Rezaie AR. Role of basic residues of the autolysis loop in the catalytic function of factor Xa. Biochemistry. 2002;41:6780–8. doi: 10.1021/bi0255367. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Manithody C, Yang L, Rezaie AR. Zymogenic and enzymatic properties of the 70-80 loop mutants of factor X/Xa. Protein Sci. 2004;13:431–42. doi: 10.1110/ps.03406904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnov MD, Esmon CT. Phosphatidylethanolamine incorporation into vesicles selectively enhances factor Va inactivation by activated protein C. J Biol Chem. 1994;269:816–9. [PubMed] [Google Scholar]
- 22.Neuenschwander PF, Bianco-Fisher E, Rezaie AR, Morrissey JH. Phosphatidylethanolamine augments factor VIla-tissue factor activity: enhancement of sensitivity to phosphatidylserine. Biochemistry. 1995;34:13988–93. doi: 10.1021/bi00043a004. [DOI] [PubMed] [Google Scholar]
- 23.Manithody C, Fay PJ, Rezaie AR. Exosite-dependent regulation of factor VIlla by activated protein C. Blood. 2003;101:4802–7. doi: 10.1182/blood-2003-01-0126. [DOI] [PubMed] [Google Scholar]
- 24.Duffy EJ, Lollar P. Intrinsic pathway activation of factor X and its activation peptide-deficient derivative, factor Xdes-143-191. J Biol Chem. 1992;267:7821–7. [PubMed] [Google Scholar]
- 25.Baugh RJ, Krishnaswamy S. Role of the activation peptide domain in human factor X activation by the extrinsic Xase complex. J Biol Chem. 1996;271:16126–34. doi: 10.1074/jbc.271.27.16126. [DOI] [PubMed] [Google Scholar]


