Abstract
This study used a novel experimental paradigm that combined associative recognition and list discrimination to study the associative deficit in older adults’ memory (M. Naveh-Benjamin, 2000). Participants viewed 2 lists of word—face pairs and were tested on recognition of pairs from the second study list. Older and young adults’ recognition was increased by repetition of individual items, but repetition of pairs of items increased recognition in young adults only. This provides converging evidence that older adults do not form associative links between items within pairs and supports the hypothesis that an associative deficit contributes to age-related memory decline.
Keywords: Controlled processing, associative deficit, list discrimination, age-related memory impairment, episodic memory
Older adults differ from young adults in certain aspects of episodic memory. For example, older adults can show a selective impairment in remembering specific details of prior events (Balota, Dolan, & Duchek, 2000; Craik, 2000; Johnson, Hashtroudi, & Lindsay, 1993; Light, 1991). One account of age-related memory decline is that older adults have difficulty creating or processing associative information (Naveh-Benjamin, 2000). This associative deficit hypothesis (ADH) holds that older adults’ inability to remember the details of prior episodes results from a failure to create and retrieve links between individual items and the contexts in which they appeared during encoding.
Much of the evidence supporting the ADH has come from associative recognition experiments in which participants study pairs of items and then undergo recognition tests for the individual items as well as the pairs of items. Experiments of this type with older and young adults have shown participants have relatively greater difficulty remembering pairs and/or the conjunctions between items than remembering individual items themselves (Bastin & van der Linden, 2006; Naveh-Benjamin, 2000; Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Naveh-Benjamin, Guez, & Shulman, 2004; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003). One potential limitation of these studies is that separate tasks often are used to assess memory for individual items and pairs. This is a problem because item- and pair-recognition tasks might differ in difficulty or in the underlying retrieval processes used by participants to perform the separate tasks. As a result, age-related differences in performance might be observed because of other factors besides associative processing. Several recent studies (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Naveh-Benjamin, Guez, & Shulman, 2004) have attempted to equate item- and pair-recognition tasks as much as possible via forced-choice paradigms. However, any task that instructs participants to remember pairs of items has some inherent differences from a task that instructs participants to remember individual items. In evaluating the ADH, it would be valuable to use a single task in which the effects of item and associative memory could both be observed. The current study did so through the use of an associative recognition paradigm, devised by Criss and Shiffrin (2005), in which the familiarity of individual items and pairs was independently manipulated. The separate influences of item familiarity and pair familiarity were then examined within a single pair-recognition task.
Criss and Shiffrin (2005) investigated the role of associative information in young adult memory by combining associative recognition with list discrimination. In their experiment (Experiment 1), participants studied two sets of word—face pairs. Some of the words and faces appeared on only one study list; other words and faces appeared on both study lists but in different combinations; and another group of words and faces appeared on both study lists in the same combinations. Participants were then shown a third set of word—face pairs and asked to identify as “old” the pairs that had appeared on the second study list. The results showed effects of both item and pair familiarity. Even though the participants’ task was to identify pairs and not individual items, an overall increase in “old” responses was observed for pairs containing items that had appeared on both study lists. Additionally, an influence of pair familiarity was observed as an increase in old/new discrimination for pairs whose items had been seen in the same combinations across the two study lists versus pairs whose items had been seen in different combinations across the two study lists. Because the individual items in these conditions appeared on both study lists, the improvement in performance was due to memory for the particular combination of items in each pair.
The current study replicated the findings of Criss and Shiffrin (2005) in young adults and used their design to test the ADH for older adults. In doing so, it also built upon previous studies of aging and memory in which the effects of item repetition on list discrimination (Jacoby, 1999) and associative recognition (Light, Patterson, Chung, & Healy, 2004) were examined. We hypothesized that if associative processing is specifically affected by aging, then age groups should differ more in the effects of pair repetition than in the effects of item repetition on memory for pairs.
Method
Participants
A specific effort was made to recruit older adults who were representative of the general aged population. Sixty-one older adults (41 women and 20 men; mean age = 82.2 years, age range = 61–96 years; mean education = 13.8 years, education range = 9–20 years) were recruited from the Pittsburgh region, including retirement communities and churches. The older adults received $7 compensation for participation. Ninety young adults (54 women and 36 men; mean age = 21.2 years, age range = 18–39 years; mean education = 14.3 years, education range = 12–23 years) were recruited from the University of Pittsburgh community and from introductory psychology courses. The young adults received either $7 or psychology course credit. All participants were native English speakers, were right-handed, and reported no history of major medical, neurological, or psychiatric disorders. After the explanation of procedures and prior to testing, all participants provided written informed consent to participate using consent forms approved by the institutional review board of the University of Pittsburgh.
Stimuli
The stimuli were the same as those used by Criss and Shiffrin (2005). They consisted of 64 standardized black-and-white photographs of faces (see Criss & Shiffrin, 2005, for standardization details) and 64 abstract words (e.g., incident) of varying environmental frequency (M = 18.59, range = 1–245, SD = 24.32; Kucera & Francis, 1967) and low imageability (M = 341.69, range = 129–400, SD = 43.13; Colthart, 1981). The stimulus set did not include any words that might describe a face, a person, or a characteristic of either.
Design
The design of the study and test lists is illustrated in Table 1. There were four conditions. The List 1 and List 2 conditions consisted of word—face pairs that appeared only on List 1 and List 2, respectively. The Lists-1-and-2-exact condition consisted of word—face pairs that appeared on both study lists. The Lists-1-and-2-rearranged condition consisted of words and faces that appeared on both study lists in different pair combinations. Items from each of these four conditions were then used to create both old and new pairs for the test list. The conditions differed in the repetition both of individual items and pairs of items. Target pairs in the List 2 condition were composed of items that were seen in pairs on the second study list only. Target pairs in the Lists-1-and-2-rearranged condition consisted of items that were seen in pairs on both study lists but whose pairings changed from one list to the other list. Target pairs in the Lists-1-and-2-exact condition consisted of items that were seen on both study lists in the same pair combination on each of the study lists. Each of these conditions also had lure pairs that were novel pairs composed of items that had been presented on the study lists in the same manner as the target pairs in their respective conditions. Finally, as an additional control, the List 1 condition consisted of intact and rearranged pairs from the first study list. These items were always lures as the memory task required participants to judge which pairs had been presented on the second study list.
Table 1.
An Example of Each Study and Test Condition
| Condition label | Study List 1 | Study List 2 | Test List | Pair type |
|---|---|---|---|---|
| Lists-1-and-2-exact | ||||
| Word1—Face1 | Word1—Face1 | Word1—Face1 | Target | |
| Word2—Face2 | Word2—Face2 | Word2—Face3 | Lure | |
| Word3—Face3 | Word3—Face3 | Word3—Face2 | ||
| Lists-1-and-2-rearranged | ||||
| Word4—Face4 | Word4—Face5 | Word4—Face5 | Target | |
| Word5—Face5 | Word5—Face4 | Word5—Face4 | ||
| Word6—Face6 | Word6—Face7 | Word6—Face8 | Lure | |
| Word7—Face7 | Word7—Face8 | Word7—Face6 | ||
| Word8—Face8 | Word8—Face6 | Word8—Face7 | ||
| List 2 | ||||
| n/a | Word9—Face9 | Word9—Face9 | Target | |
| n/a | Word10—Face10 | Word10—Face11 | Lure | |
| Word11—Face11 | Word11—Face10 | |||
| List 1 | ||||
| Word12—Face12 | n/a | Word12—Face12 | Pseudotarget | |
| Word13—Face13 | n/a | Word13—Face14 | Lure | |
| Word14—Face14 | Word14—Face13 |
Note. In the experiment, there were equal numbers of pairs in all conditions (not illustrated here to conserve space). List 1 targets are labeled as “pseudo-targets” because participants were instructed to say “yes” only to pairs appearing on List 2. Table adapted from Criss and Shiffrin (2005). n/a = not applicable.
Procedure
Participants received two study lists. Both study lists contained 48 pairs of items. On each trial of each list, participants performed an incidental task that involved rating each pair on the following question: “Are these items pleasant or unpleasant?” Participants were instructed to consider the entire pair when making their decision. Items were presented until the participant responded or for a maximum of 5,000 ms. Each study trial was separated by a 500-ms interstimulus interval (ISI). At the end of the first list, participants were reminded that they had just seen the first of two study lists. Participants were given a 3-min break during which they completed a number search task and then advanced to the second study list, which was presented in the same manner as the first study list. Following the second study list, participants engaged in a 1-min math task before being informed that they would take an unexpected memory test. Prior to the presentation of this 64-trial test list, participants were given examples of all the possible types of targets and lures and instructed to respond “yes” only if they had seen intact pairs from List 2 during the study session and to respond “no” to all other pairs.
In order to make the experiment accessible for the older adults, we tested them in off-campus settings such as retirement homes. To provide a similar experimental setting for young adults, we also tested them in locations outside the laboratory, such as offices. The testing environment for both groups was always an isolated room with adequate lighting and closed doors to decrease distraction.
Results
Response Times
Overall, older adults had slower response times (RTs) than young adults. Mean RT for correct responses was 2,224 ms (SD = 574) for older adults and 1,723 ms (SD = 437) for young adults. Mean RT for incorrect responses 2,202 ms (SD = 595) for older adults and 1,909 ms (SD = 472) for young adults. These differences in RT across age groups were significant both for correct responses, t(147) = 6.04, p < .001, d = .98, and incorrect responses, t(147) = 3.35, p = .001, d = .55.
Accuracy
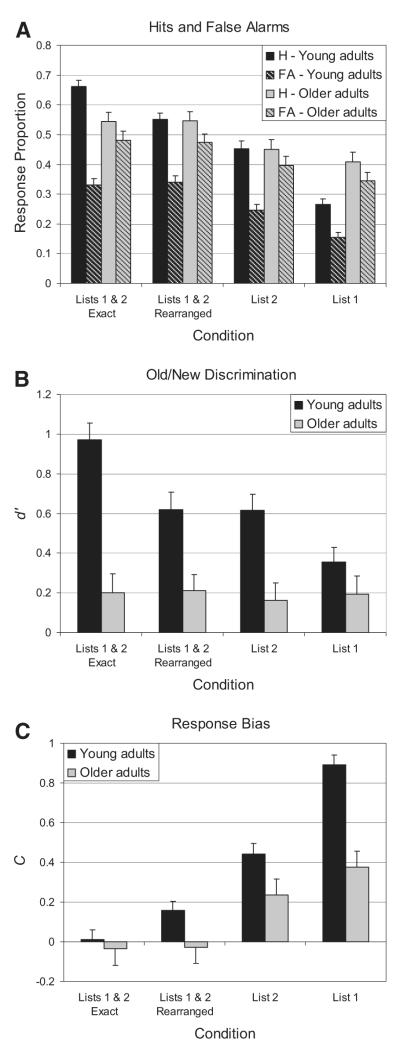
Panel A of Figure 1 displays the proportion “old” responses given by young and older adults to studied (old) pairs from each condition as well as new pairs that consisted of rearranged items from studied pairs. Separate 4 (condition) × 2 (old vs. new) repeated-measures analyses of variance (ANOVAs) were performed on the proportion “old” data.
Figure 1.
A. Proportion “old” responses to studied pairs (i.e., targets and List 1 pseudo-targets) and new pairs (i.e., lures) across the four conditions by young adults and older adults. B. Old/new discrimination, as measured by d’, across the four conditions for young adults and older adults. C. Response bias, as measured by criterion, C, across the four conditions for young adults and older adults. In all three panels, error bars represent standard error of the mean.
For young adults, the main effect of condition was significant, F(3, 264) = 110.9, p < .001, ; the main effect of old versus new pairs was significant, F(1, 88) = 187.6, p < .001, ; and the interaction of Condition × Old Versus New Pairs was significant, F(3, 264) = 13.5, p < .001, . For older adults, the main effect of condition was significant, F(3, 177) = 16.9, p < .001, , and the main effect of old versus new pairs was significant, F(1, 59) = 27.7, p < .001, , but the interaction was not significant, F(3, 177) < 1. The significant main effects of old versus new pairs indicate that both age groups were capable of the basic task of identifying studied pairs as old more often than new pairs. The significant main effects of condition indicate further that both age groups were influenced by the manipulation of conditions in the experiment. The presence of a Condition × Old Versus New Pairs interaction among young adults reflects an increase in “old” responses to studied pairs, but not to new pairs, in the Lists-1-and-2-exact condition versus the Lists-1-and-2-rearranged condition. Since the Lists-1-and-2-exact and Lists-1-and-2-rearranged conditions differed only in whether studied pairs remained intact across the two study lists, the increase in “old” responses to studied pairs indicates a benefit of pair repetition, independent of the items within the pairs. This benefit was not observed among older adults, suggesting that they did not use associative information about each pair as a whole.
We directly compared the young adult and older adult groups using independent-samples t tests. The older adults gave more “old” responses than young adults to new pairs in all conditions: Lists-1-and-2-exact, t(147) = 4.14, p < .001, d = .68; Lists-1-and-2-rearranged, t(147) = 3.82, p < .001, d = .63; List 2, t(147) = 4.44, p < .001, d = .72; and List 1, t(147) = 6.33, p < .001, d = 1.02. This suggests that in general, older adults found it difficult to reject novel pairs of items. For studied pairs, older adults gave more “old” responses than young adults to pairs from List 1, t(147) = 4.00, p < .001, d = .65, suggesting that they had difficulty with the task of responding “old” only to those pairs that appeared on the second study list. Proportion “old” responses did not differ between young and older adults in the Lists-1-and-2-rearranged condition, t(147) = 0.166, p = .87, d = .03, or in the List 2 condition, t(147) = 0.09, p = .93, d = .02. However, older adults gave fewer “old” responses than young adults to studied pairs in the Lists-1-and-2-exact condition, t(147) = -3.22, p = .002, d = .53, reflecting the observation that pair repetition increased pair recognition in young adults but not in older adults.
Old/New Discrimination and Response Bias
Additional analyses were performed to assess the separate contributions of old/new discrimination and response bias to performance in both age groups. For each participant, the proportion of hits and false alarms were used to compute d’ (Macmillan & Creelman, 1991). The false-alarm rates used for d’ were taken from the “old” responses to lures within each condition, so that d’ here specifically reflects discrimination of old versus new pairs, rather than old versus new items. The means of d’ across age groups and conditions are displayed in Panel B of Figure 1. A 4 (condition) × 2 (age group) ANOVA on the d’ data revealed significant main effects of condition, F(3, 441) = 4.53, p = .004, , and age group, F(1, 147) = 46.7, p < .001, . There was a significant Condition × Age Group interaction, F(3, 441) = 4.21, p = .006, , reflecting the result that older adults’ old/new discrimination did not differ across conditions. In contrast, young adults’ discrimination increased in the Lists-1-and-2-exact condition relative to other conditions, reflecting the benefit of repeating intact pairs across both study lists.
Because older adults had such low discrimination overall, the question arises whether the lack of a pair-repetition benefit was due to floor effects. That is, perhaps the task was simply too difficult for older adults, and they responded at chance levels across all conditions. Directional t tests indicated that d’ for older adults was greater than 0 in each of the four conditions: Lists-1-and-2-exact, t(59) = 2.17, p = .017, d = .28; Lists-1-and-2-rearranged, t(59) = 2.57, p = .007, d = .33; List 2, t(59) = 1.80, p = .039, d = .28; and List 1, t(59) = 2.07, p = .022, d = .22. Thus, although older adults’ performance was poor, their discrimination was sufficiently above chance that any benefit of pair repetition should have been observed.
A measure of response bias, C, was also computed for each participant (Macmillan & Creelman, 1991). The means of C across conditions and age groups are presented in Panel C of Figure 1. Positive values of C indicate strict criteria and tendency to respond “new,” whereas negative values of C indicate lax criteria and a tendency to respond “old.” Criteria are high in the List 1 condition due to the task instruction to reject pairs viewed only on the first study list. An independent-samples t test showed a difference between the two age groups in C for the List 1 condition, t(147) = 5.98, p < .001, d = .97, which reflects older adults’ difficulty rejecting List 1 pairs. Differences in C among the other three conditions were examined using a 3 (condition) × 2 (age group) ANOVA. There was a main effect of condition, F(2, 294) = 39.0, p < .001, , seen partly as a shift toward higher criteria in the List 2 condition. List 2 target and lure pairs consisted of items that were studied only once, whereas target and lure pairs in the Lists-1-and-2-exact and Lists-1-and-2-rearranged conditions consisted of items that were studied twice. Thus, participants’ tendency to respond “old” to studied and new pairs at test was influenced by repetition of items within the pairs across study lists. The main effect of age group was marginally nonsignificant, F(1, 147) = 3.73, p = .055, . Finally, there was no Condition × Age Group interaction, F(2, 294) = 2.12, p = .12, . Thus, the shift in criterion across the two conditions did not differ between groups. This suggests that the influence of individual item memory on task performance was similar across the two age groups.
Discussion
The current results provide new, converging evidence for an associative deficit in older adult memory. The current study showed the associative deficit in a novel experimental paradigm. Additionally, a large sample size of 61 older adults was tested, and the use of off-campus locations made the experiment accessible to a representative sample of the older adult population.
According to the ADH, the differences between young and older adults in episodic memory ability are caused by a specific age-related decline in the ability to form associative links between items and their contexts. This hypothesis has previously been tested with paired-associate memory tasks in older adults (e.g., Naveh-Benjamin, 2000). The current study provided a further test of associative memory through use of a novel experimental paradigm that combined associative recognition and list discrimination into a single task (Criss & Shiffrin, 2005). In their study, Criss and Shiffrin found young adults had increased recognition of word—face pairs that were repeated across two study lists, compared with recognition of pairs that were studied only once but contained items that had been studied twice. The benefit of pair repetition was therefore attributed to memory for associative information for whole pairs. The present experiment replicated these findings in young adults and extended the method into the domain of normal cognitive aging.
Older adults in the current study differed from young adults in several important ways. First, compared with young adults, older adults showed a decreased ability to reject novel pairs, consistent with previous studies of associative recognition in aging (e.g., Naveh-Benjamin, 2000). Second, older adults also showed a decreased ability to reject pairs from one of two study lists, consistent with previous studies of list discrimination in aging (e.g., Jennings & Jacoby, 1993). Finally, and most important, older adults did not show increased recognition of pairs based on repetition of the pairs across study lists, which suggests that they failed to form or use associative links between the items in each pair. This difference between age groups in the effect of pair repetition contrasts with the similarity between age groups in the effect of item repetition. Across the two conditions that differed only in item repetition (List 2 and Lists-1-and-2-rearranged), both age groups displayed increases in hits as well as false alarms. This was reflected in analyses of response bias, which indicated similar criterion shifts for the two age groups across these conditions. Thus, in the current study, greater age-related changes were observed in pair memory than in item memory within a single task, reinforcing earlier findings from studies in which separate tasks had been used for associative recognition and item recognition (e.g., Naveh-Benjamin, 2000).
It was argued in the Introduction that previous studies’ use of separate tasks for item and pair memory left open the possibility that the two tasks differed in some additional cognitive processes other than the use of associative information. Of course, the use of a single task does not necessarily rule out the possibility that more than one cognitive process may be involved or may differ between age groups. For example, many theorists have argued that in recognition memory tasks, participants receive contributions from two separate underlying memory processes: recollection and familiarity (see Yonelinas, 2002, for a review). According to this theory, familiarity is a graded process in which a current item is judged in terms of how well it matches all previous items stored in memory, whereas recollection is an all-or-none process that involves the retrieval of specific details of the encoding episode. Numerous studies indicate that older adults are impaired in the use of recollection but not familiarity, and that they consequently rely on familiarity to a greater extent than young adults in memory tasks (e.g., Jennings & Jacoby, 1993). This age-related decline of recollection has also been used to account for older adults’ associative deficit (Yonelinas, 2002). Therefore, it might be argued that the age-related differences observed in the current study are due to young adults’ ability to use recollection to identify studied pairs and older adults’ tendency to rely on familiarity.
Such an explanation, however, cannot account for the current results. The task in the current experiment was to respond “old” only to pairs studied on the second study list and to respond “new” to pairs studied only on the first list as well as unstudied pairs. Thus, in this task, only the study episodes from the second list are relevant to the response choice. However, target pairs in the Lists-1-and-2-exact and Lists-1-and-2-rearranged conditions differ only whether they were presented intact on the first study list. Recollection of study episodes from the second study list should not differ between the two conditions and should not have any effect on “old” responses to target pairs. That is, recollection should not contribute to the pair-repetition benefit we observed (in fact, recollection might be expected to work against the pair-repetition benefit, since Lists-1-and-2-exact target pairs could cue episodes from the first study list and lead participants to incorrectly reject those pairs). In contrast, familiarity would be expected to contribute to the pair-repetition benefit seen in the current results because target pairs in the Lists-1-and-2-exact condition were studied twice, unlike pairs in the Lists-1-and-2-rearranged condition. Familiarity is thought to be preserved in older adults, so they should still be expected to display a benefit of pair repetition in the current task. Nonetheless, older adults did not display the pair-repetition benefit. Thus, it is not likely that older adults were impaired in familiarity-based processes per se, but rather that associative information about word-face pairs was not available to familiarity-based retrieval processes because associative information was poorly encoded during the study episodes.
On a related note, Light et al. (2004) found that older adults had more false alarms to lure pairs in associative recognition when the items within those pairs had been repeated across several study lists. A similar effect was observed in young adults when recollection was reduced through response deadlines (see also Benjamin, 2001; Jacoby, 1999). In the current study, item repetition increased false alarms (and hits) in both age groups, as seen in the comparison between the List 2 and Lists-1-and-2-rearranged conditions. Thus, responses of young adults in the current study were consistent with reduced recollection (perhaps due to task difficulty), providing further evidence that the current findings are not due to better recollection among young adults.
In summary, the current findings are consistent with previous research suggesting that the associative deficit is distinct from other age-related cognitive changes, such as controlled-processing impairment (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Naveh-Benjamin, Guez, & Shulman, 2004). However, these results do not rule out age-related effects of controlled processing in associative recognition tasks (e.g., Caldwell & Masson, 2001). Rather, we suggest that an associative deficit may occur in addition to controlled-processing changes. Other age-related memory changes may also be relevant, such as increased reliance on meaning-based processing over perceptual processing (Koutstaal et al., 2001, 1999; Koutstaal & Schacter, 1997). Further research is needed to examine how these factors work together to produce age-related differences in memory and other cognitive processes.
Acknowledgments
This research was supported in part by the Center for the Neural Basis of Cognition at Carnegie Mellon University and the University of Pittsburgh. Portions of this work were presented at the 2006 Cognitive Aging Conference, Atlanta, Georgia, and in the doctoral dissertation of Amy Overman. We are grateful to Amy Criss for kindly sharing experimental materials.
Contributor Information
Amy A. Overman, Department of Psychology and Center for the Neural Basis of Cognition, University of Pittsburgh
James T. Becker, Departments of Psychiatry, Neurology, and Psychology and Center for the Neural Basis of Cognition, University of Pittsburgh.
References
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy older adults. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford University Press; New York: 2000. pp. 395–409. [Google Scholar]
- Bastin C, van der Linden M. The effects of aging on the recognition of different types of associations. Experimental Aging Research. 2006;32:61–77. doi: 10.1080/03610730500326291. [DOI] [PubMed] [Google Scholar]
- Benjamin AS. On the dual effects of repetition on false recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:941–947. [PubMed] [Google Scholar]
- Caldwell JI, Masson ME. Conscious and unconscious influences of memory for object location. Memory & Cognition. 2001;29:285–295. doi: 10.3758/bf03194922. [DOI] [PubMed] [Google Scholar]
- Colthart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Craik FIM. Age-related changes in human memory. In: Park DC, Schwarz N, editors. Cognitive aging: A primer. Psychology Press; Philadelphia: 2000. pp. 75–99. [Google Scholar]
- Criss AH, Shiffrin RM. List discrimination in associative recognition and implications for representation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1199–1212. doi: 10.1037/0278-7393.31.6.1199. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychology and Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay D. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. Journal of Memory and Language. 1997;37:555–583. [Google Scholar]
- Koutstaal W, Schacter DL, Brenner C. Dual-task demands and gist-based false recognition of pictures in younger and older adults. Journal of Memory and Language. 2001;44:399–426. [Google Scholar]
- Koutstaal W, Schacter DL, Galluccio L, Stofer KA. Reducing gist-based false recognition in older adults: Encoding and retrieval manipulations. Psychology and Aging. 1999;14:220–237. doi: 10.1037//0882-7974.14.2.220. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis W. Computational analysis of present-day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Memory & Cognition. 2004;32:1182–1193. doi: 10.3758/bf03196891. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. Cambridge University Press; New York: 1991. [Google Scholar]
- Naveh-Benjamin M. Adult-age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: Further support using face-name associations. Psychology and Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Shulman S. Older adults’ associative deficit in episodic memory: Assessing the role of decline in attentional resources. Psychonomic Bulletin & Review. 2004;11:1067–1073. doi: 10.3758/bf03196738. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: Further support for an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]