Abstract
Purpose of Review
While traditional disciplinary research theory and methods have focused separately on how social and physical environmental factors affect children’s health, evolving research underscores important integrated effects.
Recent findings
This review outlines the specific reasons why social determinants should be considered mainstream in children’s environmental health research with particular focus on interactive effects between social and physical hazards. These include (a) sensitivity of overlapping physiological systems, via epigenesis, programming, and plasticity to social and physical environmental moderation that may impact health across the life span; (b) ways in which social environmental vulnerabilities moderate the effects of physical environmental factors providing specific examples related to respiratory health and neurodevelopment; (c) overlapping exposure distribution profiles; and (d) relevance to pediatric health disparities.
Summary
Because of the covariance across exposures and evidence that social stress and other environmental toxins (e.g., pollutants, tobacco smoke) may influence common physiological pathways (e.g., oxidative stress, pro-inflammatory immune pathways, autonomic disruption), understanding the potential synergistic effects promises to more completely inform children’s environmental health risk. While this discussion focuses around the respiratory and neurological systems, these concepts extend more broadly to children’s psychological and physical development.
Keywords: children, social toxins, physical hazards, health disparities
Introduction
An area of particular interest in children’s environmental health is the search for mechanisms responsible for health disparities across economic and ethnic groups. Recent consensus statements by both the National Academy of Science1 and the National Institutes of Environmental Health Sciences2 support the position that examining disparities in environmental health requires attention to both physical environmental hazards and social conditions. Yet, while contemporary accounts acknowledge this, traditional disciplinary research has focused separately, by in large, on the influence of social and physical factors. Indeed, far more attention has been given to physical toxins including tobacco smoke, air pollutants, allergens, and metals (e.g., lead, mercury)3-5.
This review outlines specific reasons why social determinants should be considered mainstream in children’s environmental health research with particular focus on interactive effects between social and physical hazards. This is discussed in light of recent studies focused on leading pediatric public health outcomes (i.e., respiratory disorders, neurodevelopment) which illustrate situations in which social stressors influence susceptibility to future environmental exposures and when contemporaneously exposed, how social x physical environmental interactions may account for more variance in explaining risk than main effects. Conversely, socially enriched environments may protect children from the toxic effects of other environmental hazards which may have implications for prevention and intervention.
Social Toxins
Increasingly, it has been recognized that children are being raised in social contexts that may be as detrimental to their development as these physical factors. References to “socially toxic environments” have existed in the psychology literature for some time. James Garbarino, an American psychologist, coined the term in the 1970s to describe rearing conditions such as violence, poverty and other economic pressures on parents and their children6. Others including Urie Bronfenbrenner have been issuing increasingly serious “social smog alerts” since first sounding the alarm in Two Worlds of Childhood7. While a number of theoretical models explaining how social conditions “get into the body” to impact health more broadly, the psychosocial stress model has been increasingly adopted in this regard8-12. In this framework, psychological stress can be conceptualized as a social toxin/pollutant that can be ‘breathed’ into the body resulting in the disruption of a number of key integrated physiological systems similar to how air pollutants and other physical toxicants lead to adverse health risks.
Developmental Plasticity and Life Course Epidemiology
The conceptualization of a life course approach to epidemiology is only briefly introduced here albeit is central to our discussion (for more details see 13, 14). These authors provide a concise overview of the life course epidemiological approach bridging biological, psychological and social models of disease causation relevant to health disparities. They use chronic respiratory disease and/or impaired respiratory function as a specific example. Plasticity is a consequence of environmental exposures during critical life periods affecting key physiological systems that operate in orchestrating underlying developmental processes15. Children are particularly vulnerable to disruption of developmental processes during relatively narrow time windows. Exposure to environmental toxicants during prenatal and/or early postnatal development may alter the normal course of morphogenesis and maturation, resulting in changes that affect both structure and function of multiple organ systems including the respiratory and neurological systems15. Moreover, when normal development is altered, the early effects may persist into adult life, magnifying the public health impact 16, 17.
Respiratory and cognitive function may operate under common regulatory processes and thus have shared vulnerabilities to a host of environmental factors during development18. While the mechanisms of early life environmental influences are not completely understood, evidence suggests that the developmental origins of the structural and functional organization of the respiratory and neurological systems involve, in part, the coordinated maturation of the immune, neural, and endocrine systems19, 20. Mechanisms underlying perinatal programming related to physical factors have been previously described in the environmental health literature and will not be reiterated here4, 21, 22. Instead, the evidence for stress effects on such programming is described in order to make the case that because stress and physical hazards (e.g., pollutants, tobacco smoke) may disrupt common physiological pathways (e.g., oxidative stress, pro-inflammatory immune pathways, autonomic disruption), we need to understand their integrated effects to more fully understand children’s environmental health risk.
Environmental Programming of Key Physiological Systems
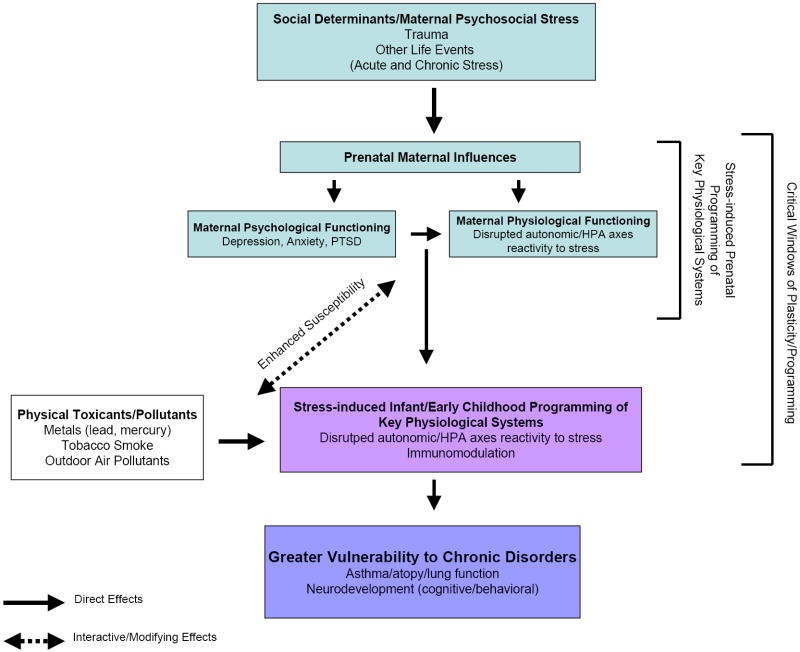
Figure 1 depicts a conceptual model for pathways linking stress experienced during critical periods of development (i.e., perinatal programming) and enhanced vulnerability to concomitant and subsequent environmental toxicant exposures. The following discussion provides a background for the model. Stressors influence pathogenesis by causing dysregulated biobehavioral states [e.g., depression, anxiety, posttraumatic stress disorder] which, in turn, exert lasting effects on physiological processes that influence disease risk23. In response to stress, physiological systems may operate at higher or lower levels than in normal homeostasis. It is the disturbed balance of these systems that is relevant to disease. Immune and neuroendocrine defensive biological responses important for the short-term response to stress, may produce long-term damage if not checked and eventually terminated20.
Figure 1.
Conceptual Model for Pathways Linking Perinatal Stress to Altered Vulnerability to Physical Environmental Toxins and Health Risk
The hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system [sympathetic-adrenal-medullary (SAM) system] seem particularly susceptible to early-life programming in relation to stress and other environmental toxins. Disturbed regulation of these systems due to maternal stress may, in turn, modulate immune and autonomic function in the offspring beginning in utero24, 25. That is, offspring may inherit a biological vulnerability to disrupted stress regulatory systems altering the child’s reactivity to subsequent challenges 26 (i.e., both subsequent stressors as well as other environmental toxins).
Both early and long-term developmental effects related to prenatal stress result, in part, from altered maternal and/or fetal glucocorticoid exposure25. Animal models as well as human studies support the connection between an adverse intrauterine environment and experiences in early postnatal life and alterations of autonomic nervous system balance (e.g., sympathovagal balance) as well 27-29. While these in utero responses may be adaptive in the short term, geared toward coping with anticipated environmental challenges, ultimately they may exact a toll in contributing to increased risk of diseases in later life.
In addition, the acquisition of the ability to regulate one’s response to stress (“self regulation”) progresses through several stages in the early years of development30. The HPA system starts to become organized between 2 and 6 months of age through transactions between the child and caregiver 31. The infants’ autonomic responses show developmental changes with relative stability between 6 to 12 months of age 32. Studies have consistently demonstrated that the quality of caregiving that the child receives during early development predicts the emergence of later self-regulation abilities, with sensitive caregiving associated with more adaptive self-regulatory abilities and more optimal functioning of the child’s HPA system33. Increased maternal stress, in turn has been associated with lower levels of parenting sensitivity and higher levels of negative parenting behaviors 34, 35. A number of correlates of perinatal maternal stress have also been associated with poor stress regulation and other negative outcomes in both animal and human offspring36-39. Essex and colleagues demonstrate prospectively how how early life stress may alter vulnerability to subsequent experiences of stress as indexed by cortisol and behavioral stress responses.
Such neurohormonal alterations have been subsequently linked to fetal and early life immunomodulation8. Aberrant or excessive pro-inflammatory immune responses as well as oxidant-induced changes, both locally and systemically, are a central determinant of structure-function changes in both the respiratory 40-42 and neurological systems43-46.
Shared Vulnerability Pathways
The notion that stress and other environmental toxins operate through overlapping mechanisms has been previously reviewed47. For example, tobacco smoke exposure in early development influences immune function as indexed by alterations in cytokine production by the fetoplacental unit and in cord blood, patterns of fetal mononuclear cell responses in vitro, and altered signaling through Toll-like receptors48, 49. Air pollution exposures have been linked to disruption of neuroimmune responses45, 50 and autonomic reactivity (particularly increasesd parasympathetic tone) even in young healthy subjects51. Moreover, both tobacco smoke and air pollutants may generate reactive oxidative species to influence health through oxidative stress pathways similar to psychological stressors47. It is thus plausible that the biologically compromised system(s) related to early life stress may be more vulnerable to subsequent environmental toxins and vice versa (life course perspective).
Examples from Leading Pediatric Developmental Disorders
The subsequent discussion focuses on specific examples demonstrating the independent and interactive effects of correlates of social environmental stress on trajectories of disease expression in the respiratory and neurological systems.
Respiratory System
While the origins of chronic lung diseases are multi-factorial, the central underlying mechanisms leading to reduced lung function and exaggerated responsiveness to bronchoconstirctor stimuli involve chronic airway inflammation associated with a cycle of injury, repair and remodeling40, 52. The fundamental cause of the underlying airway inflammation is aberrant and/or excessive immune responses to various environmental agents 40 and oxidant-induced inflammation42. Airway inflammation and early remodeling occur and progress even in the presymptomatic and/or subclinical state53, i.e. lung function may be ‘set’ in the first years of life54.
Research continues to delineate the relationships among early environmental influences, immunodeviations, and developmental outcomes in the lungs 41, 42, 55. The most common cause of chronic airway inflammation in early childhood is asthma. Although diverse pathogenic mechanisms are likely involved in different forms of asthma and the consequential airway changes [i.e., airway hyperreactivity (AHR), which is thought to be present in all patients with asthma 56], there has been a major focus on the influence of the systemic propensity for type 2 T-helper (Th2) allergic responses and eosinophils 57. These mechanisms have their roots in early life with an immunological bias towards a Th2 phenotype in utero41, 58. Perinatal stress may influence the evolving systemic propensity for type Th2 responses (i.e., enhanced adaptive immunity)8. Antigen-independent responses including innate immune cells (e.g., bronchial epithelial cells, alveolar macrophages, and dendritic cells) may also be important in modifying airway inflammation 59. Factors, including stress, that slow maturation of local immune networks (e.g., dendritic cells [DCs], epithelial cells [ECs], regulatory T cells) may predispose to a Th2 phenotype 60.
The balance between functional parasympathetic and sympathetic activity in relation to stress, emotional stimuli, and immune function may also be important for the expression of atopic disorders as well as early airway inflammation and reactivity 47. A bidirectional network of interactions between the central nervous system, the endocrine system and the immune system is well documented61. The immune and nervous system are closely related in both physiological and pathological reactions in the lung. Communications between neurons and immune cells resulting in airway inflammation and the development of airway hyperreactivity are a consequence of neuronal dysregulation62, 63. Finally, animal studies suggest that neural control of airway smooth muscle and the irritant receptor systems are established during early life and sensitive to environmental programming 27. Lung and airway reflexes through the central nervous system (CNS) are crucial in maintaining airway and respiratory function as well as defensive mechanisms. These central pathways (e.g., nucleus tractus solitarius (NTS)] have been shown to undergo neuroplasticity under a variety of conditions (e.g., exposures to environmental tobacco smoke, pollutants, stress)64.
Perinatal stress and emotional arousal may influence airway narrowing through inflammatory pathways and imbalance in sympathovagal activity. Nogueira and colleagues65 were first to demonstrate that prenatal stress increased allergen-induced airway inflammation in adult mice offspring. Similarly, allergen aerosol challenge has been associated with increased airway hyperresponsiveness in mice exposed to prenatal stress66. Mice exposed to prenatal stress were also more likely to express a Th2 adaptive immune response.
More recent evidence suggests that stress modifies the response to other environmental toxins to influence the expression of respiratory phenotypes. We recently demonstrated an association between traffic-related air pollution (NO2) and risk for childhood asthma in an urban sample only among children who were also exposed to elevated social stress67. No main effects were evident for either NO2 or the stressor in this study. Similarly, Chen and colleagues68 showed that chronic traffic-related pollution exposure and stress interacted in predicting both increased asthma symptoms and heightened inflammatory profiles in adolescents with asthma.
Neurodevelopment/Behavior/Cognition
Social stress and physical environmental toxins impact overlapping biological processes which determine adaptive plasticity in early neurodevelopment as well. Developmental CNS organization into functional neuronal and synaptic networks is determined by environmental signals which modify neuronalgenesis, synaptic formation and synaptic pruning69. Environmental factors can promote or disrupt this process depending on whether they are positive (social supports, good nutrition, etc.) or negative (psychosocial stress, chemical toxicants, malnutrition, trauma, etc.). While plasticity allows recovery from short term toxic exposures, the neural mechanisms underlying the plasticity of the developing brain exposed to chronic stress could induce permanent structural or organizational changes via altered neuronal growth and/or synaptogenesis/pruning. As the hippocampus is the brain region with the highest density of glucocorticoid receptors which modulate the process of neuron and synaptogenesis, it is not surprising that with regard to the neurological effects of chronic stress, the primary functional endpoint appears to be changes in the development and formation of memory. While acutely, stress may enhance memory formation70, chronic stress inhibits it. In animals, chronic stress induces atrophy of apical dendrites in the hippocampus71, 72 and a reduction in dendritic length and branching density73. Animal behavioral studies have confirmed the adverse effects of pre- and post-natal chronic stress on memory and learning74, 75.
More recent evidence suggests that stress modifies the response to environmental toxins to influence the expression neurobehavioral phenotypes76, 77. Studies in animals have demonstrated that combined exposure to maternal lead and stress in the prenatal environment may act synergistically to enhance behavioral and neurochemical toxicity in the offspring78 with evidence of mediation through HPA disregulation79, 80. In addition, recent epidemiologic evidence supports the role of stress as a modifier of physical toxins (e.g., lead, ETS exposure). Rauh et al81 measured pre-and post-natal exposure to ETS and Bayley Scales of infant development in urban children enrolled during pregnancy and followed longitudinally. Prenatal ETS exposure predicted a 5 point decrement in the Bayley MDI scores (p=0.02). Material hardship predicted a 3 point decrement in MDI (p=0.07). The children with both prenatal hardship and ETS performed an average of 10 points lower on the Bayley MDI than children with neither exposure.
Converse Protective Effects
Animal studies have also shown that environmental enrichment can reverse the effects of early stress experiences on stress reactivity82. For example, Schneider83 and Guilarte et al,84 demonstrated that animals raised in social isolation were more sensitive to the neurotoxic effects of lead than animals raised in an enriched environment. Laviola et al and Morley-Fletcher et al have shown that environmental enrichment eliminates the outcomes of prenatal stress on corticosterone response and reactivity to an immune-suppressive agent in offspring later in life 85, 86. Similarly, Francis et al has shown that environmental enrichment can reverse the effects of early stress (maternal separation) on HPA activation and behavioral response to stress in rat offspring87.
There is also evidence from a range of studies in humans to suggest that maternal psychosocial functioning (e.g., stress, anxiety, depression, self-esteem) has a significant effect on the mother-infant relationship and parenting, and that this in turn can have consequences for both the short and long-term health and functioning of the child33. A recent prospective study in humans demonstrates that postnatally, maternal sensitivity can modify the effects of prenatal stress experiences and maternal psychological functioning on infant stress reactivity 88. We have previously linked intimate partner violence (IPV) in the home during early development (a particular social stressor) to adverse respiratory outcomes in children including increased risk for asthma89 and reduced lung function in early childhood90. More recent analysis show that, while maternal IPV is associated with increased childhood asthma risk, factors contributing to a supportive caregiving environment appear to buffer the maternal IPV-asthma association91. Also, parental social support which may buffer stress experiences, has been shown to be inversely associated with asthma prevalence among children 92. Another recent study demonstrates the direct positive effect of higher levels of maternal self-esteem on children’s cognitive functioning as assessed using the Bayley’s Scale of Infant Development at age 24 months93. Moreover, there was evidence that enhanced maternal self-esteem attenuated the negative effects of lead exposure on cognitive functioning in these children. And finally, Thahn and colleagues94 investigated the relationships among neonatal stress, cognitive indices and basal cortisol levels in very low gestational age infants and found that maternal factors (parenting stress, interactive behaviors) ameliorated adverse effects of stress on cortisol and focused attention in these infants. These studies have important implications for prevention and intervention studies.
It is worth mentioning in this context that the potential programming effects of stress on childhood health outcomes may occur at an even more fundamental level, i.e., through epigenetic programming95, 96. A variety of environmental factors have been identified which influence DNA methylation including stress97. Epigenetic mechanisms may even explain why maternal behavior toward young offspring affects the size of the offspring’s hippocampus in adulthood, depending on the offspring’s genotypes98.
Implications for Health Disparities
The environmental hazards discussed herein co-occur. Marginalized populations of lower-socioeconomic position that are disproportionately exposed to irritants (e.g., tobacco smoke), pollutants (e.g., diesel-related particles) and indoor allergens (e.g., cockroach, mouse allergen) may also live in communities that are increasingly socially toxic which, in turn, may be related to increased experience of psychosocial stress67, 99, 100. Thus, those living in disadvantaged social circumstances may be most at risk for synergistic effects.
Summary
Taken together, these lines of evidence point toward the need to consider social environmental factors as mainstream in children’s environmental epidemiology. The likelihood of multiple mechanistic pathways with complex interdependencies must be considered when examining the integrative influence of social and physical environmental toxins on children’s environmental health. Because these factors tend to cluster in the most socially disadvantaged, this line of research may better inform the etiology of growing health disparities. While the focus of this discussion has been around the respiratory and neurological systems, these concepts extend more broadly to children’s psychological and physical development. Design of future epidemiologic studies and effective intervention programs will need to address environmental toxicants and social stress jointly to impact public health most effectively101, 102.
Acknowledgments
During preparation of this manuscript RJ Wright was supported by the National Institutes of Health (R01 HL080674).
References
- 1.Genes, behavior, and the social environment: Moving beyond the nature/nurture debate. Washington DC: Institute of Medicine of the National Academies; 2006. [PubMed] [Google Scholar]
- 2.Summary of the Symposium on Genetic Variation and Gene Environment Interaction in Human Health and Disease. National Institute of Environmental Health Sciences (NIEHS), National Human Genome Research Institute (NHGRI) and National Institute of Alcohol Abuse and Alcoholism (NIAAA) 2003 [Google Scholar]
- 3.Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113:1037–1043. [PubMed] [Google Scholar]
- 4.Wang L, Pinkerton KE. Air pollutant effects on fetal and early postnatal development. Birth Defects Research C: Embryo Today. 2007;81(3):144–154. doi: 10.1002/bdrc.20097.. * Reviews evidence linking perinatal air pollution and tobacco smoke exposures and respiratory and neurodevelopmental outcomes. In addition they review evidence linking perinatal pollutant exposure and programming of immune function.
- 5.Bellinger DC. Very low lead exposures and children’s neurodevelopment. Current Opinions in Pediatrics. 2008;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 6.Garbarino J. Raising children in a socially toxic environment. San Francisco: Jossey-Bass; 1995. [Google Scholar]
- 7.Two worlds of childhood [computer program]. Version. New York, NY: Simon & Schuster; 1972. [Google Scholar]
- 8.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Pediatric and Perinatal Epidemiology. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 9.Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public health research: models to explain health disparities. Annual Review of Anthropology. 2005;34:231–252. [Google Scholar]
- 10.Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson B, Kubzansky LD, Wright RJ. Linking perceived unfairness to physical health: the perceived unfairness model. Review General Psychology. 2006;10(1):21–40. [Google Scholar]
- 12.Wright RJ. Health effects of socially toxic neighborhoods: The violence and urban asthma paradigm. Clinics in Chest Medicine. 2006;27(3):413–421. doi: 10.1016/j.ccm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Shlomo Y. Rising to the challenges and opportunities of life course epidemiology. International Journal of Epidemiology. 2007;36(3):481–483. doi: 10.1093/ije/dym116. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–293. [PubMed] [Google Scholar]
- 15.Feinberg AP. Phenotypic plasticity and the epigentics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 16.Bavis RW, Mithcell GS. Long-term effects of the perinatal environment on respiratory control. Journal of Applied Physiology. 2008;104(4):1220–1229. doi: 10.1152/japplphysiol.01086.2007. [DOI] [PubMed] [Google Scholar]
- 17.von Mutius E. Childhood experiences take away your breath as a young adult. American Journal of Respiratory & Critical Care Medicine. 2002;165:1467–1468. doi: 10.1164/rccm.2204011. [DOI] [PubMed] [Google Scholar]
- 18.Franco Suglia S, Wright RO, Schwartz J, Wright RJ. Association between lung function and cognition among children in a prospective birth cohort study. Psychosomatic Medicine. 2008;70(3):356–362. doi: 10.1097/PSY.0b013e3181656a5a.. * The first prospective study to link reduced lung function to impaired cognitive function in school-aged children and discusses shared vulnerability pathways.
- 19.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–994. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 21.Yu M, Zheng X, Peake J, Joad JP, Pinkerton KE. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. Journal of Allergy and Clinical Immunology. 2008;122(3):640–647. doi: 10.1016/j.jaci.2008.04.038.. * Demonstrates that tobacco smoke exposure influences early immune development, a pathway that overlaps with other environmental hazards (e.g., stress, air pollution).
- 22.Kumar R. Prenatal factors and the development of asthma. Current Opinions in Pediatrics. 2008;20(6):682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 24.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy – a review. Neuroscience & Biobehavioral Reviews. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? Journal Internal Medicine. 2007;261(5):453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 26.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research. 2008;167:121–134. doi: 10.1016/S0079-6123(07)67009-5.. ** First prospective study linking maternal experience of trauma and consequent PTSD symptoms and disrupted behavioral and physiological response (i.e., cortisol) in their infants.
- 27.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. Journal of Neuroscience. 2005;25(40):9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pryce CR, Ruedi-Bettschen D, Dettling AC, Feldon J. Early life stress: long-term physiological impact in rodents and primates. News Physiological Sciences. 2002:17150–5. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- 29.Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Experimental Neurology. 2004;190(Suppl 1):S8–21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Rifkin-Graboi A, Borelli JL, Bosquet M. Neurobiology of stress in infancy. In: Zeanah CHJ, editor. Handbook of infant mental health. 3. New York: Guilford; in press. [Google Scholar]
- 31.Wright RJ, Bosquet Enlow M. Maternal stress and perinatal programming in the expression of atopy. Expert Reviews in Clinical Immunology. 2008;4(5):535–538. doi: 10.1586/1744666X.4.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkon A, Lippert S, Vujan N, Rodriguez ME, Boyce WT, Eskenazi B. The ontogeny of autonomic measures in 6- and 12-month-old infants. Developmental Psychobiology. 2006;48(3):197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- 33.Barlow J, Coren E. Parent-training programmes for improving maternal psychosocial health. Cochrane Database Systematic Reviews. 2004:CD002020. doi: 10.1002/14651858.CD002020.pub2. Published Last Modified Date. Accessed Dated Accessed. [DOI] [PubMed] [Google Scholar]
- 34.Belsky J. The determinants of parenting: A process model. Child Development. 1984;55:83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Juanin L, Borghini A, Ansemet F. Prematurity, maternal stress, and mother-child interactions. Early Human Development. 2004;79:145–158. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 37.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 38.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Progress in Brain Research. 2007;167:137–149. doi: 10.1016/S0079-6123(07)67010-1.. ** This article demonstrates how early programming of the HPA axis may be modified through parental caregiving experiences either buffering the child or rendering them more vulnerable to future stress experiences. Such changes may also make children more vulnerable to other environmental exposures (pollutants, metals). Also see Essex et al., 2002.
- 39.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 40.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: Implications for development of asthma prevention strategies. Journal Allergy Clinical Immunology. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Prescott SL. The development of respiratory inflammation in children. Paediatric Respiratory Reviews. 2006;7:89–96. doi: 10.1016/j.prrv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. Journal of Allergy and Clinical Immunology. 2008;122(3):456–468. doi: 10.1016/j.jaci.2008.08.004.. * Reviews the role of oxidant imbalance in chronic lung disease and airway inflammation.
- 43.Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Frontiers in Bioscience. 2005;10:675–680. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- 44.Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity: antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Hertz-Picciotto I, Park HY, Dostal M, Kocan A, Trnovec T, Sram R. Prenatal exposure to persistent and non-persistent organic compounds and effects on immune system development. Basic & Clinical Pharmacology & Toxicology. 2008;102(2):146–154. doi: 10.1111/j.1742-7843.2007.00190.x.. ** This reviews developmental milestones of the immune system in the perinatal period including examples of environmentally induced alterations in immune markers. The report also underscores the coordinated development of the immune system and the central nervous system highlighting the plausibility that disruption of critical events in immunomodulation play a role in neurobehavioral development.
- 46.Gomez-Mejiba SE, Zhai Z, Akram H, et al. Inhalation of environmental stressors & chronic inflammation: autoimmunity and neurodegeneration. Mutation Research. 2008 doi: 10.1016/j.mrgentox.2008.09.016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Current Opinions in Allergy and Clinical Immunology. 2005;5(1):23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatric Respiratory Reviews. 2008;9(1):3–9. doi: 10.1016/j.prrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. European Respiratory Journal. 2006;28(4):721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 50.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic Pathology. 2008;36(2):289–310. doi: 10.1177/0192623307313011.. ** These authors examined whether residence in cities with high air pollution was associated with neuroinflammation and neurodegenerative changes in the CNS in a sample of healthy children and young adults who had died suddenly.
- 51.Zareba W, Couderc JP, Oberdorster G, et al. ECG paremeters and exposure to carbon ultrafine particles in young healthy subjects. Inhalation Toxicology. 2008 doi: 10.1080/08958370802492407. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holgate ST. Asthma: a dynamic disease of inflammation and repair. In: Chadwick DJ, Cardew G, editors. The Rising Trends in Asthma. West Sussex, England: John Wiley & Sons; 1997. pp. 5–34. [DOI] [PubMed] [Google Scholar]
- 53.Pohunek P, Warner JO, Tuyrzikova J, Kudrmann J, Roche WR. Markers of esosinophilic inflmmation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatric Allergy & Immunology. 2005;16(1):43–51. doi: 10.1111/j.1399-3038.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 54.Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. American Journal of Respiratory and Critical Care Medicine. 2005;172(10):1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heaton T, Rowe J, Turner S, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–149. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 56.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 57.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nature Immunology. 2002;3(8):715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 58.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002 Jan;32(1):43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 59.Suarez CJ, Parker NJ, Finn PW. Innate immune mechanisms in allergic asthma. Current Allergy & Asthma Reports. 2008;8(5):451–459. doi: 10.1007/s11882-008-0085-8. [DOI] [PubMed] [Google Scholar]
- 60.Joachim RA, Noga O, Sagach V, et al. Correlation between immune and neuronal parameters and stress perception in allergic asthmatics. Clinical & Experimental Allergy. 2008;38(2):283–290. doi: 10.1111/j.1365-2222.2007.02899.x. [DOI] [PubMed] [Google Scholar]
- 61.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cellular Immunology. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veres TZ, Rochlitzer S, Shevchenko M, et al. Spatial interactions between dendritic cells and sensory nerves in allergic airway inflammation. American Journal of Respiratory and Critical Care Medicine. 2007;37(5):553–561. doi: 10.1165/rcmb.2007-0087OC. [DOI] [PubMed] [Google Scholar]
- 63.Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. Journal of Allergy and Clinical Immunology. 2006;117:67–71. doi: 10.1016/j.jaci.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 64.Bonham AC, Chen C-Y, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. Journal of Applied Physiology. 2006;101:322–7. doi: 10.1152/japplphysiol.00143.2006.. * This reviews existing evidence for potential targets for plasticity in the NTS environmental conditions that may influence plasticity, and the impact of this plasticity on lung and airway reflexes.
- 65.Nogueira PJ, Ferreira HHA, Antunes E, Teixeira NA. Chronic mild prenatal stress exacerbates the allergen-induced airway inflammation in rats. Mediators of Inflammation. 1999;8:119–122. doi: 10.1080/09629359990621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pincus-Knackstedt MK, Joachim RA, Blois SM, et al. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. Journal of Immunology. 2006;177:8484–8492. doi: 10.4049/jimmunol.177.12.8484. [DOI] [PubMed] [Google Scholar]
- 67.Clougherty J, Levy JI, Kubzansky LD, Ryan B, Canner Jacobson M, Franco Suglia S, Wright RJ. The effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863.. ** This is the first population-based study demonstrating an interaction between a community-level stressor (violence) and outdoor air pollution exposure on the risk for developing asthma in an urban community. An example of social x physical environmental interaction accounting for more of the variance than main effects.
- 68.Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental Health Perspectives. 2008;116(7):970–975. doi: 10.1289/ehp.11076.. ** These authors demonstrate the interactive effects of social stress and air pollution exposure on exacerbations of asthma in adolescents as indexed by symptoms and peak expiratory flow rates. Interactions also showed higher stress associated with heightened inflammatory profiles as pollution levels decreased. Interactions were more robust than main effects.
- 69.Ledoux J. The sympathetic self: how our brains become who we are. New York: Penguin Group; 2002. [Google Scholar]
- 70.Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychiatry. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Nelson CA, C LJ. The effects of stress and trauma on brain and memory: a view from developmental cognitive neuroscience. Developmental Psychopathology. 1998;10:793–809. doi: 10.1017/s0954579498001874. [DOI] [PubMed] [Google Scholar]
- 72.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience & Behavioral Physiology. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 73.Sousa N, Almeida OF. Corticosteroids: sculptors of the hippocampal formation. Reviews Neuroscience. 2002;13:59–84. doi: 10.1515/revneuro.2002.13.1.59. [DOI] [PubMed] [Google Scholar]
- 74.Zaharia MD, Kulzczycki J, Shanks N, Meaney MJ, Anisman H. The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: genetic and maternal factors. Psychopharmacology. 1996;128:227–223. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]
- 75.Frisone DR, Frye CA, Zimmerberg B. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behavioral Brain Research. 2002;128:153–160. doi: 10.1016/s0166-4328(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 76.Hubbs-Tait L, Nation JR, Krebs NF, Bellinger DC. Neurotoxicants, micronutrients, and social environments: individual and combined effects on children’s development. Psychological Science in the Public Interest. 2005;6:57–121. doi: 10.1111/j.1529-1006.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 77.Wright RO. Neurotoxicology: what can context teach us? Journal of Pediatrics. 2008;152(2):155–157. doi: 10.1016/j.jpeds.2007.10.040.. * A recent comment on the independent and combined influences of social context and physical hazards on child neurodevelopment.
- 78.Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29(5):812–827. doi: 10.1016/j.neuro.2008.03.003.. ** Examines the interactive and potentially synergistic effects of lead and stress in experimental rodent models.
- 79.Rossi-George A, Virgoline MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: A potential biological unifying mechanism for their corresponding disease profiles. Toxicology & Applied Pharmacology. 2008 doi: 10.1016/j.taap.2008.10.003. Epub ahead of print. ** Examines the interactive and potentially synergistic effects of lead and stress in experimental rodent models and influences on HPA axis disruption.
- 80.Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008;29(6):928–939. doi: 10.1016/j.neuro.2008.09.010.. ** Examines the interactive and potentially synergistic effects of lead and stress in experimental rodent models on subsequent stress response in offspring.
- 81.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology Teratology. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002.. ** A prospective study of the interaction between an index of social stress and tobacco smoke exposure on neurodevelopment in socially disadvantaged urban children.
- 82.Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiology of Disease. 2008;31(2):159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Research. 2001;896:48–55. doi: 10.1016/s0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- 84.Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Annals Neurology. 2003;53:50–56. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- 85.Moreley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18(12):3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- 86.Laviola G, Rea M, Morley-Fletcher S, et al. Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. European Journal of Neuroscience. 2004;20(6):1655–1664. doi: 10.1111/j.1460-9568.2004.03597.x. [DOI] [PubMed] [Google Scholar]
- 87.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development. 2008;84:249–245. doi: 10.1016/j.earlhumdev.2007.06.004.. ** These authors are the first to prospectively assess how antenatal stress exposure and postnatal rearing style (maternal sensitivity) act in concert to shape the offspring biobehavioral stress response in a human sample. Maternal sensitivity modulated the effects of prenatal psychiatric illness on the infant cortisol response.
- 89.Subramanian SV, Ackerson LK, Subramanyam MA, Wright RJ. Domestic violence is associated with adult and childhood asthma prevalence in India. International Journal of Epidemiology. 2007;36(3):569–579. doi: 10.1093/ije/dym007. [DOI] [PubMed] [Google Scholar]
- 90.Franco Suglia S, Ryan L, Laden F, Dockery D, Wright RJ. Violence exposure, a chronic psychosocial stressor, and childhood lung function. Psychosomatic Medicine. 2007;70(2):160–169. doi: 10.1097/PSY.0b013e318160687c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franco Suglia S, Bosquet Enlow M, Kullowatz A, Wright RJ. Maternal intimate partner violence predicts increased asthma incidence in children: Buffering effects of supportive caregiving. Archives Pediatrics & Adolescent Medicine. doi: 10.1001/archpediatrics.2008.555. in press. ** The first prospective study of the buffering effects of the quality of maternal caregiving on the link between stress and asthma expression in early childhood.
- 92.Bender-Berz J, Carter A, Wagmiller R, Horwitz S, Klein Murdock K, Briggs-Gowan MJ. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2-3 year olds. Journal of Pediatric Psychology. doi: 10.1093/jpepsy/jsj123. in press. [DOI] [PubMed] [Google Scholar]
- 93.Surkan PJ, Schnaas L, Wright RJ, et al. Maternal self-esteem, exposure to lead, and child neurodevelopment. Neurotoxicology. 2008;29(2):278–285. doi: 10.1016/j.neuro.2007.11.006.. ** A prospective study demonstrating the potential buffering effects of maternal self-esteem on the adverse effects of lead and neurodevelopment in early childhood.
- 94.Thanh Tu M, Grunau RE, Petrie-Thomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Developmental Psychobiology. 2007;49(2):150–164. doi: 10.1002/dev.20204.. ** This study investigated the relationships among neonatal pain-related stress, cognitive indices (focused attention), and basal cortisol levels in VLGA infants at 8 months (corrected age) and the potential role of maternal factors (parenting stress, interactive behaviors) in ameliorating adverse effects of stress on cortisol and focused attention in these infants.
- 95.Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environmental and Molecular Mutagenesis. 2008;49:4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- 96.Miller RL, Ho S-M. Environmental epigentics and asthma: Current concepts and call for studies. American Journal of Respiratory and Critical Care Medicine. 2008;177:567–573. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environmental and Molecular Mutagenesis. 2008;49(1):46–60. doi: 10.1002/em.20357.. ** Reviews experimental evidence demonstrating that the egipgenomic state of a gene (glucocorticoid receptor promoter) can be established through behavioral programming.
- 98.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004:7847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 99.Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008;29(5):28–32. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Neill MS, Jerrett M, Kawachi I, et al. Health, wealth, and air pollution: advancing theory and methods. Environmental Health Perspectives. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Subramanian SV, Wright RJ. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132:757–69. doi: 10.1378/chest.07-1904.. * This overviews a multilevel approach that recognizes the embedding of asthma within its biological, psycho-socioeconomic, environmental and community contexts. Examples of practical statistical approaches to multi-level modeling are also provided.
- 102.Wright RJ, Franco Suglia S, Levy JI, et al. Transdisciplinary research strategies for understanding socially patterned disease: The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) Project as a case study. Ciencia & Saude Coletiva. 2008;13(6):1729–1742. doi: 10.1590/s1413-81232008000600008.. * Overviews the design of a multilevel study considering the interaction of social and physical environmental factors on urban asthma risk.