Abstract
The beginnings of pig domestication in Southwest Asia are controversial. In some areas, it seems to have occurred abruptly ca. 10,500 years ago, whereas in nearby locations, it appears to have resulted from a long period of management of wild boar starting at the end of the Late Pleistocene. Here, we present analyses of suid bones from Akrotiri Aetokremnos, Cyprus. This site has provided the earliest evidence for human occupation of the Mediterranean islands. Morphological analysis and direct radiocarbon dating of both degraded collagen and apatite of these bones reveal that small-sized suids were living on Cyprus 11,400–11,700 years ago. We demonstrate that these suids were introduced by humans and that, at this early date, their small size must result from island isolation. This sheds light on the early Holocene colonization of Cyprus and on pre-Neolithic Mediterranean seafaring. We further argue that wild boar were managed on the mainland before their introduction to Cyprus (i.e., before the beginning of the Neolithic and at least 1 millennium before the earliest known morphological modifications attributable to domestication). This adds weight to the theory that pig domestication involved a long period of wild boar management that started about the time of the Pleistocene/Holocene transition.
Keywords: domestication, island, Near East, radiocarbon dating, Sus
Among the various places where pigs have been independently domesticated through the Old World, Eastern Anatolia (Turkey) is the earliest (1). In the upper Euphrates basin, at Nevalı Çori, a rapid decrease in animal size ca. 10,500 calibrated radiocarbon date (cal.) B.P. suggests an abrupt event and a constant and intensive breeding pressure (2). On the other hand, in the long chronological sequence of Çayönü in the Tigris valley, evidence suggests that domestication began earlier but evolved more slowly, with fully domestic pigs emerging around ca. 9,000 cal. B.P (3). This suggests the existence of an intermediate stage between “hunting” and “breeding.” Although disputed, wild boar at Hallan Çemi (4, 5) in the Tigris Basin might have been managed in the wild as early as 13,000–12,700 cal. B.P. in a way similar to the way they are managed by some modern New Guineans (6). In this controversial context, the presence of a small number of suid bones at the Cypriot site of Akrotiri Aetokremnos dated to ca. 12,500 cal. B.P. deserves further attention.
Cyprus has never been connected to the mainland. Today, the minimal distance to the mainland is 69 km, and the last Pleniglacial marine regression only reduced it to 63 km, with an islet in-between (7). As a result of this long isolation, the last Late Glacial Cypriot terrestrial mammal community was characterized by a small number of endemic species: dwarf elephants and hippopotami, a genet, and 1 or 2 species of mice (8, 9). The earliest evidence for human presence on the island came from Aetokremnos, a small collapsed rock shelter on the island's southern coast (9–11). The site is composed of 3 principal layers. The lowest (layer 4) produced a huge number of pygmy hippopotami (Phanourios minutus) as well as smaller amounts of other fauna and lithic artefacts. Layer 3 is nearly sterile. Layer 2 yielded numerous lithic artefacts, shellfish and bird remains, and a few hippopotami bones (9). Radiocarbon dating of charcoal from layers 2 and 4 attests that humans were present on Cyprus over 12,000 years ago (i.e., shortly before the beginning of the Holocene) (9). The exact role played by humans in hippopotamus extinction remains controversial, because the reliability of 14C dating carried out on hippopotamus bones is problematic (10, 12–15). However, most scholars agree that the hippopotami did not survive after the beginning of the Holocene some 11,800 years ago.
Pertinent to this essay was the interpretation of 18 bones, originally identified as pig (14 bones) and deer (4 bones) (16). They were spread throughout the 3 layers. No evidence for the presence of suid or deer has been documented in the faunally rich Pleistocene deposits of Cyprus (8). The earliest presence on the island of both taxa is associated with the Early Prepottery Neolithic period, dated to 10,400–10,000 cal. B.P (17). The identification of the bones (18) and whether they date to the occupation of Akrotiri or are more recent intrusions (18, 19) were in question. Here, we report a previously undescribed taxonomic study of this assemblage, including comparisons with faunal samples from the mainland as well as radiocarbon dating of the suid remains.
Results
Anatomical and Taxonomic Revision.
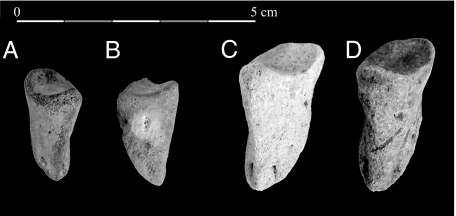
Anatomical and morphometrical analyses demonstrate that the 4 phalanges originally attributed to the Mesopotamian fallow deer (Dama mesopotamica) (16) are actually the first and third axial phalanges of suids (Sus scrofa) (Fig. 1). Evidence for the revision of the identifications is supplied in (SI Text and Figs. S1–S3). Therefore, the incisor and the 17 metapodials and phalanges all belong to suids (Table S1). They indicate the presence of at least 2 individuals (aged 18–24 months old and more than 25 months old at death). The very unbalanced pattern of the representation of skeletal parts (Fig. S4) argues for selection by a predator, either animal or human (assuming that no suid bones were left unidentified among the nearly 220,000 hippopotamus bones recovered from the site; SI Text). Only the bearded vulture (Gypaetus barbatus) is able to accumulate bones with such a high rate of phalanges (20), but it always leaves numerous digestion marks, which are missing at Aetokremnos. However, such a pattern may fit butchery refuse (21) or bones left by humans as part of a hide (16).
Fig. 1.
Dorsal views of abaxial (A and B) and axial (C and D) third phalanges of suids from Aetokremnos: FN367 N97E87–88 (A), FN385 N99E87 (B), FN914 N96E89 (C), and FN340 N96E91 (D). C and D were previously attributed to the fallow deer (16). Photograph courtesy of J.-D.V.
Radiocarbon Dating.
In the absence of preserved bone collagen in the unburnt bones, we dated the organic and inorganic (biogenic apatite or bioapatite) fractions of burnt bones. When exposed to low temperatures, bone collagen degrades, becomes trapped in the apatite structure, and can therefore be dated. At higher temperatures, bioapatite recrystallizes and becomes more resistant to diagenetic alteration (22). Three axial third phalanges were selected, showing different degrees of burning: unburnt (FN422); charred (FN1138A), and partly calcined (FN1138B). Degraded collagen from FN1138A gave the oldest age (10,045 ± 52 B.P.; Table 1), followed by the bioapatite from FN1138B (9,842 ± 54 B.P.). The other 2 bioapatite samples gave significantly younger ages. The difference of 1,000 years in 14C age between the organic and inorganic fractions from the same bone (FN1138A) suggests that the inorganic carbon from bone apatite experienced isotopic exchange with surface waters. Therefore, we rejected the 2 dates on charred and unburnt apatite. FN1138B may have suffered from limited isotopic exchange with soil waters because of the fact that it was only partially calcined (23). In conclusion, degraded collagen provides the most accurate and reliable date, followed by partly calcined apatite. After calibration (24), our results indicate an age of 11,700–11,400 cal. B.P. (1σ). Although slightly younger than the main occupation of layer 2, this date excludes the possibility that the suid bones are a Neolithic or more recent intrusion.
Table 1.
Radiocarbon dates and calibrations from Akrotiri suid phalanges
| AA ref. | Sample | Material | Date, years | ±, years | d13C (PDB, ‰) | cal. B.P. |
|||
|---|---|---|---|---|---|---|---|---|---|
| 68.2% |
95.4% |
||||||||
| From | To | From | To | ||||||
| 79920 | FN422 | Bone apatite | 8,588 | 50 | −12.8 | 9,505 | 9,598 | 9,488 | 9,668 |
| 79921 | FN1138A | Bone apatite (charred) | 9,055 | 52 | −14.6 | 10,194 | 10,245 | 9,964 | 10,380 |
| 79922 | FN1138B | Bone apatite (partly calcined) | 9,842 | 54 | −18.7 | 11,201 | 11,285 | 11,179 | 11,390 |
| 79923 | FN1138A | Degraded collagen | 10,045 | 69 | −25.1 | 11,396 | 11,746 | 11,272 | 11,956 |
Radiocarbon (14C Accelerator mass spectrometry, AMS) dates from Aetokremnos suid phalanges. Dates are calibrated using the Radiocarbon calibration programme rev. 3.0.3. (24). Only the outer limits of the ranges are shown. For details of method, see Materials and Methods.
Morphometrical Comparisons.
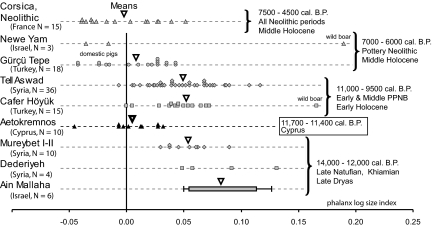
The Aetokremnos phalanges are 9 to 20% smaller than Late Dryas wild boar phalanges from the Levant (Fig. 2, SI Text, and Table S3). They are also significantly smaller than phalanges from the Early and Middle Prepottery Neolithic B (PPNB) domestic suids in this area (Fig. 2). Conversely, they are the same size as the very small domestic pigs of the Final PPNB of Gürçü Tepe (Turkey) and of the Pottery Neolithic domestic pigs in Israel. They are also the same size as the Early and Middle Neolithic domestic pigs of Corsica, which are among the smallest known Holocene suids from a Mediterranean island.
Fig. 2.
Comparison of the size of the suid phalanges at Aetokremnos with those of contemporary wild boar, PPNB early domestic suids, Pottery Neolithic pigs of the Northern and Southern Levant, and Neolithic Mediterranean island pigs from Corsica. The reference value (0.00) is the mean of the values of the pig phalanges from US 1,000, at Shillourokambos, Cyprus. References for data are listed in SI Text.
Discussion
Radiocarbon dating indicates that the Aetokremnos suid remains are Early Holocene in age. They are too small to come from any known wild population living on the continent at that time. We can also exclude a continental domestic origin, because the earliest size decrease attributable to domestication is attested at least 1 millennium later on the mainland (2, 3). Consequently, even if these phalanges were part of a hide (or of butchered provisions), they could not have been imported from the mainland, and we conclude that suids were living on Cyprus at least 11,400 years ago. At such an early date, the 9–20% size difference between Aetokremnos suids and the Levantine wild boar dated to the Younger Dryas most likely results from an island effect rather than domestication pressure.
It is unlikely that this population migrated to Cyprus very long before the 12th millennium cal. B.P., because there is no record of suids in the Pleistocene faunas of Cyprus (8). Starting from the time a large mammal population is isolated on an island, size decrease and reduction of the limb extremities occur very fast (25), especially for suids (26). During the Pleistocene, endemic hippopotami probably saturated most of the large mammal ecological niches of Cyprus, precluding the settlement of any potential competitor on the island. Their Late Pleistocene extinction released numerous niches that could, in turn, have been colonized by suids, which are known for their ecological adaptability and prolificacy (26, 27) and have biological traits close to those of hippopotami. Although good swimmers, colonization of remote islands by wild boar is not known anywhere in the world (e.g., Japan, New Guinea) (26, 27), and there is no record of suids on any of the isolated Mediterranean islands during the Pleistocene (28). It seems that early Holocene wild boar would not have crossed the 70-km wide Cyprus channel frequently enough to form a permanent population on the island. During the last Pleniglacial, the distance between Cyprus and the mainland was reduced to 63 km because of the 130-m lowering of the sea level. Bathymetric maps indicate the presence of an undersea ridge stretching from the tip of the Karpass Peninsula toward the Gulf of Iskenderun (7). At least 1 island would appear along this ridge with a lowering of 130 m, reducing the distance between Karatas and Cyprus to 2 spans of water of 45 and 18 km. However, even a 45-km journey in the open sea remains too long for wild boar. This is especially true if the currents are strong, as they should probably have been during the last Pleniglacial, because of the higher temperature gradient between the south and the north of the Eastern Mediterranean basin and because of a narrower sea arm between Cyprus and the Levantine coastline.
It is more likely that these suids were purposefully introduced by humans, who can thus be suggested as having frequented the island before the occupation of Aetokremnos. Mesolithic and Neolithic introductions of suids by humans are attested in the Baltic Sea (27); in the Mediterranean (28); in Japan (26); in Flores, Timor, and New Guinea (29); and, later, in many islands of the Indian Ocean and Pacific Ocean. Recent reports of small-sized suid bones on the Aegean islands of Youra and Kythnos during the 10th and 11th millennia cal. B.P (30) suggest that managed wild boar predated domestic pigs in this area by at least 1 millennium. The recent discovery of suid bones at the likely PPNA site of Agia Varvara Asprokremnos (31) and the dominance of suids in the earliest Cypro-PPNB phases at Shillourokambos (10,400 cal B.P.) (17) suggest that suids survived in Cyprus and continued to be a major resource throughout the Neolithic. Thus, contrary to what was previously hypothesized, the island was not lacking game between the Late Pleistocene extinction of the small hippopotami and the Early Holocene introduction of domestic animals (19). Suids are one of the most prolific and easy-to-catch animals, and their presence on Cyprus could have been the reason for visits or even permanent settlement by humans.
Anthropogenic introduction of suids to Cyprus during the 12th millennium cal. B.P. also implies that wild boar were already managed on the continent at that time (i.e., 1,500 years before the earliest attested domestication) (2). Management in the wild during the Younger Dryas has been suggested for gazelles [Natufian (32)], goats [Zarzian (33)], and wild boar [Late Natufian (4, 5)]. Genetic analyses of the existing populations of the ancestor of the domestic goat, the bezoar goat (Capra aegagrus), have recently indicated that management of this goat occurred over a very large area, from Eastern Anatolia to the Iranian Central Zagros, and probably predated domestication (34). The transportation of wild boar to Cyprus 12,000 years ago is one of the strongest cases for predomestic management. This clearly demonstrates a long period of management in the wild (6) before morphological change attributable to domestication, as observed at Nevalı Çori (2). Such an intermediate wild-domestic status in both the Upper Tigris valley (3–5) and Cyprus indicates that pre-Neolithic wild boar management could have been practiced from Eastern Anatolia to the Mediterranean coast. Even if these suids were still wild, this practice appears to be an early step in the domestication process. The ensuing rate of intensification of control of suid populations then varied from place to place, leading to fast morphological modifications at some sites of the Upper Euphrates valley (2) and slower changes at others, as in the Tigris basin (3).
Materials and Methods
Radiocarbon Dating of the Suid Remains.
In an initial attempt to date the suid remains, 2 (unburnt) samples were sent to the radiocarbon laboratory in Belfast (U.K.). They gave a collagen/bone yield of 0.3 and 0.03%, respectively, below the acceptable limit (1%) to produce a reliable 14C date based on bone collagen. This is in keeping with results previously reported (9) on Phanourios bones from the same site, suggesting that conditions here do not favor collagen preservation.
It was therefore decided to focus on the inorganic fraction of bone, carbonate hydroxylapatite or bioapatite. Bioapatite contains about 1 wt % carbon in the form of carbonate ions, which substitute for phosphate and can be used for 14C dating. The suitability of this inorganic carbon for radiocarbon dating was suggested a long time ago (35), and it was successfully applied to bones found in sub-Saharan environments (36, 37). In the absence of tooth enamel, calcined bones are the best candidate for 14C dating (22). This is because when exposed to temperatures above 600 °C, bioapatite crystals increase in size and become more resistant to chemical exchange. Calcination is associated with a decrease in δ-13C values (up to 10‰), which can be used as an indicator of the degree of recrystallization (24).
Three suid bones (all coming from layer 2) were selected for 14C dating, showing different degrees of cremation: not cremated (FN422), charred (FN1138A), and partly calcined (FN1138B). Secondary carbonates present in the porosity of bioapatite were leached under vacuum using 1 M acetic acid until there was no visible degazing. The advantage of this method is that it preferentially removes secondary carbonates without altering bioapatite carbonate (38). To test for carbon isotope exchange in bioapatite, we dated the organic fraction of FN1138A, together with the bioapatite fraction of the same bone. In charred bones, bioapatite is not recrystallized, and it is therefore comparable to unburnt bones. The organic fraction originates from degraded collagen and can be dated after acid/alcali/acid pretreatment to remove secondary carbonates and humics/humin acids. The purified sample was then heated at 450 °C in the presence of O2, and the evolved CO2 was trapped by cryogeny. For apatite samples, CO2 was liberated with 4 mL of 60% phosphoric acid (vol/vol) at 90 °C. Graphitization, AMS measurements, and data processing were performed at the University of Arizona AMS 14C Laboratory (Tucson, AZ).
Osteometric Methods and Material for Size Comparisons.
To maximize the dataset, we compared all measurable phalanges from Aetokremnos (abaxial and axial) using the log size index technique (39). As references for this, we used the mean values of measurements of abaxial and axial phalanges from the large Cypriot suid bone series from a homogeneous stratigraphic unit (US 1,000, ca. 9,500–9,000 cal. B.P.) of the third section of the Early Prepottery Neolithic site at Shillourokambos. To reduce allometric effects, we only used length measurements of the phalanges: greatest length for the first phalanx, peripheral greatest length for the second, and dorsal length for the third [measured according to von den Driesch (40)]. The dispersion coefficients (100 × mean/SD) of these measurements taken on the reference series range below 10% (5–8.2%, Table S3), indicating both a moderate natural variability and low measurement relative errors, and thus a good reliability, even for abaxial phalanges.
Because archaeozoologists usually do not take or publish these measurements, it is difficult to find large series of comparative references in the literature. We only took into consideration the measurements of the axial second and third phalanges. The references that we used are as listed in SI Text.
Supplementary Material
Acknowledgments.
We thank L. Gourichon, D. Helmer, and J. Peters for unpublished metric data on Dederyieh, Cafer Höyük, and Gürçü Tepe, respectively; S.J.M. Davis for rich discussions; and O. Bar Yosef and G. Wilcox for suggestions on an initial draft. We also thank R. Bendrey, who contributed to improve the English of the manuscript. The French School at Athens, French Ministry of Foreign Affairs, and Department of Antiquities of Cyprus approved or helped our work in Cyprus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The archaeological bones reported in this paper are temporarily deposited for study in the Muséum national d'Histoire naturelle, Paris, and will be deposited finally in the Kourion Museum at Episkopi, Cyprus.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905015106/DCSupplemental.
References
- 1.Larson G, et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci USA. 2007;104:15276–15281. doi: 10.1073/pnas.0703411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters J, von den Driesch A, Helmer D. In: The First Steps of Animal Domestication. New Archaeological Approaches. Vigne J-D, Peters J, Helmer D, editors. Oxford: Oxbow Books; 2005. pp. 96–124. [Google Scholar]
- 3.Ervynck A, Dobney K, Hongo H, Meadow R. Born free? New evidence for the status of Sus scrofa at Neolithic Çayönü Tepesi (Southeastern Anatolia, Turkey) Paléorient. 2001;27(2):47–73. [Google Scholar]
- 4.Rosenberg M, Nesbitt R, Redding RW, Peasnall BL. Hallan Çemi, pig husbandry, and post-Pleistocene adaptations along the Taurus-Zagros Arc (Turkey) Paléorient. 1998;24(1):25–41. [Google Scholar]
- 5.Redding R. In: The First Steps of Animal Domestication. New Archaeological Approaches. Vigne J-D, Peters J, Helmer D, editors. Oxford: Oxbow Books; 2005. pp. 41–48. [Google Scholar]
- 6.Redding R, Rosenberg M. In: Ancestors for the Pigs: Pigs in Prehistory. Nelson SM, editor. Philadelphia: University of Pennsylvania; 1998. pp. 65–76. [Google Scholar]
- 7.Held SO. Colonization cycles on Cyprus 1: The biogeographic and paleontological foundations of early prehistoric settlement. Reports of the Department of Antiquities, Cyprus (Nicosia) 1989:7–28. [Google Scholar]
- 8.Boekschoten GJ, Sondaar PY. On the fossil mammalia of Cyprus. Proc K Ned Akad Wet B. 1972;75(4):306–338. [Google Scholar]
- 9.Simmons AH. Faunal Extinction in an Island Society. New York: Kluwer Academic–Plenum Publishers; 1999. [Google Scholar]
- 10.Simmons AH. Extinct pygmy hippopotamus and early man in Cyprus. Nature. 1988;333:554–557. [Google Scholar]
- 11.Simmons AH. In: Neolithic Revolution! New Discoveries in the Neolithic of Cyprus. Peltenburg E, Wasse A., editors. Oxford: Oxbow Books; 2004. pp. 1–14. Levant Suppl Ser 1. [Google Scholar]
- 12.Bunimovitz S, Barkai R. Ancient bones and modern myths: Ninth millennium BC hippopotamus hunters at Akrotiri Aetokremnos, Cyprus? Journal of Mediterranean Archaeology. 1996;9(1):85–96. [Google Scholar]
- 13.Olsen S. In: Faunal Extinction in an Island Society. Simmons A, editor. New York: Kluwer Academic–Plenum Publishers; 1999. pp. 230–238. [Google Scholar]
- 14.Ammerman AJ, Noller S. New light on Aetokremnos. World Archaeol. 2005;37:533–543. [Google Scholar]
- 15.Simmons AH, Mandel R. Not such a new light: A response to Ammerman and Noller. World Archaeol. 2007;39:475–482. [Google Scholar]
- 16.Reese DS. In: Faunal Extinction in an Island Society. Simmons AH, editor. New York: Kluwer Academic–Plenum Publishers; 1999. pp. 164–167. [Google Scholar]
- 17.Vigne J-D, Carrère I, Guilaine J. Unstable status of early domestic ungulates in the near east: The example of Shillourokambos (Cyprus, IX–VIIIth millennia cal. B.C.) Bulletin Correspondance Héllenique. 2003;(Suppl 43):239–251. [Google Scholar]
- 18.Poplin F, Vigne J-D. footnote 1 of reference 16. In: Simmons AH, editor. Faunal Extinction in an Island Society. New York: Kluwer Academic–Plenum Publishers; 1999. p. 167. [Google Scholar]
- 19.Wasse A. In: On the Margins of Southwest Asia: Cyprus During the 6th to 4th Millennia BC. Clarke J, editor. Oxford: Oxbow Books; 2007. pp. 44–63. [Google Scholar]
- 20.Robert I, Vigne J-D. The bearded vulture (Gypaetus barbatus) as an accumulator of archaeological bones. Late glacial assemblages and present day reference data in Corsica (Western Mediterranean) J Archaeol Sci. 2002;29:763–777. [Google Scholar]
- 21.Binford L. Bones: Ancient Man and Modern Myths. New York: Academic; 1981. [Google Scholar]
- 22.Lanting JN, Aerts-Bijma AT, van der Plicht J. Dating cremated bone. Radiocarbon. 2001;43(2A):249–254. [Google Scholar]
- 23.Olsen J, et al. Characterisation and blind testing of radiocarbon dating of cremated bone. J Archaeol Sci. 2008;35:791–800. [Google Scholar]
- 24.Stuiver M, Reimer PJ. Radiocarbon calibration programme rev. 3.0.3. Radiocarbon. 1993;35:215–230. [Google Scholar]
- 25.Blondel J. Evolution Biogeography. Paris: Masson; 1986. in French. [Google Scholar]
- 26.Hongo H, Anezaki T, Yamazaki K, Takahashi O, Sugawara H. In: Pigs and Humans, 10,000 Years of Interaction. Albarella U, Dobney K, Ervynck A, Rowley-Conwy P, editors. Oxford: Oxford Univ Press; 2007. pp. 109–130. [Google Scholar]
- 27.Rowley-Conwy P, Dobney K. In: Pigs and Humans, 10,000 Years of Interaction. Albarella U, Dobney K, Ervynck A, Rowley-Conwy P, editors. Oxford: Oxford Univ Press; 2007. pp. 131–155. [Google Scholar]
- 28.Vigne J-D. In: The Holocene History of the European Vertebrate Fauna. Benecke N, editor. Berlin: Deutsches Archäologisches Institut; 1999. pp. 295–322. [Google Scholar]
- 29.Larson G, et al. Phylogeny and ancient DNA of Sus provide insights into Neolithic expansion in Island Southeast Asia and Oceania. Proc Natl Acad Sci USA. 2007;104:4834–4839. doi: 10.1073/pnas.0607753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trantalidou K. In: A Colloquium on the Prehistory of the Cyclades. Brodie N, Doole J, Gavalas G, Renfrew C, editors. Cambridge, UK: McDonald Institute Monographs; 2008. pp. 19–27. [Google Scholar]
- 31.McCartney C, Manning SW, Sewell D, Stewart ST. The EENC 2006 field season: Excavations at Agia Varvara-Asprokremnos and survey of the local early Holocene landscape. Reports of the Department of Antiquities, Cyprus (Nicosia) 2007:27–44. [Google Scholar]
- 32.Legge AJ. In: Papers in Economic Prehistory. Higgs ES, editor. Cambridge, UK: Cambridge Univ Press; 1972. pp. 119–124. [Google Scholar]
- 33.Hole F. In: The Origins and Spread of Agriculture and Pastoralism in Eurasia. Harris DR, editor. Washington: Smithsonian Institution Press; 1996. pp. 263–281. [Google Scholar]
- 34.Naderi S, et al. The goat domestication process inferred from large-scale mitochondrial DNA analysis of wild and domestic individuals. Proc Natl Acad Sci USA. 2008;105:17659–17664. doi: 10.1073/pnas.0804782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan AA, Termine JD, Haynes CV., Jr Mineralogical studies on bone apatite and their implications for radiocarbon dating. Radiocarbon. 1977;19(3):364–374. [Google Scholar]
- 36.Saliège JF, Person A, Paris F. Preservation of 12C/13C original ratio and 14C dating of the mineral fraction of human bones from Saharan tombs, Niger. J Archaeol Sci. 1995;22:301–312. [Google Scholar]
- 37.Sereno PC, et al. Lakeside cemeteries in the Sahara: 5000 years of Holocene population and environmental change. PLoS ONE. 2008;3:e2995. doi: 10.1371/journal.pone.0002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balter V, Saliège JF, Bocherens H, Person A. Evidence of physicochemical and isotopic modifications in archaeological bones during controlled acid etching. Archaeometry. 2002;44:329–336. [Google Scholar]
- 39.Meadow RH. In: History of Animals from Bones, Jubilee for Angela von den Driesch. Becker C, Manhart H, Peters J, Schibler J, editors. Rahden, Westfaly: Leidorf; 1999. pp. 285–300. in German. [Google Scholar]
- 40.von den Driesch A. A Guide to the Measurement of Animal Bones from Archaeological Sites. Cambridge, MA: Harvard University, Peabody Museum of Archaeology and Ethnology; 1976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.