Abstract
Yeast vacuole fusion requires 4 SNAREs, 2 SNARE chaperone systems (Sec17p/Sec18p/ATP and the HOPS complex), and 2 phosphoinositides, phosphatidylinositol 3-phosphate [PI(3)P] and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. By reconstituting proteoliposomal fusion with purified components, we now show that phosphoinositides have 4 distinct roles: PI(3)P is recognized by the PX domain of the SNARE Vam7p; PI(3)P enhances the capacity of membrane-bound SNAREs to drive fusion in the absence of SNARE chaperones; either PI(3)P or PI(4,5)P2 can activate SNARE chaperones for the recruitment of Vam7p into fusion-competent SNARE complexes; and either PI(3)P or PI(4,5)P2 strikingly promotes synergistic SNARE complex remodeling by Sec17p/Sec18p/ATP and HOPS. This ternary synergy of phosphoinositides and 2 SNARE chaperone systems is required for rapid fusion.
Intracellular membrane fusion is a conserved reaction, vital for vesicle trafficking, hormone secretion, and neurotransmission. Fusion is regulated by NSF (N-ethylmaleimide-sensitive factor)/Sec18p, αSNAP (soluble NSF attachment protein)/Sec17p, SNAREs (SNAP receptors), Sec1p/Munc18–1p family (SM) proteins, Rab GTPases, and Rab:GTP-binding proteins, termed “Rab effectors” (1–3). Lipids, including phosphoinositides, sterols, diacylglycerol (DAG), and phosphatidic acid (PA), have specific roles in fusion (4–14). Proteins and lipids cooperate for their enrichment in membrane fusion microdomains (6, 8, 15, 16).
SNARE proteins are integral or peripheral membrane proteins required for membrane fusion. SNAREs have either a Q or R residue at the center of their SNARE domain and associate in 4-helical QabcR complexes in cis (anchored to one membrane) or in trans (anchored to apposed membranes), where a, b, and c are families of related Q-SNAREs (2, 17, 18). Reconstituted proteoliposomes (RPLs) bearing Q-SNAREs fuse with RPLs bearing an R-SNARE through trans-SNARE-complex assembly (19, 20). This fusion has slow kinetics, requires nonphysiologically high SNARE densities, and causes substantial leakage of luminal contents of the RPLs (21–24).
We study membrane fusion with yeast vacuoles (lysosomes). Vacuole fusion (25) requires 3 Q-SNAREs (Vam3p, Vti1p, and Vam7p) and 1R-SNARE (Nyv1p) (26, 27), two SNARE chaperone systems, Sec17p/Sec18p/ATP (28), and the HOPS (homotypic fusion and vacuole protein sorting)/Vps Class C complex (29, 30), the Rab-GTPase Ypt7p (31), and chemically minor but functionally vital “regulatory lipids”: ergosterol (ERG), DAG, PI(3)P, and PI(4,5)P2 (8). Inactive 4SNARE cis-complexes on isolated organelles are disassembled by Sec17p/Sec18p/ATP (27). The heterohexameric HOPS complex, containing the SM protein Vps33p as a subunit, promotes and proofreads SNARE-complex assembly (32–34). HOPS can physically interact with the Q-SNAREs [Vam7p (35) and Vam3p (36, 37)], 4SNARE cis-complexes (32), GTP-bound Ypt7p (29), and phosphoinositides (35). PI(3)P supports the membrane association of the Qc-SNARE Vam7p, which has no transmembrane domain, through binding its PX domain (38). SNAREs, HOPS, Ypt7p, and regulatory lipids assemble in an interdependent fashion to form a fusion-competent membrane microdomain, the “vertex ring” (8, 16, 39). Trans-SNARE complexes are essential for fusion (26), yet fusion can be accelerated by SNARE-associating factors such as HOPS (14, 35) and by cycles of SNARE complex disassembly and reassembly, termed “remodeling” (40).
Membrane fusion has been reconstituted with all purified yeast vacuolar components, including 4SNAREs, vacuolar lipids, 2 SNARE chaperone systems, and phosphoinositides (14). We now show distinct functions of phosphoinositides in RPL fusion: the PX-domain of the SNARE Vam7p recognizes PI(3)P, as reported (38); PI(3)P activates the 3Q-SNAREs to be more fusogenic in the absence of SNARE chaperones; either PI(3)P or PI(4,5)P2 accelerates fusion by promoting the synergy between Sec17p/Sec18p and HOPS, although this synergy is not a function of the membrane recruitments of these SNARE chaperones. This ternary synergy between phosphoinositides and SNARE chaperones is essential for the assembly and remodeling of SNARE complexes.
Results
Phosphoinositides Stimulate SNARE- and SNARE-Chaperone-Dependent Membrane Fusion.
Vacuolar SNARE-RPLs without phosphoinositides (supporting information (SI) Fig. S1), SNARE chaperones (Sec17p/Sec18p and HOPS), and either diC8-PI(3)P or diC8-PI(4,5)P2 but not both (Fig. 1, Figs. S2 A and B and S3A) were incubated and assayed for fusion by a lipid-mixing assay, using the fluorescence resonance energy transfer (FRET) between NBD-PE [N-(7-nitro-2,1,3-benzoxadiazole-4-yl)-phosphatidylethanolamine] and Rh-PE [N-(lissamine rhodamine B sulfonyl) phosphatidylethanolamine] (19, 41). Water-soluble diC8-phosphoinositides partition into the outer monolayers of RPLs, mimicking the cytoplasmic leaflets of vacuolar membranes. RPL lipid mixing was stimulated 74- or 10-fold by diC8-PI(3)P or diC8-PI(4,5)P2, respectively (Fig. 1 A and B). Thus sufficient levels of either phosphoinositide can support RPL fusion (Fig. 1, Figs. S2, and S3). Phosphoinositide-stimulated fusion requires the Qa-SNARE Vam3p, Sec17p, Sec18p, ATP, and HOPS (Fig. 1), indicating that diC8-phosphoinositides, whatever their effect on the lipid bilayer, promote RPL fusion through the vacuolar SNARE- and SNARE-chaperone-dependent pathway.
Fig. 1.
Either PI(3)P or PI(4,5)P2 supports membrane fusion. (A and B) Requirements for lipid mixing in the presence of diC8-PI(3)P (A) or diC8-PI(4,5)P2 (B). Assays had 4SNARE-RPLs (450 μM total lipids, 300–750 nM of each SNARE), 1 mM ATP, 2 mM MgCl2, 620 nM Sec17p, 510 nM Sec18p, 28 nM HOPS, 1 μM anti-Vam3p (αVam3p), and 90 μM diC8-phosphoinositide, where indicated. Each panel shows representative data from at least 3 experiments.
SNARE proteins alone can cause the leakage of luminal contents during fusion (21, 24, 42). To test for lysis, we used dithionite (S2O42−), a membrane-impermeable reducing agent that destroys the fluorescence of NBD-PE (43), as described (14, 44). The fluorescence of exposed NBD-PE was rapidly diminished by S2O42− added either 32 min or 6 min before the addition of Sec17p/Sec18p/HOPS (Fig. S2 C and D). Because the S2O42− that was added 6 min before the SNARE chaperones was largely active and capable of destroying any exposed NBD fluorophore whereas S2O42− added 32 min before was fully inactivated (14), the equivalent rates of lipid mixing seen in each sample (Fig. S2 C and D) indicate that Sec17p, Sec18p, HOPS, and diC8-phosphoinositides promote fusion with little accompanying lysis of the SNARE-RPLs.
Are PI(3)P and PI(4,5)P2 the only phosphoinositides that can promote fusion? We tested diC4-PI(3)P, diC4-PI(4,5)P2, and 5 diC8-phosphoinositides, which comprise all of the phosphoinositides of Saccharomyces cerevisiae (45). DiC8-PI(3)P and diC8-PI(4,5)P2 were the most active; the diC4-phosphoinositides did not stimulate at all, and either diC8-PI(4)P or diC8-PI(3,5)P2 stimulated less than either diC8-PI(3)P or diC8-PI(4,5)P2, respectively (Fig. 2A; Fig. S4). In each case, comparable levels of soluble fusion proteins were reisolated with the SNARE-RPLs by floatation from fusion reactions (Fig. 2B; Fig. S4). Thus, with 4-SNARE RPLs, PI(3)P and PI(4,5)P2 must act on fusion per se rather than simply recruiting peripheral membrane proteins.
Fig. 2.
Phosphoinositide specificity. (A) Lipid mixing between 4SNARE-RPLs was assayed as in Fig. 1 with diC8- or diC4-phosphoinositides. Curves are representative of more than 3 experiments. (B) Membrane association of Vam7p (soluble Qc-SNARE), Sec17p, Sec18p, and HOPS is not affected by phosphoinositides. 4SNARE-RPLs were incubated as in A and then reisolated by Histodenz ultracentrifugation. Proteins that floated with the RPLs were analyzed by immunoblotting.
Only PI(3)P Supports Fusion in the Absence of SNARE Chaperones.
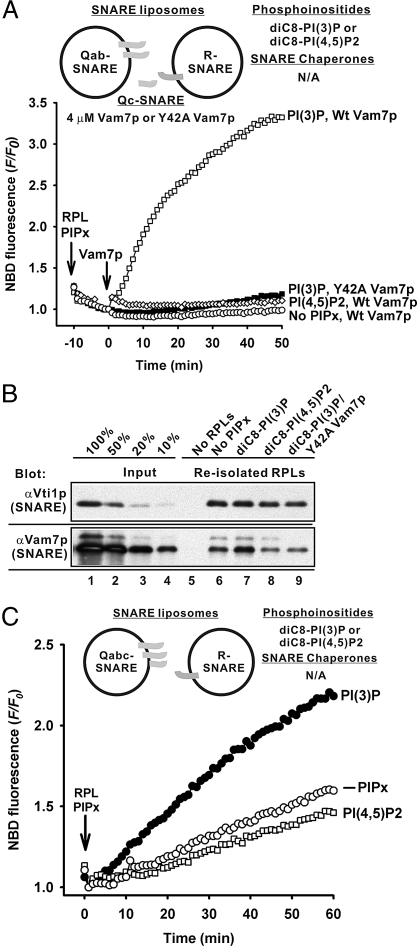
Although vacuoles bear all 4 SNAREs (46), they fuse through trans-SNARE pairing between the 3 Q-SNAREs and the R-SNARE, as shown with isolated organelles (26, 33) and RPLs (14, 47). We therefore used proteoliposomes with Q-SNAREs and R-SNARE on distinct fusion partners (Fig. S1) to explore the interplay of phosphoinositides with SNAREs and SNARE chaperones. Without SNARE chaperones, PI(3)P and added Vam7p (4 μM) promote fusion between Qab-SNARE RPLs and R-SNARE RPLs (Fig. 3A, open squares). PI(3)P enhances Vam7p binding to RPLs (Fig. 3B, lane 7). The Y42A Vam7p mutant, with little affinity for PI(3)P because of a point mutation in its PI(3)P-specific PX-domain (38), was unable to cooperate with PI(3)P to bind to RPLs or promote fusion (Fig. 3 A and B). Little fusion and less Vam7p association with the RPLs were obtained with either no phosphoinositides or with PI(4,5)P2 instead of PI(3)P (Fig. 3 A and B), showing its unique role in supporting fusion through Vam7p recognition (7, 38). An additional specific function of PI(3)P was shown by lipid-mixing assays with the Qabc-SNARE RPLs and R-SNARE RPLs (Fig. 3C). Although Vam7p had been associated during liposome preparation with the other 2 Q-SNAREs, PI(3)P still significantly stimulated the fusion between the Qabc-SNARE RPLs and R-SNARE RPLs (Fig. 3C, solid circles), while PI(4,5)P2 had no effect (open squares). Thus PI(3)P, and not PI(4,5)P2, has a unique role in promoting fusion, which is unrelated to its capacity to bind Vam7p.
Fig. 3.
Effects of phosphoinositides on fusion in the absence of SNARE chaperones. (A) PI(3)P promotes fusion without Sec17p/Sec18p and HOPS. Lipid mixing between Qab-SNARE RPLs (400 μM total lipids, 490–750 nM of each SNARE) and R-SNARE RPLs (50 μM total lipids, 65 nM of SNARE) were assayed with ATP (1 mM), MgCl2 (2 mM), either wild-type (WT) Vam7p or Y42A Vam7p (4 μM), and diC8-phosphoinositides (90 μM), where indicated. The experiment shown is representative of more than 3 experiments. (B) PI(3)P enhances the association of Vam7p with RPLs through its PX domain. RPLs were reisolated from the reactions in A, and RPL-bound Vam7p was analyzed by immunoblotting. (C) PI(3)P enhances fusion between R- and Qabc-SNARE RPLs. Lipid mixing between the Qabc-SNARE RPLs (400 μM total lipids, 300–780 nM of each SNARE) and R-SNARE RPLs (50 μM total lipids, 65 nM of SNARE) was assayed as in A but without exogenous Vam7p. The data are representative of more than 3 experiments.
Interplay Between Phosphoinositides and SNARE Chaperones Promotes SNARE Complex Assembly and Remodeling.
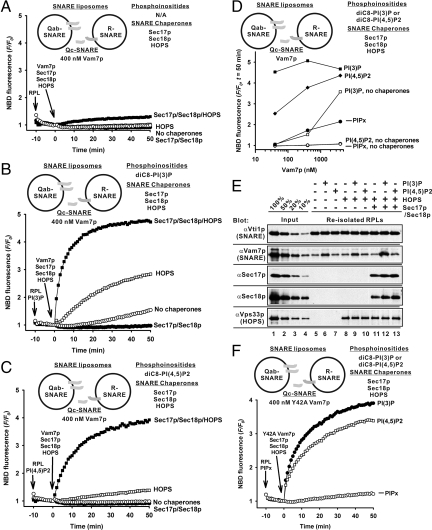
Without phosphoinositides, Qab-SNARE RPLs and R-SNARE RPLs, which are incubated with exogenous Vam7p (400 nM, comparable to the concentrations of the other 2 Q-SNAREs), Sec17p/Sec18p, and HOPS, only exhibit very slow lipid mixing (Fig. 4A, solid squares). However, with either PI(3)P or PI(4,5)P2, these SNARE chaperones synergistically stimulated the initial rates of RPL lipid mixing 35- or 17-fold, respectively, in comparison to the reaction in the absence of phosphoinositides and 8- or 26-fold in comparison to the reactions with HOPS alone (Fig. 4 A–C). Without HOPS, Sec17p/Sec18p alone abolished RPL lipid mixing, even in the presence of phosphoinositides (Fig. 4 A, B, and C, solid circles). The requirement for the interplay between chaperones and phosphoinositides is stricter at 40 nM Vam7p, a low and physiological (48) concentration (Fig. 4D). Phosphoinositides do not significantly promote the proteoliposomal association of HOPS or Sec17p/Sec18p (Fig. 4E, lanes 11–13), although PI(3)P enhances Vam7p binding (Fig. 4E, lanes 6, 9, and 12). However, in RPL lipid-mixing reactions with Y42A, a Vam7p mutant protein without affinity for PI(3)P, PI(3)P still strongly stimulated the lipid mixing in the presence of SNARE chaperones (Fig. 4F). Thus the interplay between SNARE chaperones and phosphoinositides does not simply support the membrane recruitment of soluble fusion proteins but rather directly participates in fusion per se. The striking interplay between SNARE chaperones and phosphoinositides was also seen in lipid-mixing reactions with Qabc-SNARE and R-SNARE RPLs (Fig. 5), in which the soluble Qc-SNARE Vam7p had been reconstituted with the other 2 Qab-SNAREs and was bound on RPL membranes in a complex. In this condition, the fusion rate in the absence of phosphoinositides but with both SNARE chaperones was less than the rate with HOPS alone or with no chaperones (Fig. 5A, open squares). Either PI(3)P or PI(4,5)P2 is necessary and sufficient for promoting the synergy of the SNARE chaperones to drive optimal fusion between these RPLs (Fig. 5 B and C, open squares). Thus it is likely that the interplay of the SNARE chaperones and phosphoinositides promotes either trans-SNARE pairing between preassembled Qabc-SNARE complexes and R-SNAREs or subsequent SNARE-complex remodeling (40).
Fig. 4.
Interplay of SNARE chaperones and phosphoinositides. (A, B, and C) In the presence of Sec17p/Sec18p and HOPS, either PI(3)P or PI(4,5)P2 significantly enhances fusion between the Qab- and R-SNARE RPLs with exogenous Vam7p. Lipid mixing between Qab- and R-SNARE RPLs was assayed as in Fig. 3A but with 400 nM Vam7, 620 nM Sec17p, 510 nM Sec18p, and 28 nM HOPS, where indicated, in the absence of phosphoinositides (A), or the presence of 90 μM diC8-PI(3)P (B), or diC8-PI(4,5)P2 (C). (D) Vam7p concentration dependence of fusion. Fusion was assayed as in A, B, and C with exogenous Vam7p (400, 40 nM, or 4 μM). (E) Association of Vam7p, Sec17p, Sec18p, and HOPS with RPLs. RPLs in fusion reactions as in A, B, and C were reisolated and bound proteins were analyzed. (F) PI(3)P strongly stimulates RPL fusion with Y42A Vam7p, which has little affinity for PI(3)P, in the presence of Sec17p/Sec18p and HOPS. Lipid mixing was assayed as in A, B, and C but with 400 nM Y42A Vam7p instead of WT Vam7p.
Fig. 5.
Either PI(3)P or PI(4,5)P2 supports the synergy between Sec17p/Sec18p and HOPS. (A, B, and C) Lipid mixing between Qabc- and R-SNARE RPLs was assayed as in Fig. 3C but with 1.2 μM Sec17p, 1.0 μM Sec18p, and 55 nM HOPS, where indicated, without phosphoinositides (A), or with 90 μM diC8-PI (3)P (B), or diC8-PI(4,5)P2 (C). The data are representative of more than 3 experiments.
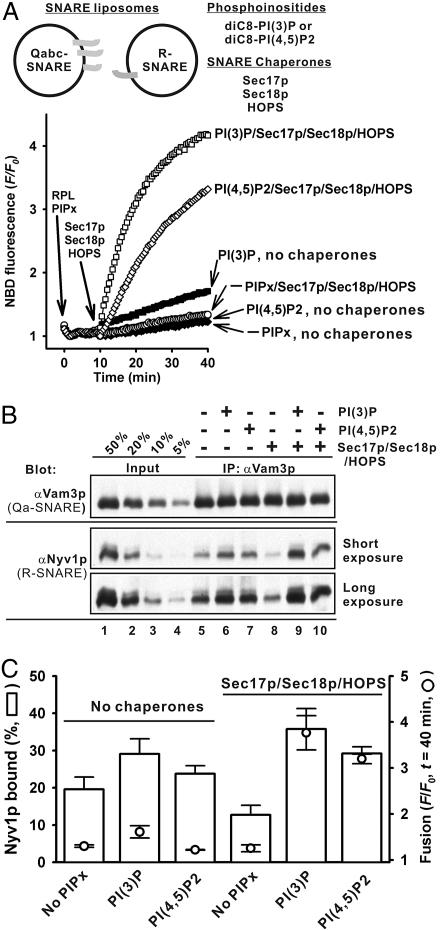
Do SNARE chaperones and phosphoinositides directly assemble and remodel SNARE complexes? To address this issue, we used a SNARE-complex assembly assay to measure the R-SNARE Nyv1p association with the Qa-SNARE Vam3p during fusion (Fig. 6). This assay contained Qabc- and R-SNARE RPLs, phosphoinositide [PIPx, either diC8-PI (3)P or diC8-PI(4,5)P2], and soluble proteins (Sec17p/Sec18p/HOPS). After incubation, proteins were immunoprecipitated with anti-Vam3p antibodies (αVam3p) and immunoblotted for Vam3p and Nyv1p (Fig. 6 and Fig. S5). Control experiments (Fig. S5) show that this assay measures Vam3p:Nyv1p association during the fusion reaction. Both SNARE chaperones and phosphoinositides were required for rapid fusion between Qabc- and R-SNARE RPLs (Fig. 6 A and C). Without SNARE chaperones, there was substantial Nyv1p association with Vam3p (Fig. 6B, lanes 5, 6, and 7, and Fig. 6C), comparable to the Nyv1p association in the presence of both phosphoinositides and SNARE chaperones (Fig. 6B, lanes 9 and 10, and Fig. 6C), even though there is very little fusion without SNARE chaperones (Fig. 6 A and C). Thus, the substantial new SNARE complexes must be in trans rather than postfusion cis conformation. The trans-SNARE complexes formed with those RPLs, SNARE chaperones, and phosphoinositides are far more fusogenic than the trans-complexes formed under the same conditions but in the absence of SNARE chaperones, suggesting that trans-SNARE complex does not suffice for fusion and that either SNARE remodeling by the interplay between SNARE chaperones and phosphoinositides is also essential or that a SNARE chaperone such as HOPS can directly participate along with SNAREs in rapid fusion (23).
Fig. 6.
Phosphoinositides and SNARE chaperones promote fusion more than SNARE complex assembly. (A and B) SNARE chaperones and either PI(3)P or PI(4,5)P2 are required to enhance the fusion competence of newly assembled SNARE complexes. (A) Lipid mixing between Qabc- and R-SNARE RPLs was assayed with either diC8-PI(3)P or diC8-PI(4,5)P2, Sec17p/Sec18p, and HOPS, where indicated, as in Fig. 5. (B) Nyv1p bound to Vam3p in the reactions in A. (C) Means and standard deviations of the fusion signals (F/F0, open circles) in A and Nyv1p bound (%, open bars) in B.
In contrast, during fusion between Qab-SNARE and R-SNARE RPLs with exogenous Qc-SNARE Vam7p (Fig. S6), SNARE chaperones and phosphoinositides synergistically promote the assembly of fusion-competent vacuolar SNARE complexes, as rapid RPL lipid mixing (Fig. S6 D and F) and robust Vam3:Nyv1p association (Fig. S6 E and F) required both SNARE chaperones and phosphoinositides. Thus SNARE chaperones are required for SNARE complex assembly when Vam7p has to be recruited to the membrane (Fig. 6 vs. Fig. S6), perhaps through the direct affinity of HOPS for both Vam7p and phosphoinositides (35).
Discussion
It has been suggested that SNARE proteins constitute the minimal machinery of membrane fusion: v-SNARE/R-SNARE on one membrane and t-SNAREs/Qabc-SNAREs on the apposed membrane assemble spontaneously to form trans v-t-SNARE/QabcR-SNARE complexes, which suffice for fusion (1, 2, 19, 20). Meanwhile, studies with yeast vacuoles, by genetic screening for altered organelle morphology in vivo (49, 50) and by in vitro fusion assays with isolated organelles, have shown that the Rab GTPase Ypt7p (31), the HOPS complex (29, 30, 35), Sec17p/Sec18p (28), and “regulatory lipids” (8) are all required, in addition to cognate SNAREs, for fusion. To connect these different approaches, we developed a reconstitution of vacuolar SNARE-proteoliposomal fusion, which requires SNARE chaperone systems, vacuolar lipids including phosphoinositides, and SNAREs (14). Our current studies support 4 major conclusions: (i) Either PI(3)P or PI(4,5)P2 alone can promote SNARE- and SNARE-chaperone-dependent fusion, and PI(3)P gives the more robust stimulation (Figs. 1 and 2). (ii) Phosphoinositides have multiple distinct functions for fusion: PI(3)P supports the membrane association of the Qc-SNARE Vam7p through the PI(3)P-binding PX domain (Fig. 3 A and B), as reported (38); only PI(3)P activates membrane-bound SNAREs to be more fusogenic in the absence of SNARE chaperones (Fig. 3C); and either PI(3)P or PI(4,5)P2 triggers a synergy of SNARE chaperones for rapid fusion (Figs. 4 and 5). (iii) The interplay between synergistic SNARE chaperones and phosphoinositides is independent of the membrane recruitment of soluble fusion proteins (Figs. 2B and 4 E and F). (iv) SNARE chaperones and phosphoinositides synergistically assemble and remodel SNARE complexes to trigger rapid fusion (Fig. 6 and Fig. S6).
Although phosphoinositides often recognize phosphoinositide-specific membrane binding modules (51), it had been unclear whether they have additional functions in membrane fusion. In reconstituted fusion, either PI(3)P or PI(4,5)P2 stimulates fusion by driving the synergy between Sec17p/Sec18p and HOPS, although phosphoinositides do not enhance the steady-state association of SNARE chaperones with membranes (Figs. 2, 4, and 5). Thus, phosphoinositides are not merely binding targets for membrane fusion proteins, but rather are essential to gather membrane-bound SNAREs and SNARE chaperones at the fusion-competent membrane microdomains and guide chaperones to act synergistically on SNAREs. In the absence of SNARE chaperones (Fig. 3), only PI(3)P supports the membrane recruitment of Vam7p and activates membrane-bound SNAREs. This helps to explain why PI(3)P exhibits a more robust stimulation of fusion when SNARE chaperones are present. Other PI(4,5)P2-specific functions may require other possible fusion proteins, such as phosphoinositide-specific phospholipase C (9). Phosphoinositide interactions with reconstituted, prenylated Ypt7p (52) remain to be explored.
How do SNARE chaperones, phosphoinositides, and SNAREs cooperate for fusion? SNARE chaperones and phosphoinositides are required for the fusion-competent SNARE-complex assembly with Qab-SNARE and R-SNARE RPLs plus soluble Qc-SNARE Vam7p (Fig. 4 and Fig. S6). Thus, Qab-SNAREs on one RPL membrane, R-SNARE on another membrane, and Qc-SNARE Vam7p in solution cannot assemble by themselves into trans-SNARE complexes on apposed membranes without SNARE chaperones and phosphoinositides (Figs. 3, 4, and Fig. S6). On the other hand, substantial SNARE-complex assembly can be driven even in the absence of SNARE chaperones (Fig. 6 B and C) if the soluble Qc-SNARE Vam7p was associated with the other Q-SNAREs during proteoliposome preparation. Since rapid fusion still requires SNARE chaperones and phosphoinositides under these conditions (Fig. 6 A and C), preassembled Qabc-SNAREs and the R-SNARE may spontaneously assemble into trans-SNARE complexes that are nonproductive, either because of incorrect composition, incorrect spatial disposition (e.g., on boundary membrane rather than at vertex ring), or for unknown reasons. Sec17p/Sec18p, HOPS, and either PI(3)P or PI(4,5)P2 may synergistically remodel these trans-SNARE complexes for fusion.
Materials and Methods
Protein isolation, preparation of SNARE proteoliposomes, proteoliposome lipid mixing assay, and flotation assays of liposome association are presented in SI Materials and Methods.
SNARE-Complex Assembly Assay.
RPL lipid-mixing reactions, with either Qabc-SNARE RPLs or Qab-SNARE RPLs, R-SNARE RPLs, either diC8-PI(3)P or diC8-PI(4,5)P2 (90 μM), Sec17p (1.2 μM), Sec18p (1.0 μM), HOPS (55 nM), and Vam7p (400 nM), where indicated, were incubated at 27 °C for 40 min while monitoring NBD fluorescence, and then transferred to ice for 10 min, mixed with αVam3p (1.7 μM), incubated on ice for 10 min, mixed with Protein A-Sepharose (GE Healthcare) in RB150 with 1% Triton X-100, and nutated at 4 °C for 30 min. Protein A-Sepharose beads were washed with RB150 with 1% Triton X-100 3 times, and then proteins were eluted with 5× SDS/PAGE sample buffer, analyzed by SDS/PAGE, and immunoblotted.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant GM23377. J.M. was supported by TOYOBO BIO Foundation long-term research grants and the Osaka University Life Science Young Independent Researcher Support Program (Japan Science and Technology Agency). We thank Amy Burfeind, Nathan Margolis, Naomi Thorngren, and Holly Jakubowski for expert assistance.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908694106/DCSupplemental.
References
- 1.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Scheller R. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer A, et al. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell. 2000;11:807–817. doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato M, Wickner W. Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. EMBO J. 2001;20:4035–4040. doi: 10.1093/emboj/20.15.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang T, et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeddinghaus C, Merz AJ, Laage R, Ungermann C. A cycle of Vam7p release from and PtdIns 3-P-dependent rebinding to the yeast vacuole is required for homotypic vacuole fusion. J Cell Biol. 2002;157:79–89. doi: 10.1083/jcb.200112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fratti R, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun Y, Fratti RA, Wickner W. Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE-dependent yeast vacuole fusion. J Biol Chem. 2004;279:53186–53195. doi: 10.1074/jbc.M411363200. [DOI] [PubMed] [Google Scholar]
- 10.Vicogne J, et al. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci USA. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Wilson KA, Rice-Stitt T, Neiman AM, McNew JA. In vitro fusion catalyzed by the sporulation-specific t-SNARE light-chain Spo20p is stimulated by phosphatidic acid. Traffic. 2007;8:1630–1643. doi: 10.1111/j.1600-0854.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- 12.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miaczynska M, Zerial M. Mosaic organization of the endocytic pathway. Exp Cell Res. 2002;272:8–14. doi: 10.1006/excr.2001.5401. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Seeley ES, Wickner W, Merz A. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 17.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 18.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15871–15876. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 20.Nickel W, et al. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, et al. SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J. 2006;90:2062–2074. doi: 10.1529/biophysj.105.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–349. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Smith EA, Chapman ER, Weisshaar JC. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1–5 ms resolution. Biophys J. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrowicz CW, Meiringer CTA, Ungermann C. Yeast vacuole fusion: A model system for eukaryotic endomembrane dynamics. Autophagy. 2008;4:5–19. doi: 10.4161/auto.5054. [DOI] [PubMed] [Google Scholar]
- 26.Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 27.Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- 29.Seals DF, Eitzen G, Margolis N, Wickner WT. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24:1775–1786. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins KM, Wickner WT. trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA. 2007;104:8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroupe C, Collins KM, Fratti RA, Wickner WT. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 37.Dulubova I, Yamaguchi T, Wang Y, Südhof T, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 38.Cheever ML, et al. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol. 2001;3:613–618. doi: 10.1038/35083000. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol. 2003;160:365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun Y, Xu H, Thorngren N, Wickner W. Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion. EMBO J. 2007;26:4935–4945. doi: 10.1038/sj.emboj.7601915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struck DK, Hoekstra D, Pagano RE. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- 42.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci USA. 2007;10:13551–13558. doi: 10.1073/pnas.0704741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30:11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- 44.Meers P, Ali S, Erukulla R, Janoff AS. Novel inner monlayer fusion assays reveal differential monolayer mixing associated with cation-dependent membrane fusion. Biochim Biophys Acta. 2000;1467:227–243. doi: 10.1016/s0005-2736(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 45.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ungermann C, et al. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol. 1999;145:1435–1442. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda R, et al. Functional architecture of an intracellular membrane t-SNARE. Nature. 2000;407:198–202. doi: 10.1038/35025084. [DOI] [PubMed] [Google Scholar]
- 48.Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ. A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion. EMBO J. 2004;23:2765–2776. doi: 10.1038/sj.emboj.7600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G. Genomic analysis of homotypic vacuole fusion. Mol Biol Cell. 2002;13:782–794. doi: 10.1091/mbc.01-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada Y, Ohsumi Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J Biol Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- 51.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 52.Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284:16118–16125. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.