Nuclear transfer is an effective reprogramming strategy that can redirect the utilization of nuclear instructions and ultimately phenotypes (1). When oocytes are used as the host, a somatic cell nucleus can be reprogrammed to activate the earliest stages of embryonic development (2, 3). Nuclear transfer embryos can develop until the stage when they are ready to implant into the uterus or shortly thereafter and then most degenerate. Very few nuclear transfer embryos successfully progress to term. Those that do mature are characterized by abnormalities in placentation, which in the mouse is placentomegaly (ref. 4 and Fig. 1). Recent reports, including one appearing in this issue of PNAS (5), investigate the etiology of this placentation defect in the mouse and arrive at somewhat different conclusions (5, 6).
Fig. 1.
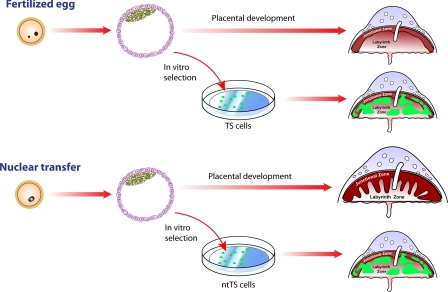
Placental development and TS cell derivation from fertilized and nuclear transfer embryos. Fertilized embryos developing in vivo generate placentas with two well-defined compartments: junctional zone and labyrinth zone. Nuclear transfer embryos developing past midgestation are rare events. However, those that do mature exhibit placentomegaly with a prominent expansion of the junctional zone. TS cells derived from either fertilized or nuclear transfer embryos are quite similar, including their abilities to successfully incorporate and function within a normally developing placenta (5). The implication from the research is that the trophoblast defects observed in nuclear transfer embryos are non-cell autonomous. Some issues potentially impacting this interpretation include in vitro cell selection.
In the mouse, a population of stem cells can be isolated from embryonic day 3.5 blastocysts, or somewhat later from a structure referred to as extraembryonic ectoderm, and expanded in vitro (7). These cells are called trophoblast stem (TS) cells. They exhibit the capacity to self-renew and differentiate into each of the trophoblast cell lineages existing in the placenta (7). The latter point is most convincingly demonstrated by the reintroduction of marked TS cells into a blastocyst and observation of the developmental capabilities of these cells after blastocyst transfer to a pseudopregnant mouse. In this issue of PNAS, Oda et al. (5) investigated this important cell population in embryos generated by either nuclear transfer or fertilization. TS cell lines were readily established from both types of embryos and shown to possess similar capacities to differentiate (Fig. 1). Gene expression profiles and epigenetic marks, such as DNA methylation, did not differ between the nuclear transfer TS (ntTS) cells and TS cells derived from embryos generated by fertilization. Comparison of the ntTS and TS cell lines was extensive and led to the conclusion that placentomegaly associated with nuclear transfer was not caused by defects intrinsic to the trophoblast lineage. A recent report from Rielland et al. (6) requires some reconsideration of this conclusion. Those scientists also investigated the derivation of TS cells from embryos produced by nuclear transfer and fertilization, but in contrast to Oda et al. (5) they show that ntTS cell lines were easier to establish and depended less on exogenous growth factors than were TS cell lines arising from fertilized embryos (6). Such observations are consistent with earlier tetraploid complementation experiments suggesting that ntTS cells have an in vivo growth advantage over TS cells derived from fertilized embryos (8, 9). Rielland et al. proposed that placentomegaly associated with nuclear transfer embryos could be caused by a number of factors, including the unique growth characteristics of ntTS cells. How do we reconcile these apparently disparate findings and how do they impact our understanding of nuclear transfer-associated placentomegaly?
Experimental protocols for the reports by Oda et al. (5) and Rielland et al. (6) were not identical. Differences existed in the donor nuclei [cumulus cell versus embryonic stem (ES) cell] and the genetic background of the mice used in the nuclear transfer experiments. Such procedural variations may influence efficiencies in TS cell derivation but would not appear to be factors in the development of placentomegaly with nuclear transfer embryos.
Insights into the etiology of nuclear transfer-associated placentomegaly have also been derived from tetraploid recombination experiments (8, 9). These experiments are based on (i) the ability to produce one-cell tetraploid embryos from two-cell embryos by electrofusion, (ii) the competence of embryos generated by aggregating tetraploid and euploid embryonic cells to generate live offspring, and (iii) the developmental restriction of tetraploid embryonic cells within the newly formed chimeric embryos to extraembryonic lineages (10). Aggregation of fertilized embryos with tetraploid nuclear transfer embryos results in normal placentation, whereas aggregation of nuclear transfer embryos with tetraploid-fertilized embryos yields placentomegaly (9). A caveat to these tetraploid recombination experiments concerns the unknown impact of the tetraploid genome on the trophoblast lineage. Tetraploidy may be inert as implied, or instead it may act to stabilize the trophoblast lineage and mask deficiencies associated with nuclear transfer. Nonetheless, the implication from the tetraploid complementation experiments is that the nuclear transfer trophoblast lineage is passive in the process leading to placentomegaly and is responding to defective signals originating from the inner cell mass (ICM) and its derivatives.
As is well known, ES cells can be derived from mouse blastocysts and exhibit some similarities to the ICM. Two independent research teams previously generated ES cells from nuclear transfer and fertilized blastocysts and found them to be equivalent (11, 12). Thus, if both ntTS and nuclear transfer ES (ntES) cells are normal then why do nuclear transfer embryos have so many problems?
In vitro TS and ES cell line derivation represents selection processes dictated by culture conditions. Optimal in vitro environments may normalize the expanding cell populations selecting for epigenetic and genetic features that promote proliferation and homogenize cellular phenotypes. The correspondence of in vitro versus in vivo phenotypes for TS and ES cells is difficult to test empirically. Within the developing embryo, both stem cell populations are present in small numbers and are transient. Although stem cell lines established from fertilized and nuclear transfer embryos may behave similarly, the in vivo niche established within nuclear transfer embryos may not permit the expansion of “normal” populations of TS or ES cells and their derivatives.
Nuclear transfer embryos must exhibit some level of normalcy, at least, among a subset of the cells that give rise to TS and ES cells. Another cell type or event could subsequently interfere with normal development. Primitive endoderm and its derivatives have significant regulatory roles in embryogenesis (13). Extraembryonic endoderm (XEN) stem cells resemble primitive endoderm and represent a third stem cell population that can be derived from the mouse blastocyst (14). A comparison of XEN stem cells derived from nuclear transfer and fertilized embryos may have some merit. Alternatively, the critical factor may be heterogeneity of the cellular constituents of the nuclear transfer embryo leading to disrupted signaling as the proportion of “aberrant” cells increases. Consistent with the idea of cellular heterogeneity is the observation made by Oda et al. (5) that their ntTS cell lines possessed a somewhat higher frequency of aneuploidy than did TS cell lines derived from fertilized embryos. More subtle genetic alterations could account for the increased success of Rielland et al. (6) in deriving ntTS cell lines and the decreased dependence of their ntTS cells for exogenous growth factors. ntES cells also exhibit higher frequencies of aneuploidy (15). Chromosomal stability is a reflection of factors intrinsic to the cell and its environment (15).
The final issue to be addressed is whether the nature of placentomegaly associated with nuclear transfer provides any clues regarding the etiology of the morphogenetic disruption. The mature mouse chorioallantoic placenta can be divided into two compartments: the junctional zone, which is comprised of trophoblast giant cells, spongiotrophoblast, and glycogen cells; and the labyrinth zone, which consists of trophoblast giant cells, syncytial trophoblast, and allantoic mesenchyme with its accompanying fetal vasculature. The junctional zone forms the maternal interface, whereas the labyrinth zone forms the fetal interface. The most striking defects in the nuclear transfer placenta are associated with the junctional zone (4, 16). The junctional zone is expanded at the expense of the labyrinth zone, exhibiting prominent increases in the number of glycogen cells and size of spongiotrophoblast cells (4, 16). Development of the junctional zone is particularly sensitive to the maternal environment (17, 18). Thus, could there be impairments in the dialogue between maternal and trophoblast compartments, which lead to placentomegaly? This may indeed be the case. Two reports published earlier this year indicate that nuclear transfer and fertilized embryos elicit distinct uterine responses (19, 20).
In summary, Oda et al. (5) demonstrate that TS cells can be readily generated from embryos established by nuclear transfer, possess a “normal” phenotype, and are not directly responsible for the placentomegaly that ensues. The latter is the consequence of being exposed to a defective niche, a view supported by tetraploid complementation experiments (9). Another recent report (6) confirms the ease of ntTS cell derivation but suggests that these cells may possess some unique features that could contribute to placentomegaly. All of these conclusions need to be tempered by the issues raised above regarding the impacts of in vitro selection and tetraploid stabilization. Nevertheless, whether or not ntTS are phenotypically unique or have “bad” neighbors their derivatives do not appear to establish an effective dialogue with cellular constituents of the maternal uterine environment. Ultimately, it is the maternal environment that is directing placentation and some fault must lie in the inability of the developing trophoblast cells to send or receive signals.
Acknowledgments.
Our work is supported by National Institutes of Health Grants HD20676, HD48861, and HD55523.
Footnotes
The authors declare no conflict of interest.
See companion article on page 16293.
References
- 1.Gurdon JB. From nuclear transfer to nuclear reprogramming: The reversal of cell differentiation. Annu Rev Cell Dev Biol. 2006;22:1–22. doi: 10.1146/annurev.cellbio.22.090805.140144. [DOI] [PubMed] [Google Scholar]
- 2.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 3.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, et al. Placentomegaly in cloned mouse concepti caused by expansion of the spongiotrophoblast layer. Biol Reprod. 2001;65:1813–1821. doi: 10.1095/biolreprod65.6.1813. [DOI] [PubMed] [Google Scholar]
- 5.Oda M, et al. Establishment of trophoblast stem cell lines from somatic cell nuclear-transferred embryos. Proc Natl Acad Sci USA. 2009;106:16293–16297. doi: 10.1073/pnas.0908009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rielland M, Brochard V, Lacroix M-C, Renard J-P, Jouneau A. Early alteration of the self-renewal/differentiation threshold in trophoblast stem cells derived from mouse embryos after nuclear transfer. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.07.022. in press. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Kunath T, Hadjantonakis A-K, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 8.Jouneau A, et al. Developmental abnormalities of NT mouse embryos appear early after implantation. Development. 2006;133:1597–1607. doi: 10.1242/dev.02317. [DOI] [PubMed] [Google Scholar]
- 9.Miki H, et al. Embryonic rather than extraembryonic tissues have more impact on the development of placental hyperplasia in cloned mice. Placenta. 2009;30:543–546. doi: 10.1016/j.placenta.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Nagy A, et al. Embyronic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 11.Bambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci USA. 2006;103:933–938. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakayama S, et al. Equivalency of nuclear transfer-derived embryonic stem cells to those derived from fertilized mouse blastocysts. Stem Cells. 2006;24:2023–2033. doi: 10.1634/stemcells.2005-0537. [DOI] [PubMed] [Google Scholar]
- 13.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries, and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 14.Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 15.Balbach ST, et al. Chromosome stability differs in cloned mouse embryos and derivative ES cells. Dev Biol. 2007;308:309–321. doi: 10.1016/j.ydbio.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Wakisaka-Saito N, et al. Chorioallantoic placenta defects in cloned mice. Biochem Biophys Res Commun. 2006;349:106–114. doi: 10.1016/j.bbrc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta. 2003;24:34–38. doi: 10.1053/plac.2002.0858. [DOI] [PubMed] [Google Scholar]
- 18.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauersachs S, et al. The endometrium responds differently to cloned versus fertilized embryos. Proc Natl Acad Sci USA. 2009;106:5681–5686. doi: 10.1073/pnas.0811841106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masouri-Attia N, et al. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc Natl Acad Sci USA. 2009;106:5687–5692. doi: 10.1073/pnas.0812722106. [DOI] [PMC free article] [PubMed] [Google Scholar]