Abstract
Sensation involves active movement of sensory organs, but it remains unknown how position or movement of sensory organs is encoded in cortex. In the rat whisker system, each whisker is represented by an individual cortical (barrel) column. Here, we quantified in awake, head-fixed rats the impact of natural whisker movements on action potential frequencies of single (identified) neurons located in different layers of somatosensory (barrel) cortex. In all layers, we found only weak correlations between spiking and whisker position or velocity. Conversely, whisking significantly increased spiking rate in a subset of neurons located preferentially in layer 5A. This finding suggests that whisker movement could be encoded by population responses of neurons within all layers and by single slender-tufted pyramids in layer 5A.
Keywords: barrel cortex, identified cells, action potential, morphology
Active scanning of the environment for sensory signals increases resolution (1), but in turn affects signal representation due to modulatory influences linked to arousal (2, 3) and through representation of movement-related signals in the sensory pathway (4). The whisker system of rodents is convenient to study cortical representation of sensory stimuli, because individual whiskers on the face are represented functionally and anatomically by identifiable individual cortical columns (5, 6) and whisker movements are readily accessible for video tracking. Movement-related information of the whiskers arrives in sensory cortex through activation of the sensory pathway by mechanoreceptors in the whisker follicle (7, 8); although corollary discharge (or efferent copy) through a monosynaptic pathway from motor cortex (9) can also affect spiking in sensory cortex (7). With respect to the sensory pathway of the trigeminal system, it has been suggested that whisker movement and whisker contact are encoded by different parallel pathways (10). Movement is encoded via the paralemniscal pathway [and possibly through a recent described pathway involving ventroposterior medial nucleus of thalamus (11)]. Contact is encoded via the extralemniscal pathway. The lemniscal pathway is thought to participate in encoding both movement and contact. These experiments, however, were conducted in anesthetized animals, and whisking was evoked by electrical nerve stimulation (10). In addition, recent thalamic recordings in anesthetized and awake animals challenged the view that the paralemniscal pathway is encoding whisker movement (12, 13). Finally, cortical recordings in awake animals during free whisking showed that movement-related information is only weakly encoded in most, but not all neurons in sensory cortex (7, 14). But because recorded neurons were not identified posthoc, the contribution of specific cell-types in encoding movement-related information during active whisking remains unknown. Thus, although cortical neurons excited by the paralemniscal pathway (e.g., Layer 5A) (15, 16) may be involved in encoding of whisker movement, this involvement actually remains to be established.
Results
Spiking Frequencies of Neurons in Awake Barrel Cortex.
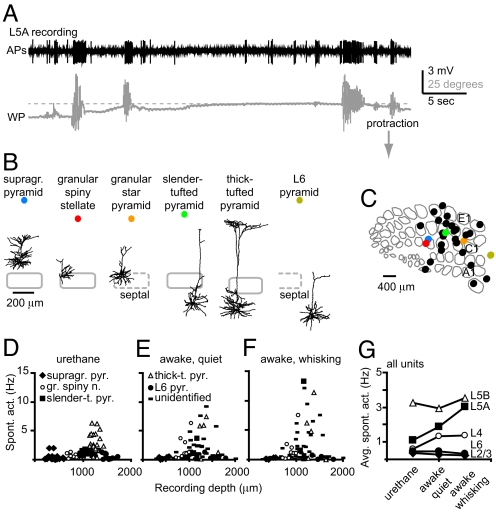
We recorded juxtasomally in different layers of barrel cortex of unanesthetized, head-fixed Wistar rats to record spiking frequencies of single neurons while tracking whisker position (Fig. 1, Fig. S1). Because cortical layers contain multiple cell types (17, 18), recorded neurons were labeled with biocytin for posthoc identification. Throughout the different layers (e.g., L2/3–L6), we recorded from neurons with irregular spiking patterns with average values in the range of 0.1–6 Hz (e.g., a supragranular pyramidal neuron in Fig. S1). The frequency of spiking was significantly lower than the whisking frequency, which typically showed a peak in the power spectrum in the range of 5–8 Hz (Fig. S1C). In addition, we did not find a correlation between occurrence of spikes and whisker position for this supragranular pyramid (Fig. S1B). However, we also recorded from pyramidal neurons that significantly increased spiking upon whisking (Fig. 1A). These neurons were preferentially located in the upper portion of the infragranular layer (L5A). In total, we recorded from 88 neurons throughout different cortical layers and identified 26 neurons posthoc. Labeled neurons were classified according to their location with respect to the cytochrome oxidase dense region, characteristic of granular L4 and were categorized as supragranular pyramids, granular spiny neurons, slender-tufted, thick-tufted, or L6 pyramids (Fig. 1B; for detailed description of classification criteria, see ref. 18; see Fig. 1C for all recording locations). Unlabeled neurons were also included and categorized into layers according to recording depth (18).
Fig. 1.
Spiking frequencies of neurons in awake barrel cortex. (A) Spiking (band-pass filtered from 300–9,000 Hz) and whisker position (WP) during juxtasomal recording of a L5A neuron in somatosensory cortex. (B) Neurolucida reconstructions of representative examples in coronal view. Gray contours illustrate barrel contours (see Fig. 1C). Thick-tufted pyramid was recorded in D2 column of urethane anesthetized animal. (C) Tangential view of barrel cortex (example map) with collected recording locations. (D–F) Spontaneous activity versus recording depth for neurons recorded under anesthetized conditions (D), awake and quiet (E), or awake and whisking (F), respectively. (G) Average spiking frequency for all units during different behavioral states. Note that behavioral state mainly affects spiking in L5A.
To study whether spiking correlated to behavioral state, we compared spiking in anesthetized animals with spiking in awake, head-fixed animals (Fig. 1 D–G). In anesthetized animals, spiking frequency was low (0.1–1 Hz), with the exception of thick-tufted pyramids (Fig. 1D). Average spiking frequencies under urethane were (in Hz ± SD): supragranular pyramids 0.39 ± 0.56 (n = 22), granular spiny neurons 0.58 ± 0.36 (n = 15), slender-tufted pyramids 1.08 ± 0.38 (n = 16), thick-tufted pyramids 3.27 ± 1.62 (n = 23), and L6 pyramids 0.47 ± 0.46 (n = 15) (18, 19). During quiet (nonwhisking) episodes in awake, head-fixed animals, spiking frequencies of identified neurons were comparable to those in urethane anesthesia (Fig. 1E, in Hz ± SD): supragranular pyramids 0.31 ± 0.21 (n = 5), granular spiny neurons 1.93 ± 2.02 (n = 9), slender-tufted pyramids 1.62 ± 1.81 (n = 3), thick-tufted pyramids 4.12 ± 3.22 (n = 5), and L6 pyramids 0.52 ± 0.47 (n = 4). During episodes of whisker movement, individual neurons showed variable and layer specific changes in spiking frequency (up to 14 Hz, Fig. 1F). Average activity during whisking episodes was (in Hz ± SD): supragranular pyramids 0.18 ± 0.16 (n = 5), granular spiny neurons 1.77 ± 2.29 (n = 9), slender-tufted pyramids 4.94 ± 7.22 (n = 3), thick-tufted pyramids 4.53 ± 4.84 (n = 5), and L6 pyramids 0.32 ± 0.38 (n = 4). When we also included unidentified neurons from head-fixed recordings, we found that average spiking frequency was comparable between all units within a layer (unlabeled neurons included) and identified units only (Fig. 1 E and F, and Table S1). Remarkably, spiking frequencies depended on the behavioral state (quiet vs. whisking) mainly for neurons located in L5A (Fig. 1G, average data from all units).
Spiking Correlates with Behavioral State for Individual Neurons.
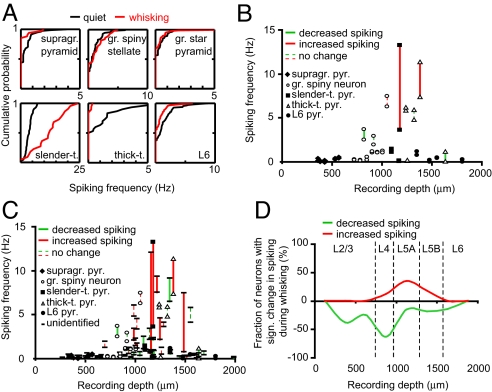
Next, we tested whether spiking and behavioral state correlated significantly for single neurons by comparing (cumulative) frequency distributions using frequencies computed for individual quiet or whisking episodes (Fig. 2A, examples correspond to reconstructions of Fig. 1B, except thick-tufted pyramid). In these individual neurons, we found either a significant decrease of spiking during whisking (supragranular pyramid, thick-tufted pyramid, L6 pyramid, Kolmogorov-Smirnov (KS), P < 0.01), no significant change (granular spiny stellate, granular star pyramid, KS, P = 0.54 and P = 0.41, respectively) or a significant increase (slender-tufted pyramid, KS, P < 0.01). Next, we analyzed spiking during quiet and whisking episodes for individual neurons in relationship to recording depth (Fig. 2 B and C and Fig. S2A) and position (Fig. S3). Only neurons with >10 episodes for each condition were selected (total of 64). Here, 26 neurons showed a significant difference between quiet and whisking episodes (39%). In all layers, we recorded from neurons that significantly reduced spiking frequency upon whisking (n = 16 neurons, Figs. 2 and 3A). We also recorded from neurons that significantly increased spiking frequency (n = 10), but these neurons were located almost exclusively (n = 7) at 1,000–1,250 μm recording depth (corresponding to L5A, Figs. 2 and 3B). At this depth, 7 out of 20 recorded neurons (35%) significantly increased spiking upon whisker movement (e.g., 1 Hz at rest, 12 Hz during whisking). Three neurons (15%) showed a significant decrease in spiking, and the remaining 50% did not significantly change spiking during whisker movement (Fig. 2D).
Fig. 2.
Effect of behavioral state on cortical spiking is layer-specific. (A) Cumulative histograms of spiking activity for individual quiet and whisking episodes for examples illustrated in Fig. 1B (except thick-tufted neuron). (B and C) Correlation of spiking frequency during quiet and whisking episodes with respect to recording depth for identified neurons only (B) or all recorded units (C). Connected symbols represent individual experiments. Red solid bar indicates a significant increase in spiking activity during whisking, green solid bar indicates a significant decrease in spiking activity during whisker movement, and dashed bars indicate no significant difference. (D) Fraction of recorded units (in %) that significantly changed spiking activity upon whisking as a function of recording depth. Green indicates decreased spiking activity and red indicates increased spiking activity, respectively. Bin size, 250 μm; smooth curve represents the fraction calculated for individual bins that significantly increased (red) or decreased (green) spiking.
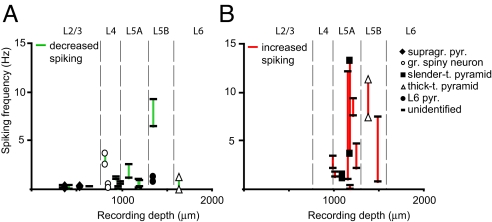
Fig. 3.
Increase in spiking during whisker movement is predominantly observed in L5A. (A and B) Correlation of spiking frequency during quiet and whisking episodes with respect to recording depth (see also Fig. 2C). In A and B, only units are shown that were characterized by either a significant decrease or significant increase in spiking during whisking, respectively. Note that a significant decrease in spiking was observed throughout the different layers of the cortical column but that a significant increase was observed almost exclusively for neurons recorded in L5A.
Spike Times and Whisker Position Are Not Tightly Coupled.
In L2/3 of sensory cortex, it has been shown that membrane potential changes correlate with whisker position (20). To study whether the occurrence of spikes is correlated to whisker position, we made cumulative distributions of whisker position at the time of spikes (Fig. S3 A and B). In all layers, spikes occurred over a large range of whisker positions. The difference between distributions of whisker angle at spike times and whisker position for all times were small for both single experiments from different layers and on the population level when all experiment were grouped (Fig. S3B). We performed the same analysis for velocity (Fig. S3C), but we did not observe a strong correlation between whisker velocity and spike times. Because individual neurons did not show a strong bias for spiking at specific whisker positions or velocity, this result indicates that in cortex both whisker position and velocity are not consistently encoded by spike times in single neurons.
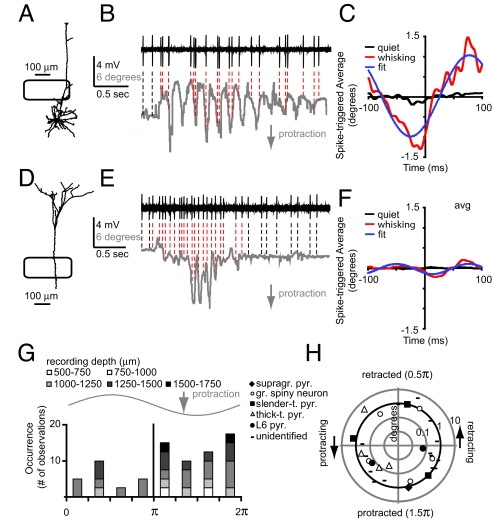
Finally, we computed spike-triggered-averages (STA) for individual units (Fig. 4 A–F) to determine the modulation depth of single units. The first example (slender-tufted pyramid; see also Figs. 1 and 2) illustrates that in addition to an increase in spiking during whisking (Fig. 2A), a large fraction of spikes occurred during the whisker protraction phase. We fitted a 4-parameter sine wave to the STA to determine the whisker phase at which spikes preferentially occurred (Fig. 4C). For this particular example, spikes occurred preferentially just after maximal protraction although the small average amplitude at t = 0 ms (0.7° protraction) still suggests a large variability in spike occurrence with respect to whisker position. The second example (thick-tufted pyramid) illustrates that an increase in spiking during whisking is not necessarily accompanied by correlated spiking with respect to the whisking cycle (Fig. 4 D–F). On the population level, the STA analysis showed that a significant number of neurons was tuned to spike during protraction (51%, Fig. 4G, KS, P < 0.05) compared with retraction (23%, remaining 26% did not correlate to whisker cycle). However, STA analysis also indicated that even when a neuron was tuned to spike at a particular whisker phase, the STA amplitude was small, suggesting broad tuning curves. A radial plot, to characterize the relationship between phase tuning and STA amplitude, showed that modulation depth for most neurons was in the order of 1° with most neurons biased toward a phase between π and 2π, i.e., during whisker protraction (Fig. 4H).
Fig. 4.
Occurrence of spikes is weakly correlated to whisking cycle. (A) Neurolucida reconstruction of slender-tufted pyramid and example traces (B) showing correlation between spike times and whisker position. (C) Spike-triggered-average (STA) of whisker position during quiet (black) and whisking (red) episodes for pyramid shown in A. (D–F) Analogous to A–C, but for thick-tufted pyramid recorded at 1382 μm from pia (L5B) (basal dendrites were not recovered). Note that spiking frequency was increased during whisking, but spiking was very weakly correlated to whisker cycle. (G) The fit on the STA (blue line in C and F) was used to determine the phase at t = 0 ms and plotted as number of neurons vs. phase of whisker cycle. (H) Scatter plot illustrating the modulation depth (log-normal scale) versus phase (linear scale) for individual units. Bold line indicates 1° amplitude.
Discussion
In conclusion, our results indicate that behavioral state affects spiking activity of cortical neurons in a layer-specific and most likely cell-type-specific way. In awake but nonwhisking animals, we found that average spiking frequencies were comparable to spiking in anesthetized animals. Upon free whisking, a subset of neurons robustly increased average spiking and these neurons were preferentially located in the upper portion of the infragranular layer [i.e., L5A, containing predominantly slender-tufted pyramids (21)]. However, even when spiking activity increased robustly upon whisker movement, we found that correlations between occurrence of spikes and whisker position or whisker velocity were weak. These weak correlations were previously observed in similar experiments for neurons of the trigeminal ganglion (22) and in extracellular recordings from unidentified units in barrel cortex (7, 23). Weak correlations (or broad tuning curves) do not imply lack of information (7, 24, 25), particularly considering low background spiking rates, but the accuracy of encoding information (in our case movement or position of a whisker) just increases significantly taking into account populations of neurons rather than single neurons (26). An alternative mechanism to encode whisker use could be that whisker phase rather than absolute whisker position is the determinant for spiking (23), although we also did not find a robust relationship between whisker phase and spike times (see Fig. 4). Remarkably, even though our data suggests the presence of a population code, electrical stimulation of single neurons in both primary motor and somatosensory cortex can evoke behavioral responses (27, 28), indicating that activity of single neurons can still significantly affect behavioral output.
Spiking Frequencies.
In awake, head-fixed animals, we found average ongoing spiking rates of 0.3–3 Hz, with obvious layer-specific differences as observed during anesthetized conditions (18). More specifically, in head-fixed animals, spiking was generally around 0.3 Hz for L2/3, 1.4 Hz for L4, and 0.5 Hz for L6. In L5, we found higher ongoing spike rates (L5A 1.9 Hz, L5B 2.9 Hz). Ongoing spike rates were affected by behavioral state only in L5A. This could be due to state-dependent changes in excitability of the medial division of posterior thalamic nucleus (POm), projecting to L5A (2).
Reported spike rates of L2/3 neurons are strikingly consistent among different studies in awake animals (20, 29, 30). We found an average spiking rate of 0.28 Hz using juxta-cellular recordings of barrel cortex L2/3 neurons in head-fixed rats. Whole-cell recordings of mice barrel cortex L2/3 neurons demonstrated an average rate of 0.61 Hz (8, 20), population imaging on rat visual cortex L2/3 neurons resulted in an average ongoing spiking rate of 0.44 Hz (30) and finally, whole-cell recordings from (hindlimb) motor cortex L2/3 neurons showed that spike rates were as low as 0.3 Hz in freely moving animals (29). Spiking frequencies in L2/3 neurons are therefore consistently low, independent of recording approach and cortical area. Unfortunately, similar spike frequency data for granular and infragranular layers do not exist because either spike rates were not consistently quantified or recorded cell-types were not identified.
Intracortical Projections of L5A Neurons.
Within the barrel column, L5A slender-tufted neurons project to L2/3 neurons (21, 31). The presence of this connection suggests that information on whisker motion, conveyed by the paralemniscal pathway to L5A, could be relayed onto L2/3 neurons. In fact, a clear correlation between membrane potential and whisker position was shown by electrophysiological recordings in barrel cortex of awake mice (20). This correlation could be caused exclusively by the L5A–L2/3 projection. Because L2/3 also receives direct input from motor cortex (9), another possibilty is that the correlation between membrane potential and whisker position in L2/3 is due to integrated input from the paralemniscal pathway and input from motor cortex. Because only membrane potential and not spiking correlates to whisker position in L2/3, the L5A–L2/3, and motor cortex–L2/3 projections may act in concert to facilitate spiking in L2/3 during for instance whisker contact (20).
Paralemniscal Pathway Encodes Whisker Motion.
Finally, recent evidence from extracellular thalamic recordings challenged the view that the paralemniscal pathway is encoding whisker movement (12). In this previous study, no evidence was found for increased spiking in the paralemniscal part of the thalamus (medial division of POm) during free whisking in awake rats. However, whisker trajectories were not consistently quantified or POm recording sites identified histologically (13). We recorded spiking of identified neurons in different layers of primary somatosensory cortex while tracking whisker position and velocity simultaneously and found that slender tufted neurons in L5A could participate in encoding whisker movement. This view is in support of the hypothesis that the paralemniscal pathway is encoding whisker movement (10, 13, 32). Our data shows that this is the case for cortical neurons of the paralemniscal pathway. The next question will be whether movement related spiking is conveyed from (i) motor cortex directly (efferent copy), from (ii) activation of the paralemniscal pathway through mechanoreceptors in the whisker follicle, or indirectly (iii) by modulation of the sensory pathway by motor cortex (33).
Methods
Awake Wistar rats (28 animals, male and female, P31–46, weight 144.7 ± 30.9 g) were housed in an enriched environment and trained for 12 days to accustom to head-fixation. During surgical procedure, the animal was anesthetized with ketamine/xylazine (10 mg ketamine, 0.05 mg xylazine/100 g body weight). Depth of anesthesia was checked by both foot and eyelid reflex and vibrissae movements. The animal's temperature was monitored with a rectal probe and maintained at 37 °C by a thermostatically controlled heating pad. Experiments were performed on the left primary somatosensory (barrel) cortex (2.5 mm posterior, 5.5 mm lateral from Bregma). The skull was cleaned using alcohol (70%) and H2O2 (1%, in H2O), the lateral muscle was partly detached, and a metal pole was fixed to the skull using dental cement (Kerr Orange, USA). The craniotomy was protected by a small plastic cylinder with a screw-cap. Juxtasomal recordings were performed using patch pipettes (6–8 MΩ) filled with (in mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.2 with NaOH, and 20 mg/mL biocytin. The pia was kept covered with 0.9% NaCl. Duration of head-fixation was <60 min to maintain stable recording conditions. Single units were searched for by monitoring electrode resistance while advancing with 1-μm steps. Using this method, unit isolation was independent of spiking frequency.
Recordings were made using an Axoclamp 2B amplifier (Axon Instruments) in combination with a Lynx 8 amplifier, band filter settings 300 and 9,000 Hz (Neuralynx). Data were acquired using the Ntrode Virtual Instrument (custom written software, R. Bruno, Columbia University, New York, NY) for Labview (National Instruments). Whisker movement and position was recorded at 60 Hz using an ETL-200 system modified for whisker tracking (ISCAN) and read into the Ntrode program. After physiological properties were measured, neurons were filled with biocytin using current pulses (34). All experimental procedures were carried out according to the animal welfare guidelines of the Max-Planck Society and the Erasmus Medical Center in Rotterdam.
Histological Procedures and Reconstruction.
All histological procedures were as described previously (18, 19). Cells were reconstructed using Neurolucida software (Microbrightfield,) or visually identified using a binocular microscope (CX31, Olympus).
Data Analysis.
Data sets were analyzed using multiple custom-made Matlab routines (courtesy of R. Enchev, Institute of Cancer Research, London, U.K.). Quiet episodes (no whisker movements) and whisking episodes were sorted manually, but the experimenter was blind for spiking data during the sorting procedure. Subsequently, spiking frequencies were calculated for specific behavioral states. To determine the correlation of spike timing with respect to whisker movement, spike-triggered-averages were determined. Spikes were separated into quiet-spikes and whisking-spikes, and whisker position was determined at the time of spike firing (in addition to 100 ms before and after the spike). Correlation to whisker position (modulation depth) was determined by fitting a 4-parameter sinusoidal curve [y = y0 + asin(2πx/b + c)] to the spike-triggered-average trace for whisker movement using Sigmaplot (Systat Software), where “y0” represents the baseline whisker position determined as the average angle during the entire recording, “a” represents modulation depth, “b” represents the whisking period as determined from the spike-triggered-average, and “c” represents the phase at time = 0. Data in Fig. 4H were plotted with modulation depth (a) as radial coordinate and phase at time 0 (c) as polar coordinate.
Anesthetized Conditions.
Recordings from urethane (1.6–1.7 g*kg−1) anesthetized Wistar rats (P26–42, male/female) were previously published (18, 19). In summary, experiments were performed on the primary somatosensory cortex of the left hemisphere (2.5 mm posterior, 5.5 mm lateral from Bregma). Spontaneous AP frequency was measured through continuous recording for 125 s.
Supplementary Material
Acknowledgments.
We thank R. Enchev (Institute of Cancer Research, London, U.K.) for analysis software; M. Kaiser, E. Stier, and E. Goedknegt for histology; R. Rödel, K. Schmidt, K. Donkersloot, and H. van der Burg for technical assistance; G. Borst for support; R. Bruno for fruitful discussions; and S. Bellanca and A. Weber for Neurolucida reconstructions. This work was supported by the Erasmus Medical Center, the Max-Planck Society, VU University Amsterdam (Center for Neurogenomics and Cognitive Research–CNCR), and by the Netherlands Organisation for Scientific Research–NWO.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904143106/DCSupplemental.
References
- 1.Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J Comp Physiol A. 2006;192:573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 2.Trageser JC, et al. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–1463. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urbain N, Deschenes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 5.Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: Description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- 6.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 7.Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol. 1997;78:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- 8.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 9.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 2006;4:e124. doi: 10.1371/journal.pbio.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbain N, Deschenes M. A new thalamic pathway of vibrissal information modulated by the motor cortex. J Neurosci. 2007;27:12407–12412. doi: 10.1523/JNEUROSCI.2914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J Neurophysiol. 2008;100:681–689. doi: 10.1152/jn.01322.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahissar E, Golomb D, Haidarliu S, Sosnik R, Yu C. Latency coding in POm: Importance of parametric regimes. J Neurophysiol. 2008;100:1152–1154. doi: 10.1152/jn.90477.2008. author reply 1155–1157. [DOI] [PubMed] [Google Scholar]
- 14.Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- 15.Alloway KD. Information processing streams in rodent barrel cortex: The differential functions of barrel and septal circuits. Cereb Cortex. 2008;18:979–989. doi: 10.1093/cercor/bhm138. [DOI] [PubMed] [Google Scholar]
- 16.Furuta T, Kaneko T, Deschenes M. Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J Neurosci. 2009;29:4089–4095. doi: 10.1523/JNEUROSCI.5393-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 18.de Kock CP, Bruno RM, Spors H, Sakmann B. Layer and cell type specific suprathreshold stimulus representation in primary somatosensory cortex. J Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kock CP, Sakmann B. High frequency action potential bursts (≥100 Hz) in L2/3 and L5B thick tufted neurons in anaesthetized and awake rat primary somatosensory cortex. J Physiol. 2008;586:3353–3364. doi: 10.1113/jphysiol.2008.155580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 21.Schubert D, Kotter R, Luhmann HJ, Staiger JF. Morphology, electrophysiology and functional input connectivity of pyramidal neurons characterizes a genuine layer va in the primary somatosensory cortex. Cereb Cortex. 2006;16:223–236. doi: 10.1093/cercor/bhi100. [DOI] [PubMed] [Google Scholar]
- 22.Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron. 2007;53:117–133. doi: 10.1016/j.neuron.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci. 2009;12:492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seung HS, Sompolinsky H. Simple models for reading neuronal population codes. Proc Natl Acad Sci USA. 1993;90:10749–10753. doi: 10.1073/pnas.90.22.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JN, et al. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J Neurosci. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- 28.Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- 29.Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nat Neurosci. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- 32.Derdikman D, et al. Layer-specific touch-dependent facilitation and depression in the somatosensory cortex during active whisking. J Neurosci. 2006;26:9538–9547. doi: 10.1523/JNEUROSCI.0918-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci. 2008;11:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.