Abstract
Understanding the mechanism by which hormone refractory prostate cancer (HRPC) develops remains a major issue. Alterations in HRPC include androgen receptor (AR) changes. In addition, the AR is activated by cytokines such as interleukin-6 (IL-6). Atypical protein kinase C (aPKCλ/ι) has been implicated in the progression of several cancers. Herein, we provide evidence that aPKCλ/ι expression correlates with prostate cancer recurrence. Experiments in vitro and in vivo revealed aPKCλ/ι to be involved in prostate cancer cell growth through secretion of IL-6. Further, aPKCλ/ι activates transcription of the IL-6 gene through NFκB and AP-1. We conclude that aPKCλ/ι promotes the growth of hormone independent prostate cancer cells by stimulating IL-6 production in an autocrine manner. Our findings not only explain the link between aPKCλ/ι and IL-6, implicated in the progression a variety of cancers, but also establish a molecular change involved in the development of HRPC. Further, aPKCλ/ι expression might be a biomarker for prostate cancer progression.
Keywords: IL-6, PSA, recurrence
Despite earlier detection and recent advances in surgery and radiation, prostate cancer is the second leading cause of cancer deaths in men in western countries (1). Hormone therapy in the form of medical or surgical castration remains the mainstay of systemic prostate cancer treatment. However, despite initial favorable responses to hormone therapy, hormone refractory tumors develop for which there is as yet no effective treatment (2). The androgen receptor (AR) and its signaling remain intact, as demonstrated by the expression of prostate specific antigen (PSA), in androgen-independent cancer cells. Alterations in these cells include AR amplification, AR point mutations, and changes in the expressions of AR co-regulatory proteins (3). In addition, AR can be activated in a ligand-independent fashion by compounds including growth factors and cytokines, such as interleukin-6 (IL-6) (4–6). Understanding the mechanism of androgen-independent prostate cancer development is essential not only for diagnosis but also more effective therapy.
Atypical protein kinase C (aPKCλ/ι and aPKCζ) is a protein kinase C isozyme distinct from other classes of this enzyme, structurally and functionally (7, 8). It plays multifunctional roles in cellular maintenance and growth of epithelial cells, for example, signal transduction and cell polarity (9–15). Studies on lung, ovary, colon, and breast cancers have demonstrated a relationship between aPKCλ/ι expression and cancer progression and suggest that aPKCλ/ι expression might predict poor survival (16–22). There are several reports showing enhanced aPKC expression in human prostate cancer tissues, but the relationship between aPKCλ/ι and prostate cancer progression remains unclear (23, 24). Furthermore, the mechanism by which aPKC promotes the progression of a variety of cancers remains uncertain. Then, we focus on aPKCλ/ι expression and its roles in prostate cancer.
IL-6, a cytokine involved in immune and hematopoietic activities, has been implicated in the progression of a variety of human cancers (25). In prostate cancer, IL-6 has been suggested to play a role in cancer progression, especially that of hormone refractory cancer (26–30). Serum IL-6 is elevated in patients with prostate cancer (28). The IL-6 receptor is expressed in prostate cancer cell lines and IL-6 is secreted only by androgen independent prostate cancer cell lines (27, 29, 30). Although these observations suggest the importance of IL-6 in prostate cancer progression, the mechanism by which IL-6 expression is regulated in prostate cancer cells is not fully understood.
Herein, we investigated aPKCλ/ι expression in 29 clinical prostate cancer tissue specimens and found a correlation between aPKCλ/ι expression and PSA failure, a clinical hallmark of recurrence. Experiments, both in vitro and in vivo, on androgen-independent cancer cells, employing siRNA-mediated depletion of aPKCλ/ι, revealed this isozyme to be involved in the proliferation of prostate cancer cells. The in vitro experiments further showed aPKCλ/ι involvement in the secretion of IL-6 into the culture medium, suggesting cancer cell growth to occur via an autocrine mechanism. This finding demonstrates the link between aPKCλ/ι and IL-6, both of which have been implicated in cancer progression. We also demonstrated that aPKCλ/ι is involved in transcription of the IL-6 gene promoter through activation of NFκB and AP-1, both of which have been implicated in transcription of the IL-6 gene in prostate cancer cells. We conclude that aPKCλ/ι promotes prostate cancer cell growth in an autocrine manner via stimulation of IL-6 production and secretion.
Results
Correlation Between aPKCλ/ι Expression and PSA Failure, a Clinical Marker of Prostate Cancer Recurrence.
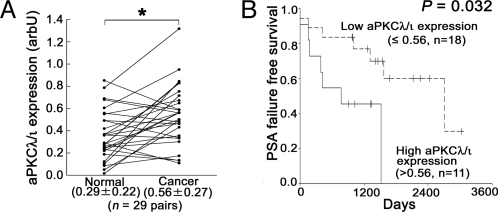
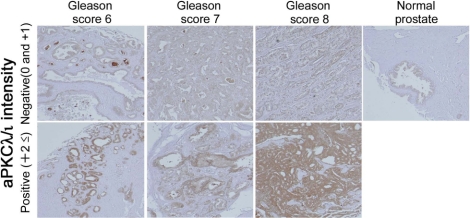
To clarify the relationship between aPKCλ/ι expression and prostate cancer, we first evaluated aPKCλ/ι expression at the mRNA levels in 29 specimens of human prostate cancer tissues. Clinicopathological features are listed at Table S1. Real-time quantitative PCR (qPCR) analyses revealed that aPKCλ/ι mRNA was more highly expressed in cancer tissue samples than in paired normal controls from same patients (Fig. 1A, P = 0.001). There were no associations between aPKCλ/ι mRNA expression and certain clinical features (Fig. S1). On the other hand, when the samples were divided into two groups by setting a cut-off at the median aPKCλ/ι value (high: >0.56, n = 11 and low: ≤0.56, n = 18), we recognized a statistically significant correlation between aPKCλ/ι mRNA expression and PSA failure (Fig. 1B, P = 0.032). There was no correlation between other clinical features and PSA failure (Fig. S2). Serum PSA was measured every 2–3 months after radical prostatectomy. PSA failure was defined as a continuous elevation with a PSA level greater than 0.2 ng/mL. PSA failure has been suggested to be associated with cancer specific death (31). Thus, aPKC may be a prognostic biomarker for prostate cancer. In univariate and multivariate analyses, only aPKCλ/ι mRNA expression showed statistical significance (Table 1, P = 0.039 in univariate and P = 0.033 in multivariate analysis). Subsequent immunohistochemical analysis of aPKCλ/ι in 43 prostate specimens (cancer tissues; n = 40, and normal tissues; n = 3) confirmed aPKCλ/ι expression at the protein level in normal and tumor tissues, with a variety of intensities (Fig. 2 and Table S2). Immunohistochemical analysis also revealed enhanced staining of aPKCλ/ι to be localized to the cytoplasm in epithelial cells of the prostate, but not in stromal cells, suggesting the importance of the specific expression of aPKCλ/ι protein in epithelial cells of the prostate.
Fig. 1.
Relationships between aPKCλ/ι expression in prostate cancer tissues. (A) aPKCλ/ι expression was compared between paired prostate cancer and normal (BPH) prostate tissues obtained from same patients (n = 29 pairs). *, P = 0.001 by paired t test. arbU: arbitrary units. Values indicate medians ± SD. (B) Kaplan-Meier and log rank analysis of aPKCλ/ι and PSA failure time. aPKCλ/ι expressions in prostate cancer tissues (n = 29) were divided into two groups according to the median value (High: n = 11, >0.56 and Low: n = 18, ≤0.56), and analyzed (P = 0.032 by log rank test).
Table 1.
Relative hazard of recurrence free survival in univariate and multivariate analysis
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| aPKCλ/ι expression | ≤ 0.56 (n = 18) | ||||||
| > 0.56 (n = 11) | 3.914 | 1.071–14.305 | 0.039 | 6.768 | 1.161–39.443 | 0.033 | |
| Stage | pT2 (n = 16) | ||||||
| pT3 (n = 13) | 2.866 | 0.747–10.992 | 0.125 | 3.928 | 0.781–19.748 | 0.097 | |
| Histology | Well (n = 5) | ||||||
| Moderately (n = 13) | 1.129 | 0.218–5.848 | 0.885 | 1.12 | 0.173–7.275 | 0.905 | |
| poorly (n = 11) | 0.892 | 0.161–4.932 | 0.895 | 0.887 | 0.081–9.669 | 0.922 | |
| Gleason score | ≤ 6 (n = 11) | ||||||
| 7 (n = 10) | 1.087 | 0.291–4.060 | 0.901 | 1.562 | 0.275–8.876 | 0.615 | |
| 8 and 9 (n = 8) | 0.842 | 0.184–3.866 | 0.825 | 4.346 | 0.346–54.581 | 0.255 | |
| Age | ≤ 68 (n = 16) | ||||||
| > 68 (n = 13) | 1.362 | 0.393–4.718 | 0.626 | 1.946 | 0.488–7.761 | 0.346 | |
| PSA | ≤ 10 (n = 15) | ||||||
| > 10 (n = 14) | 1.168 | 0.375–3.639 | 0.789 | 0.931 | 0.233–3.721 | 0.920 | |
CI, confidence interval; HR, hazard ratio.
Fig. 2.
Representative examples of immunohistochemistry of aPKCλ/ι expression. Expression intensities of aPKCλ/ι were divided in two groups (positive; +2≤ and negative; 0 and + 1). Gleason scores are indicated in the figures and aPKCλ/ι expression in normal prostate tissue is also shown in the figure.
Suppression of aPKCλ/ι Expression Reduces Prostate Cancer Cell Growth In Vitro and In Vivo.
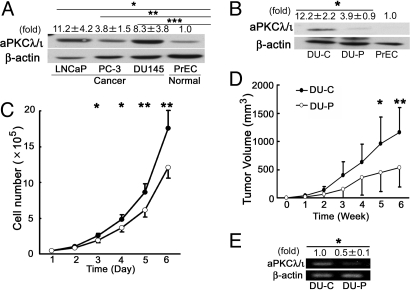
The correlation of aPKCλ/ι expression in prostate cancer tissue samples with PSA failure prompted us to clarify the role of aPKCλ/ι in prostate cancer cell lines. Western blot clearly showed aPKCλ/ι expression to be higher in prostate cancer cell lines, LNCaP, PC-3 and DU145 cells, than in normal prostate cells, PrEC, as expected (Fig. 3A). To evaluate the role of aPKCλ/ι in cell growth, we introduced siRNA for aPKCλ/ι into the DU145, an androgen-independent cell line, and established a mixture of cell lines expressing siRNA for aPKCλ/ι (DU-P), as well as vector control (DU-C) cells. As shown in Fig. 3B, we confirmed the reduced expression of aPKCλ/ι in the pooled transfectant (DU-P), as compared to control cells (DU-C). We found that DU-P cells grew more slowly than control cells (Fig. 3C, P < 0.05 at days 3 and 4, and P < 0.01 at days 5 and 6). We next transplanted the cell lines into nude mice and monitored the tumor volume in vivo. As shown in Fig. 3D, the xenografts of aPKCλ/ι-depleted DU-P cells showed slower growth than those of control DU-C cells (P = 0.04 at 5 weeks, P = 0.012 at 6 weeks). The suppression of aPKCλ/ι expression in xenografts was confirmed by RT-PCR analysis (Fig. 3E, P = 0.001). Thus, the suppression of aPKCλ/ι expression leads to the inhibition of prostate cancer growth in vitro and in vivo, clearly indicating a positive role of aPKCλ/ι in the growth of prostate cancer cells.
Fig. 3.
aPKCλ/ι expression in prostate cancer cell lines and growth inhibition of aPKCλ/ι siRNA transfected DU145 cells in vitro and in vivo. (A) aPKCλ/ι expression in prostate cell lines was analyzed by Western blot. β-actin was used as an internal control. Values indicate means ± SD at least three independent experiments (set as 1.0 in PrEC). *, P = 0.017, **, P = 0.032 and ***, P = 0.031 by unpaired t test. (B) DU145 cells transfected with siRNA for aPKCλ/ι expression vector (DU-P cells) and empty vector (DU-C cells) were confirmed by Western blot. β-actin was used as an internal control. Values indicate means ± SD from at least three independent experiments (set as 1.0 in PrEC). *, P = 0.004 by unpaired t test. (C) The inhibition of cell growth of siRNA transfected cells in vitro. DU-C (filled circles) and DU-P (open circles) cells were seeded onto 12-well plates at 4 × 104 cells and counted until day 6 (n = 4 in each group). Points and bars indicate means ± SD from at least three independent experiments. *, P < 0.05 **, P < 0.01 by unpaired t test. (D) The inhibition of cell growth of siRNA transfected cells in vivo. DU-C (filled circles) and DU-P (open circles) cells were implanted s.c. into the right and left flanks of male nude mice (n = 7 in each group) and tumor growth was calculated at 6 weeks (The day of injection was taken as week 0). Points and bars indicate means ± SD. *, P = 0.04; **, P = 0.012 by unpaired t test. (E) aPKCλ/ι mRNA expression extracted from xenografts. Total RNA was extracted from the same tumor specimens and aPKCλ/ι mRNA expression was examined. Values indicate means ± SD from at least three independent experiments (set as 1.0 in DU-C derived tumors) *, P = 0.001 by unpaired t test.
aPKCλ/ι Mediates the Growth of Prostate Cancer Cells in an Autocrine Manner Through IL-6 Secretion.
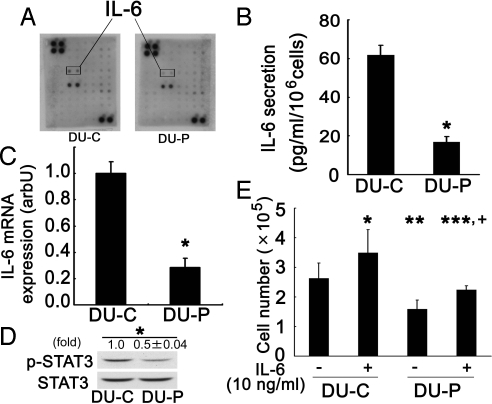
To explore the mechanism involved in aPKCλ/ι-dependent growth of prostate cancer cells, we focused on cytokines which have been implicated in the growth of a variety of cancer cells. Comparison of the conditioned medium between DU-C and DU-P cell lines by cytokine membrane array revealed IL-6 to be a candidate cytokine, that is, that its regulation might be under the control of aPKCλ/ι (Fig. 4A). The ELISA results confirmed that IL-6 is secreted into conditioned medium of control DU-C cells and decreased in aPKCλ/ι-depleted DU-P cells (Fig. 4B, P = 0.002). Similarly, qPCR revealed IL-6 mRNA expression to also be suppressed in aPKC-depleted DU-P cells (Fig. 4C, P < 0.001). These results show that aPKCλ/ι regulates IL-6 secretion in prostate cancer cells.
Fig. 4.
IL-6 expression was suppressed in siRNA transfected prostate cancer cells. (A) Conditioned media from DU-C and DU-P cells were analyzed using a membrane array. Arrows show IL-6 spots. Since this representative data are screening test only, it is performed once. This data had confirmed using ELISA and qPCR. (B) Expressions of IL-6 protein in DU-C and DU-P cells were confirmed using ELISA. Conditioned media from DU-C and DU-P (n = 2 in each group) were analyzed using an IL-6 ELISA kit from R&D Systems. Bars represent means ± SD from at least three independent experiments. *, P = 0.002 by unpaired t test. (C) Expressions of IL-6 mRNA in DU-C and DU-P cells. IL-6 mRNA expression was investigated by qPCR (n = 3 in each group). Bars represent means ± SD from at least three independent experiments. *, P < 0.001 by unpaired t test. arbU: arbitrary units. (D) STAT3 phosphorylations in DU-C and DU-P cells were analyzed by Western blot. After phospho-STAT3 protein had been detected, membranes were re-probed for STAT-3, and then used as controls. Values indicate means ± SD at least three independent experiments (set as 1.0 in DU-C). *, P = 0.002 by unpaired t test. (E) Recombinant IL-6 stimulated growth of DU-C and DU-P cells. DU-C and DU-P cells were stimulated with IL-6 (10 ng/ml). At day 3 after stimulation, cells were treated with trypsin and counted (n = 4 in each group). Bars represent means ± SD from at least three independent experiments. *, P = 0.001, **, P < 0.001 and ***, P = 0.393 (vs. DU-C without IL-6 stimulation) +, P = 0.016 (vs. DU-P without IL-6 stimulation) by ANOVA followed by Bonferroni test.
IL-6 is the cytokine reportedly expressed in androgen-independent prostate cancer cell lines including parental DU145 (26, 30). We next examined the effect of aPKCλ/ι depletion on phosphorylation of STAT3, one of the downstream mediators of the possible IL-6 involvement in prostate cancer cells (25, 29, 32, 33). As shown in Fig. 4D, STAT3 phosphorylation was down-regulated in DU-P cells as compared to DU-C cells, indicating that IL-6 signaling is altered in aPKCλ/ι-depleted cells. Given that aPKCλ/ι depletion results in the decreased production of IL-6, these results raise another question as to whether aPKCλ/ι depletion also affects IL-6 signaling, versus only IL-6 production. To clarify this issue, we next evaluated the response of cells to ectopic IL-6. When both cell types were treated with recombinant IL-6 (10 ng/ml) for 3 days, IL-6 increased cell growth, as we expected (Fig. 4E, P = 0.001 and P = 0.016). Moreover, DU-P cells treated with IL-6 showed growth similar to that of DU-C cells not treated with IL-6 (Fig. 4E, P = 0.393). Thus, aPKCλ/ι depletion does not affect the growth response of cells to IL-6. Taking these observations together, we conclude that secretion of IL-6 enhanced by aPKCλ/ι expression plays a role in promoting growth of the prostate cancer cell line DU145. Our results suggest that this growth promotion is mediated in an autocrine manner.
aPKCλ/ι Mediates IL-6 Gene Transcription Through NFκB and AP-1 in Prostate Cancer Cells.
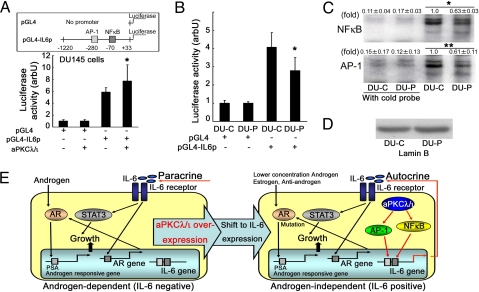
To analyze the mechanism by which aPKCλ/ι enhances IL-6 secretion, we next examined the effect of aPKCλ/ι on transcription of the IL-6 gene by luciferase reporter assay and EMSA. The luciferase reporter gene, pGL4-IL6p, contains a 1.2-kb 5′-flanking region of genomic DNA isolated from DU145 cells. This region contains the regulatory sequences recognized by AP-1, NFκB, MRE and other factors (30). As shown in Fig. 5A, overexpression of aPKCλ/ι in DU145 cells enhanced activation of the IL-6 promoter (Fig. 5A, P = 0.016). On the other hand, depletion of aPKCλ/ι (DU-P cells) resulted in decreased reporter gene expression (Fig. 5B, P < 0.001). These results strongly support the notion that aPKCλ/ι is involved in transcription of the IL-6 gene. Transcription of this gene is regulated by two major transcription factors, AP-1 and NFκB, in prostate cancer cells (30). To examine the involvement of these transcription factors in aPKCλ/ι-mediated IL-6 gene transcription, we used EMSA. The results showed both NFκB and AP-1 to be reduced upon aPKCλ/ι depletion (Fig. 5 C and D, P < 0.001 and P = 0.026). These results support the notion that both AP-1 and NFκB are involved in aPKCλ/ι-mediated transcriptional activation of the IL-6 gene.
Fig. 5.
Involvement of aPKCλ/ι in activation of the IL-6 gene promoter and its transcription factors, NFκB and AP-1. (A) IL-6 promoter activation was induced by wild type aPKCλ/ι in DU145 cells. DU145 cells were transfected with pGL4, pGL4-IL6p, empty vector and wild type aPKCλ/ι. After a 48 h incubation, luciferase activity was analyzed in a luminometer (n = 4 in each group). Each control was given a value of 1.0 and each bar indicates the means ± SD of at least three independent experiments. *, P = 0.016 (vs. pGL4-IL6p without wild-type aPKCλ/ι expression vector) by ANOVA following Bonferroni test. (B) IL-6 promoter activation was reduced in DU-P cells. DU-C and DU-P cells were transfected with pGL4 and pGL4-IL6p. After a 12-h incubation, luciferase activity was analyzed in a luminometer (n = 4 in each group). Each control was given a value of 1.0 and each bar indicates the means ± SD of at least three independent experiments. *, P < 0.001 (vs. DU-C with pGL4-IL6p transfection) by ANOVA following Bonferroni test. (C) DNA binding properties of NFκB and AP-1 were examined by EMSA. Each nuclear extract was reacted with 32P-labeled specific probes. To confirm specific binding of nuclear extracts and probes, reactions were also carried out using a cold probe. Values indicate means ± SD at least three independent experiments (set as 1.0 in DU-C). *, P < 0.001 and **, P = 0.026 by ANOVA following Bonferroni test. (D) Nuclear extracts for EMSA were confirmed by lamin B immunoblot. (E) Autocrine mechanism of prostate cancer cell growth involving aPKCλ/ι and IL-6. Overexpression of aPKCλ/ι in prostate cancer cells leads to up-regulation of IL-6 transcription through NFκB and AP-1 activation. Secreted IL-6 stimulates cell growth through STAT3 activation in an autocrine manner.
Discussion
aPKCλ/ι overexpression has been implicated in the progression and invasiveness of several tumor types including non-small cell lung cancer, ovarian cancer, breast cancer and glioma (16–22, 34, 35). Gene amplification of aPKCλ/ι is also observed in some cases (16, 18). In this study, we obtained evidence supporting a statistically significant correlation between aPKCλ/ι overexpression and a clinical marker of prostate cancer recurrence, PSA failure. A very recent study demonstrated aPKCλ/ι expression is required for cell survival (36). Thus, the aPKCλ/ι expression level and its activity might be prognostic factors for PSA failure.
IL-6, as noted above, has been implicated in the progression of a variety of tumors including prostate cancer (25–27, 29). Its overexpression has been detected in tissues (37, 38) and serum (28) from cancer patients. Preoperative plasma IL-6 level is related to PSA failure after radical prostatectomy (39). In vivo study indicates an important role of IL-6 in prostate cancer cells. Inhibition of IL-6 by anti-IL-6 antibody decreases the growth of PC-3 cells, which is one of an androgen-independent prostate cancer cells, in vivo (40). Therefore, IL-6 may play a role in androgen-independent growth of prostate cancer. Our findings on the molecular link between aPKCλ/ι and IL-6 raise an intriguing question as to the correlation between aPKCλ/ι expression and IL-6 expression in prostate cancer patients. We evaluated our tissue samples for IL-6 expression by qPCR. However, there was no statistically significant correlation between aPKCλ/ι expression and IL-6 expression (Fig. S3). One explanation is that the examined tissues contained not only cancer cells but also stromal cells. In support of this possibility, most prostate cancer tissues that express high levels of aPKCλ/ι protein (30/43, 70%) show a specific overexpression in epithelial cells but not in the surrounding stromal cells. However, IL-6 is reportedly expressed not only in prostate cancer epithelial cells, but also in stromal cells (37, 38). Thus, the use of laser capture microdissection (LCM) might be required to clarify this point. Unfortunately, we did not have sufficient sample quantities for LCM. We are currently conducting more extensive clinical studies aimed at clarifying the correlation between aPKCλ/ι expression and IL-6 expression.
Experiments using the prostate cancer cell line DU145 revealed that aPKCλ/ι is involved in prostate cancer growth both in vivo and in vitro. Furthermore, depletion of aPKCλ/ι in DU145 cells suppressed NFκB and AP-1 activities, transcription and secretion of IL-6, as well as suppressing cell growth, but not IL-6 signaling. We conclude that enhanced aPKCλ/ι expression in prostate cancer cells results in overproduction and secretion of a prostate growth factor, IL-6, at the transcriptional level. This forms an autocrine loop contributing to the growth of prostate cancer (Fig. 5E). The aPKC-dependent expression of IL-6 mRNA is also observed for another androgen-independent prostate cell line, PC-3, suggesting the generality of this regulation (Fig. S4). The specific overexpression of aPKCλ/ι in epithelial cells but not in stromal cells of the prostate further supports such an autocrine mechanism. It is known that most of androgen-independent prostate cancer tissues overexpress or/and express mutated AR that is still activated by lower concentration androgen, estrogen and anti-androgen drug (3). Taken together, the pathway might cooperate with the deregulated AR system to regulate proliferation of hormone-independent prostate cancer cells (Fig. 5E).
The following reports strongly support our conclusion. Combined constitutive activation of NFκB and AP-1 has been reported to mediate deregulated expression of IL-6 in DU145 cells (30). Ectopic expression of IL-6 in IL-6-negative LNCaP prostate cancer cells results in stimulation of the STAT3 signaling pathway as well as cell growth (29). However, the mechanism by which IL-6 expression is deregulated is not yet fully understood. As for aPKCλ/ι, several studies have shown that aPKCλ/ι activates NFκB and AP-1 (9, 10, 13–15, 41–44). Furthermore, aPKC activates NFκB in prostate cells (45). aPKC deregulates the growth of mouse prostate cancer cells (46). While the mechanism by which aPKC affects the growth of cancer cells remains obscure, the involvement of Rho B suppression in aPKC-dependent invasive properties in glioblastoma (35) and that of activation of the Rac1/Erk pathway in aPKC-dependent growth and tumorigenicity have been reported (17, 22). Our present results may explain the link between aPKCλ/ι and IL-6, two molecules implicated in the progression of a variety of malignancies, and establish a molecular mechanism underlying prostate cancer development and/or progression, thereby providing insights into the prognosis and treatment of prostate cancer.
How aPKCλ/ι expression was regulated in prostate cancer cells? Amplification of aPKCλ/ι gene is reported in lung and ovarian cancer (16, 18, 19) and amplification of chromosome 3q including aPKCλ/ι gene in prostate cancer cell lines (47). Another possibility is that aPKCλ/ι expression is up-regulated through the transcriptional activation of aPKCλ/ι promoter during hormone refractory process. Gustafson et al. reported aPKCλ/ι promoter analysis using luciferase (48). They show Bcr-Abl regulates aPKCλ/ι expression through the MEK-dependent activation of an Elk1 element within aPKCλ/ι promoter in leukemia cell line. We are ongoing the investigation of the mechanism of aPKCλ/ι overexpression involving in hormone refractory process. Further, other molecules expression, such as PAR-4, might be affected the alteration of aPKCλ/ι activity during hormone refractory process (49).
One of the most important clinical aspects of prostate cancer is dramatically decreased androgen-dependent cell growth, a typical indicator of prostate cancer progression. Possible mechanisms include androgen receptor overexpression and other mutations that result in hypersensitivity to androgens and/or other growth factors (3–6). IL-6 is overexpressed in hormone refractory prostate cancer patients and is one of the factors implicated in this process (26–28, 37). Importantly, IL-6 can stimulate androgen receptor activation independently of androgens with the induction of androgen receptor expression (4, 5). A recent study on LNCaP cells, which are sensitive to androgens and do not usually express IL-6, suggested IL-6 to be involved in the progression of prostate cancer cells from androgen dependence to androgen independence (50). It has been reported androgen-dependent prostate cancer cells obtained from xenografts treated with the anti-IL-6 antibody retained in androgen-dependence. In contrast, cancer cells obtained from xenografts untreated with the anti-IL-6 antibody are converted to androgen-independence in vitro and in vivo experiments, which means that IL-6 contributes to the development of androgen independence in prostate cancer (51). Taking these observations and our results together, we speculate that the molecular link between aPKCλ/ι and IL-6 revealed in the present study supports the notion that aPKCλ/ι is involved in this transition from androgen dependent to androgen independent growth of prostate cancer. As for the relationship between androgens and aPKC, there is an interesting report suggesting that aPKCζ, another aPKC isotype, is involved in the growth of androgen dependent LNCaP cells (52). In LNCaP cells, androgen stimulates aPKCζ through an unknown mechanism, and AILNCaP, a LNCaP subline established after androgen depletion, showed constitutive activation of aPKCζ. Another study on a breast cancer cell line, MCF7, showed the involvement of aPKCζ in estradiol-dependent cell growth (53). These reports further suggest a close relationship between aPKC and hormone-dependent cell growth. Our present findings provide additional evidence clarifying this point and are anticipated to facilitate understanding the progression of hormone-related malignancies including prostate cancer. Furthermore, aPKCλ/ι expression might be a biomarker for prostate cancer progression.
Materials and Methods
Cell Lines, Patients, and Tissues Sample.
LNCaP, PC-3, and DU145 cells were obtained from the American Type Culture Collection. PrEC cells were obtained from Clonetics. All cell lines were maintained with suitable medium (F-12 supplemented with 10% FCS (FCS) for LNCaP and PC-3, MEM supplemented with 10% FCS for DU145, PrEGM for PrEC) under 5% CO2.
Paired human untreated primary prostate cancer tissues and normal (or benign prostatic hypertrophy (BPH)) (n = 29) tissues from same patients were obtained during radical prostatectomy at Yokohama City University Hospital and its affiliates. The sampling and usage of all prostate tissues in this study were approved by the ethical committee of Yokohama City University Graduate School of Medicine and performed only after obtaining informed consent from each patient. For details, see SI Text and Table S1.
Reagents.
Human recombinant IL-6 was purchased from R&D Systems. G418 was purchased from Invitrogen Corp. Anti-aPKCι antibody was purchased from BD Biosciences. Anti-lamin-B antibody (c-19) was purchased from Santa Cruz Biotechnology Inc. Anti-actin antibody (AC-15) was purchased from Sigma-Aldrich. Anti-phospho-STAT3 and anti-STAT3 (#9131 and #9132, respectively) antibodies were purchased from Cell Signaling Technology Inc. Anti-rabbit and anti-mouse horseradish peroxidase conjugates were purchased from GE Healthcare U.K. Ltd.
Generation of Stable Transfectant-Induced siRNA for aPKCλ/ι.
To investigate the role of aPKCλ/ι in prostate cancer, we generated aPKCλ/ι knock-down cells using siRNA for aPKCλ/ι. The pEB6-Super vector (54) encoding the shRNA sequence for aPKCλ/ι RNAi with the target sequence 5′-CAA GTG TTC TGA AGA GTT T-3′ (DU-P cells) or empty vector (DU-C cells) was transfected into DU145 cells using Nucleofactor electroporation methods (Amaxa AG). Then, transfected cells were selected by G418 (800 μg/mL) over a 3-week period. After the specific down-regulation of aPKCλ/ι had been confirmed by Western blot, the cells were used for further experiments.
RNA Extraction and Real-Time Quantitative PCR (qPCR).
Total RNA from cell lines, prostate tissues and xenografts were extracted using ISOGEN (NipponGene) according to the manufacturer's instructions. After cDNA had been synthesized with random hexomers and MMLV (Moloney Murine Leukemia Virus), qPCR was performed with an iCycler and SYBR Green Supermix (Bio-Rad). For details, see SI Text.
Immunohistochemistry.
Immunohistochemistry was performed for aPKCλ/ι protein expression according to the previous report (20). For details, see SI Text.
Cell Growth Analysis.
DU-C or DU-P cells (4 × 104) were incubated in 12-well plates (day 0). Incubated cells were harvested with trypsin and counted till 6 days (from day 1 to day 6) using a hematocytometer (Beckman Coulter, Inc.). For the IL-6 stimulation experiment, 4 × 104 DU-C or DU-P cells were seeded onto 12-well plates and incubated for 24 h. The medium was then changed to phenol red-free RPMI1640 with 0.1% BSA (BSA), IL-6 (10 ng/mL) was added and incubation was continued for another 3 days. Then, cells were harvested with trypsin and counted.
In Vivo Tumor Growth.
5 × 106 cells (DU-C and DU-P cells) were injected into the flank regions of athymic nude mice (4–6 weeks old, n = 7). Tumors were measured weekly with a caliper (for comparison with the week 0 value). The tumor volume was calculated using the formula: tumor volume (mm3) = 0.5 × length × (width)2. After 6 weeks, tumors were isolated and aPKCλ/ι expression was confirmed by RT-PCR.
Cytokine Membrane Array.
Cytokines in the conditioned medium were detected using Human Cytokine Array III (Ray Biotech) according to the manufacturer's instructions. For details, see SI Text.
ELISA (ELISA) for IL-6 Secretion.
IL-6 secretion in the collected medium was measured using a human IL-6 ELISA kit according to the manufacturer's instructions (R&D Systems). For details, see SI Text.
Western Blot.
Cell lysates were prepared and subjected to Western blot. For details, see SI Text.
Luciferase Assay.
Approximately 1.2 kb of the IL-6 5′-flanking region was generated using PCR from genomic DNA extracted from DU145 cells, and cloned into the pGL4.0 [luc2] vector (pGL4-IL6p) (Promega). Wild type aPKCλ/ι was obtained as described in previous reports (9, 10). phRL-SV40 was used as the internal control for the luciferase assay (Promega). After cells transfected each plasmid vector were incubated and lysed, luciferase activity was measured using the dual-luciferase reporter assay system (Promega) and a luminometer, TD-20/20 (Turner Design). For details, see SI Text.
Electrophoretic Mobility Shift Assay (EMSA).
Nuclear proteins of DU-C or DU-P were extracted using NE-PER (Pierce Biotechnology Inc.) and subjected to EMSA using gel shift assay systems (Promega). For details, see SI Text.
Statistical Analysis.
All statistical analyses were performed using SPSS for windows (SPSS Inc.). For details, see SI Text.
Supplementary Material
Acknowledgments.
We thank R. Shimizu, Y. Nakamura, T. Yamaki, Y. Imano, T. Taniguchi, H. Soeda, C. Kondo, and A. Ishiyama for technical and secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Science, and Technology; a COE Research Grant from the Japan Society for the Promotion of Science; and a Grant for Strategic Research Promotion from Yokohama City University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907044106/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Calabrò F, Sternberg CN. Current indications for chemotherapy in prostate cancer patients. Eur Urol. 2007;51:17–26. doi: 10.1016/j.eururo.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Taplin ME, Balk SP. Androgen receptor: A key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 4.Culig Z, Bartsch G, Hobisch A. Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth. Mol Cell Endocrinol. 2002;197:231–238. doi: 10.1016/s0303-7207(02)00263-0. [DOI] [PubMed] [Google Scholar]
- 5.Lin DL, Whitney MC, Yao Z, Keller ET. Interleukin-6 induces androgen responsiveness in prostate cancer cells through up-regulation of androgen receptor expression. Clin Cancer Res. 2001;7:1773–1781. [PubMed] [Google Scholar]
- 6.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akimoto K, et al. A new member of the third class in the protein kinase C family, PKC lambda, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994;269:12677–12683. [PubMed] [Google Scholar]
- 8.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 9.Akimoto K, et al. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 10.Akimoto K, et al. Atypical protein kinase Cλ binds and regulates p70 S6 kinase. Biochem J. 1998;335:417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A, Akimoto K, Ohno S. Protein kinase C λ/ι (PKCλ/ι): aPKC isotype essential for the development of multicellular organism. J Biochem. 2003;133:9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- 12.Ohno S. Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Meco MT, et al. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by PB1 domain interactions. Mol Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NFκB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regala RP, et al. Atypical protein kinase C ι is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- 17.Regala RP, et al. Atypical protein kinase C ι plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280:31109–31115. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- 18.Eder AM, et al. Atypical PKC iota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006;66:4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]
- 20.Kojima Y, et al. The overexpression and altered localization of the atypical Protein kinase C lambda/iota in breast cancer correlates with the pathological type of these tumors. Human Pathol. 2008;39:824–831. doi: 10.1016/j.humpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, et al. Correlation of aPKC-iota and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:70–75. [PubMed] [Google Scholar]
- 22.Murray NR, et al. Protein kinase C is required for Ras transformation and colon carcinogenesis in vivo. J cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornford P, et al. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999;154:137–144. doi: 10.1016/S0002-9440(10)65260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koren R, et al. Expression of protein kinase C isoenzymes in benign hyperplasia and carcinoma of prostate. Oncol Rep. 2004;11:321–326. [PubMed] [Google Scholar]
- 25.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 27.Corcoran NM, Costello AJ. Interleukin-6: Minor player or starring role in the development of hormone-refractory prostate cancer ? BJU Int. 2003;91:545–553. doi: 10.1046/j.1464-410x.2003.04025.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima J, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 29.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor κB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–2215. [PubMed] [Google Scholar]
- 31.Freedland SJ, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich PC, et al. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam L, et al. Expression levels of the JAK/STAT pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br J Cancer. 2007;97:378–383. doi: 10.1038/sj.bjc.6603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R, et al. Involvement of PKC-iota in glioma proliferation. Cell Prolif. 2008;41:122–135. doi: 10.1111/j.1365-2184.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin RM, Parolin DA, Lorimer IA. Regulation of glioblastoma cell invasion by PKC iota and RhoB. Oncogene. 2008;27:3587–3595. doi: 10.1038/sj.onc.1211027. [DOI] [PubMed] [Google Scholar]
- 36.Win HY, Acevedo-Duncan M. Role of protein kinase C-iota in transformed non-malignant RWPE-1 cells and androgen-independent prostate carcinoma DU-145 cells. Cell Prolif. 2009;42:182–194. doi: 10.1111/j.1365-2184.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobisch A, et al. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Royuela M, et al. Immunohistochemical analysis of the IL-6 family of cytokines and their receptors in benign, hyperplasic, and malignant human prostate. J Pathol. 2004;202:41–49. doi: 10.1002/path.1476. [DOI] [PubMed] [Google Scholar]
- 39.Shariat SF, et al. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith PC, Keller ET. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001;48:47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, et al. Inhibition of atypical PKC blocks ultraviolet-induced AP-1 activation by specifically inhibiting ERKs activation. Mol Carcinog. 2001;27:65–75. [PubMed] [Google Scholar]
- 42.Lu Y, Jamieson L, Brasier AR, Fields AP. NF-kappaB/RelA transactivation is required for atypical protein kinase C iota-mediated cell survival. Oncogene. 2001;20:4777–4792. doi: 10.1038/sj.onc.1204607. [DOI] [PubMed] [Google Scholar]
- 43.Bonizzi G, Piette J, Schoonbroodt S, Merville MP, Bours V. Role of the protein kinase C λ/ι isoform in nuclear factor-κB activation by interleukin-1β or tumor necrosis factor-α: cell type specificities. Biochem Pharmacol. 1999;57:713–720. doi: 10.1016/s0006-2952(98)00353-0. [DOI] [PubMed] [Google Scholar]
- 44.Lallena MJ, Diaz-Meco MT, Bren G, Payá CV, Moscat J. Activation of IκB kinase β by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Win HY, Acevedo-Duncan M. Atypical protein kinase C phosphorylates IKKαβ in transformed non-malignant prostate cell survival. Cancer Lett. 2008;270:302–311. doi: 10.1016/j.canlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh PM, Bedolla R, Mikhailova M, Kreisberg JI. RhoA-dependent murine prostate cancer cell proliferation and apoptosis: Role of protein kinase Czeta. Cancer Res. 2002;62:2630–2636. [PubMed] [Google Scholar]
- 47.Nupponen NN, Hyytinen ER, Kallioniemi AH, Visakorpi T. Genetic alterations in prostate cancer cell lines detected by comparative genomic hybridization. Cancer Genet Cytogenet. 1998;101:53–57. doi: 10.1016/s0165-4608(97)00060-5. [DOI] [PubMed] [Google Scholar]
- 48.Gustafson WC, et al. Bcr-Abl regulates protein kinase Cι (PKCι) transcription via an Elk1 site in the PKCι promoter. J Biol Chem. 2004;279:9400–9408. doi: 10.1074/jbc.M312840200. [DOI] [PubMed] [Google Scholar]
- 49.Gurumurthy S, Rangnekar VM. Par-4 inducible apoptosis in prostate cancer. J Cell Biol. 2004;91:504–512. doi: 10.1002/jcb.20000. [DOI] [PubMed] [Google Scholar]
- 50.Lee SO, Chun JY, Nadiminty N, Lou W, Gao AC. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67:764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- 51.Wallner L, et al. Inhibition of interleukin-6 with CNTO328, an anti-inteleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phonotype in orchiectomized mice. Cancer Res. 2006;66:3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- 52.Inoue T, et al. Requirement of androgen-dependent activation of protein kinase Cζ for androgen-dependent cell proliferation in LNCaP cells and its roles in transition to androgen-independent cells. Mol Endocrinol. 2006;20:3053–3069. doi: 10.1210/me.2006-0033. [DOI] [PubMed] [Google Scholar]
- 53.Castoria G, et al. Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells. Mol Cell Biol. 2004;24:7643–7653. doi: 10.1128/MCB.24.17.7643-7653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki A, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.