Abstract
Heterotrimeric G proteins in physiological and pathological processes have been extensively studied so far. However, little is known about mechanisms regulating the cellular content and compartmentalization of G proteins. Here, we show that the association of nucleoside diphosphate kinase B (NDPK B) with the G protein βγ dimer (Gβγ) is required for G protein function in vivo. In zebrafish embryos, morpholino-mediated knockdown of zebrafish NDPK B, but not NDPK A, results in a severe decrease in cardiac contractility. The depletion of NDPK B is associated with a drastic reduction in Gβ1γ2 dimer expression. Moreover, the protein levels of the adenylyl cyclase (AC)-regulating Gαs and Gαi subunits as well as the caveolae scaffold proteins caveolin-1 and -3 are strongly reduced. In addition, the knockdown of the zebrafish Gβ1 orthologs, Gβ1 and Gβ1like, causes a cardiac phenotype very similar to that of NDPK B morphants. The loss of Gβ1/Gβ1like is associated with a down-regulation in caveolins, AC-regulating Gα-subunits, and most important, NDPK B. A comparison of embryonic fibroblasts from wild-type and NDPK A/B knockout mice demonstrate a similar reduction of G protein, caveolin-1 and basal cAMP content in mammalian cells that can be rescued by re-expression of human NDPK B. Thus, our results suggest a role for the interaction of NDPK B with Gβγ dimers and caveolins in regulating membranous G protein content and maintaining normal G protein function in vivo.
Keywords: cAMP, cardiac contractility, G proteins, NDPK, zebrafish
Signaling through the activation of G proteins represents the most widely used signaling pathway in mammalian biology (1). A variety of G protein-coupled receptors (GPCRs) mediate extracellular signals via heterotrimeric G proteins, which are composed of a guanine nucleotide binding α-subunit (Gα), as well as a β-subunit (Gβ) and a γ-subunit (Gγ). Upon GPCR activation, the bound GDP in Gα is exchanged for GTP and both the GTP-liganded Gα and the stable dimer Gβγ regulate downstream effectors (2).
Nucleoside diphosphate kinases (NDPKs), which catalyze the transfer of γ-phosphate between NTPs and NDPs, represent a family of multifunctional proteins encoded by nine human nm23 genes. The two major isoforms, NDPK A and B (17–21 kDa), play crucial roles in a wide array of cellular processes [for review see (3)]. Despite their high sequence homology and the well known formation of heterohexamers to perform their house keeping enzyme activity (4), NDPK A and B have distinct cellular functions, which are based on the possibility of both isoforms contributing to multimeric protein complexes like the SET complex (5) and a complex formed with Ca2+-activated potassium channel KCa3.1 (6). In such complexes, NDPK not only supplies NTPs but also acts as protein kinase (6). We have previously shown that NDPK B, but not NDPK A, forms a complex with Gβγ (7, 8) and acts as a histidine kinase for Gβ. The high energetic phosphate on Gβ can be specifically transferred to GDP and the GTP that is formed, induces G protein activation (3). Recent in vitro findings suggest that this phosphorelay can regulate Gs protein activity independently of GPCRs in cardiomyocytes, thereby stimulating cAMP synthesis and contractility (9). However, data supporting a role of the NDPK B/Gβγ complex in G protein signaling in vivo are still missing.
Therefore, we used the zebrafish model to selectively decrease the expression of NDPK A, B and the zebrafish Gβ1 isoforms by injection of morpholino-modified antisense oligonucleotides. In addition, we analyzed embryonic fibroblasts (MEFs) from NDPK A and B double knockout mice to explore the function of the NDPK B/Gβγ complex in mammals. We report that heterotrimeric G proteins and NDPK B depend on each other in the maintenance of their membranous protein content and additionally regulate caveolin expression. Apparently, NDPK B is an essential partner of heterotrimeric G proteins, indispensable for intact G protein content and function in vivo.
Results
Morpholino-Mediated Knockdown of the Zebrafish NDPK A and B.
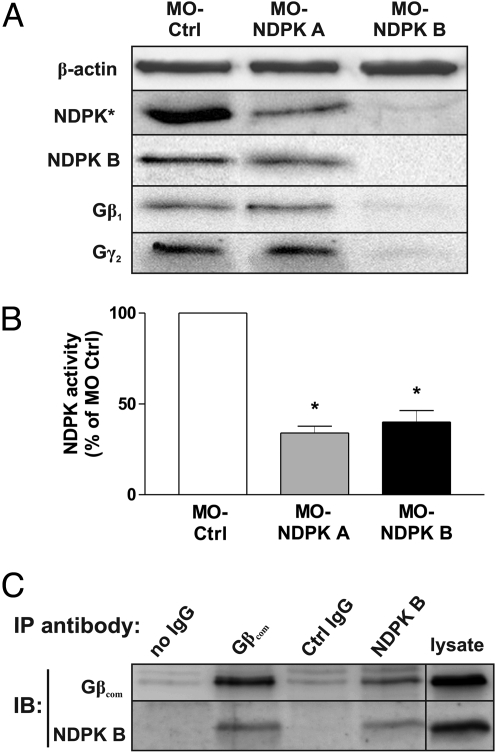
To investigate the role of the NDPK B/Gβγ complex in vivo, we used antisense-morpholino oligonucleotides (MO) to inhibit the gene expression of the two major NDPK isoforms in zebrafish embryos. Zebrafish NDPK B (NDPK Z1) and NDPK A (NDPK Z2) exhibit 85% and 77% amino acid sequence identity to their human orthologs (Fig. S1A), and are thus evolutionarily conserved. Specific MOs, targeted against the translational start site of zebrafish NDPK B or NDPK A, were injected into the embryo at one-cell-stage. The resulting morphants were analyzed at 48–72 h post-fertilization (hpf). Wild-type (WT) embryos and embryos injected with a standard control morpholino (MO-Ctrl) served as controls. As shown in Fig. 1A, injection of both, MO-NDPK A and MO-NDPK B strongly reduced total NDPK content. As an antibody specifically recognizing zebrafish NDPK A is not available, we analyzed total NDPK expression by an antibody recognizing both isoforms. Using an NDPK B specific antibody, the decreased NDPK B expression by MO-NDPK B injection could be demonstrated, while MO-NDPK A did not diminish the NDPK B content. In agreement with the reduced abundance of the protein, the MO-injection against NDPK A or B largely reduced the total NDPK phosphate transfer activity to 34 ± 4% and 40 ± 6% of WT activity, respectively (Fig. 1B). Most important, the protein levels of Gβ1 and Gγ2 were markedly reduced upon depletion of NDPK B, but not upon depletion of NDPK A. In contrast, the expression of β-actin (Fig. 1A) was not altered. To verify the previously detected complex formation of NDPK B with Gβγ (7, 8) in zebrafish, we performed co-immunoprecipitation experiments. As shown in Fig. 1C, an anti-Gβ antibody precipitated both endogenous Gβ and NDPK B from zebrafish lysates. Reciprocally, NDPK B and Gβ were co-immunoprecipitated with an anti-NDPK B antibody. These data confirm the association of NDPK B with Gβγ in the zebrafish.
Fig. 1.
NDPK A and B depletion in zebrafish embryos. Zebrafish embryos were injected with MO-Ctrl (4 ng), MO-NDPK A (8 ng) and MO-NDPK B (4 ng). (A) Immunoblot analysis of lysates prepared 72 hpf using specific antibodies against total fish NDPK (NDPK*), NDPK B, Gβ1 and Gγ2. β-actin served as loading and specificity control. (B) Relative NDPK activity (normalized to MO-Ctrl) was quantified as formation of 3H-GTP from 3H-GDP and ATP in lysates of embryos injected as indicated. Data are means ± SEM, n = 3, *, P < 0.05 vs. MO-Ctrl. (C) Co-immunoprecipitation of NDPK B and Gβ in zebrabrafish lysates. Endogenous Gβ or NDPK B was immunoprecipitated (IP) from zebrafish lysates. Association of NDPK B or Gβ was detected by immunoblotting (IB). Representative immunoblots are shown.
Cardiac Dysfunction in NDPK B-Depleted Zebrafish Embryos.
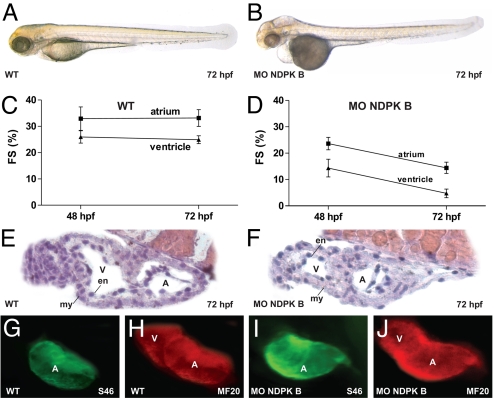
Despite the reduction of protein expression and total NDPK activity, zebrafish depleted of NDPK A did not reveal any obvious phenotypic alterations (Fig. S2) and were indistinguishable from wild-type (WT, Fig. 2A and Movie S1) or MO-Ctrl (see Fig. 4A) injected animals. In contrast, the NDPK B morphants showed severe alterations, such as smaller eyes and an impairment of the heart function (Fig. 2B). A decrease in cardiac contractility was associated with pericardial edema, insufficient blood flow and decelerated heart rate (Fig. 2B and Movie S2). Quantitative analysis of cardiac contractility revealed a progressive diminution in atrial and ventricular fractional shortening (FS): in the atrial chamber we found a reduction from 33 ± 4.4% in WT embryos to 23.6 ± 6.7% and 14.4 ± 7.1% in NDPK B morphants at 48 and 72 hpf, respectively. In the ventricle, fractional shortening was reduced from 25%±3% in control animals to 14.3 ± 3.4% (48 hpf) and 4.7 ± 1.6% (72 hpf) in embryos with NDPK B depletion (Fig. 2 C and D). Aside from the reduced contractility, the hearts of MO-NDPK B injected embryos appeared morphologically normal with two distinct heart chambers. At 72 hpf, appropriate growth of an epicardial, myocardial as well as an endocardial layer was observed by HE-staining similar to WT and MO-Ctrl embryos (Fig. 2 E and F). As shown by immunofluorescence, the expression of the heart-specific thick myofilament components, such as atrial and ventricular myosin heavy chain (MHC) was also not affected by NDPK B knockdown (Fig. 2 G–J). Altogether, these data indicate a structurally intact development of the heart in NDPK B morphants.
Fig. 2.
Cardiac dysfunction in NDPK B-depleted zebrafish embryos. Cardiac phenotype of wild-type (WT) (A) and NDPK B knockdown (B) embryos at 72 hpf. Fractional shortening (FS) of the atrial and ventricular chamber of WT (C) and NDPK B morphants (D) at 48 and 72 hpf. Data are means ± SEM, n = 11–21. Histology sections of a WT (E) and NDPK B knockdown (F) heart region stained with hematoxylin/eosin. A, atrium, V, ventricle, en, endocardial, my, myocardial layer. (G–J) Whole-mount immunofluorescence following staining with specific antibodies against atrial myosin heavy chain [S46, (G) and (I)] and entire heart tube myosin heavy chain [MF20, (H and J)] showing unaltered expression of structure proteins.
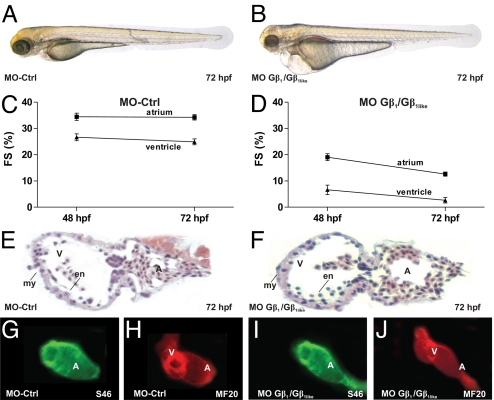
Fig. 4.
Loss of zebrafish Gβ1 phenocopies NDPK B morphants. Cardiac phenotype of control morpholino-injected (MO-Ctrl) (A) and MO-Gβ1/Gβ1like double-injected (B) embryos at 72 hpf. Fractional shortening (FS) of the atrial and ventricular chamber of MO-Ctrl fishes (C) and Gβ1/Gβ1like morphants (D) at 48 and 72 hpf. Data are means ± SEM, n = 10–12. Histology sections of a MO-Ctrl (E) and Gβ1/Gβ1like knockdown (F) heart region stained with hematoxylin/eosin. A, atrium, V, ventricle, en, endocardial, my, myocardial layer. (G–J) Whole-mount immunofluorescence following staining with specific antibodies against atrial myosin heavy chain [S46, (G and I)] and entire heart tube myosin heavy chain [MF20, (H and J)] showing unaltered expression of structure proteins.
To confirm that the cardiac phenotype is specific to NDPK B knockdown, we performed RNA rescue experiments (Fig. S3) by co-injection of a zebrafish NDPK B mRNA, that is morpholino-resistent due to silent mutations in the MO target site. Up to 53% of the embryos co-injected with MO-NDPK B and the mutated mRNA displayed a wild-type-like phenotype (Fig. S3 B–E) and restored NDPK B expression as well as cardiac contractility (Fig. S3F).
Morpholino-Mediated Knockdown of Zebrafish Gβ1 Isoforms.
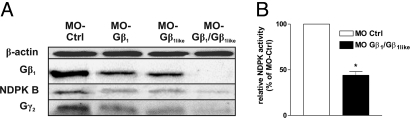
In zebrafish, two Gβ1 orthologs have been identified, Gβ1 and Gβ1like, which differ in only three amino acids outside known functional domains. Both are >98% identical to the human Gβ1 protein (Fig. S1C). Whole-mount in situ hybridization using specific probes against the 5′-untranslated regions of Gβ1 or Gβ1like revealed an identical mRNA-pattern (Fig. S4), indicating that both genes might be functionally relevant. G protein β1-subunit forms a complex with NDPK B in mammalian cells (3, 7, 8) and the co-immunoprecipitation experiments shown in Fig. 1C indicate an identical association in zebrafish. We therefore sought to suppress the expression of the Gβ1 isoforms by two specific MOs, MO-Gβ1 and MO-Gβ1like, targeted against the translational start site. The MOs were injected singly or in combination into zebrafish embryos. Immunoblot analyses of the lysates revealed that injection of a single MO (MO-Gβ1 or MO-Gβ1like) reduced the total Gβ1 ortholog expression by about 50%, whereas the double-knockdown by co-injection of both MOs (MO-Gβ1/Gβ1like) led to a nearly complete loss of zebrafish Gβ1 (Fig. 3A). In accordance with mRNA data from the in situ hybridization, these data show that total zebrafish Gβ1 is equally composed of both Gβ1 and Gβ1like. As Gγ subunits form heterodimers with Gβ1 and only Gβγ dimers are stable protein entities (2), corresponding reduction in Gγ2 protein expression was detected (Fig. 3A). To further substantiate the specificity of the knockdown effects, we performed Gβ1 knockdown using a splice MO [MO(s)-Gβ1like] instead of the translation start MO. Injection of MO(s)-Gβ1like caused a similar phenotype as MO-Gβ1like. Immunoblot analysis confirmed the depletion of Gβ1 and RT-PCR verified altered splicing (10) in MO(s)-Gβ1like injected embryos (Fig. S5).
Fig. 3.
Gβ1 and Gβ1like depletion in zebrafish embryos. Zebrafish embryos were injected with MO-Ctrl (4 ng), MO-Gβ1 (3 ng) and MO-Gβ1like (3 ng) singly or in combination (3 + 3 ng). (A) Immunoblot analysis of lysates prepared 72 hpf using specific antibodies against NDPK B, Gβ1 and Gγ2. β-actin served as loading and specificity control. (B) Relative NDPK activity (normalized to MO-Ctrl) was quantified as formation of 3H-GTP from 3H-GDP and ATP in lysates of embryos injected as indicated. Data are means ± SEM, n = 3, *, P < 0.05 vs. MO-Ctrl.
Most importantly, the knockdown of the Gβ1 isoforms by either approach proportionally decreased the amount of the NDPK B protein (Fig. 3A). In accordance with the data obtained by NDPK B knockdown, the total NDPK activity in the zebrafish lysates following Gβ1/Gβ1like knockdown was reduced to 44 ± 4% of control (Fig. 3B).
Loss of Gβ1 in Zebrafish Embryos Phenocopies NDPK B Morphants.
Injection of MOs targeted against the translation start of Gβ1 or Gβ1like as well as of the splice MO against Gβ1like causes identical cardiac phenotypes, which were very similar to those obtained in NDPK B morphants. Consistent with the findings on the protein level, the double-knockdown of both Gβ1 isoforms caused the most severe phenotype (Fig. 4B) characterized by significantly impaired cardiac contractility. The fractional shortening of the atrial and ventricular chamber is severely decreased from 34.4 ± 3.9% and 24.9 ± 3.7% in MO-Ctrl to 12.6 ± 3.7% and 2.6 ± 1.1%, respectively, in MO-Gβ1/Gβ1like double-injected embryos at 72 hpf (Fig. 4 C and D). Similar to NDPK B knockdown embryos, the heart of the Gβ1/Gβ1like morphants appeared normally developed and structurally intact (Fig. 4 E–J).
Together with the strong reduction of Gβ1γ2 expression by NDPK B depletion and the co-immunoprecipitation experiments (Fig. 1), these data argue for a close association of NDPK B and Gβγ in the zebrafish. To further substantiate this hypothesis, we injected subeffective doses of either MO-NDPK B (2 ng) or MO-Gβ1/Gβ1like (1 ng each) and observed no significant effect on cardiac contractility (Fig. S6). However, co-injection of both MOs at these subeffective doses resulted in a severe reduction of atrial and ventricular fractional shortening at 72 hpf (Fig. S6) pointing to a synergistic role of NDPK B and Gβ for cardiac contractility in the zebrafish.
Impairment of G Protein Signaling by NDPK B/Gβγ Depletion in Zebrafish Embryos.
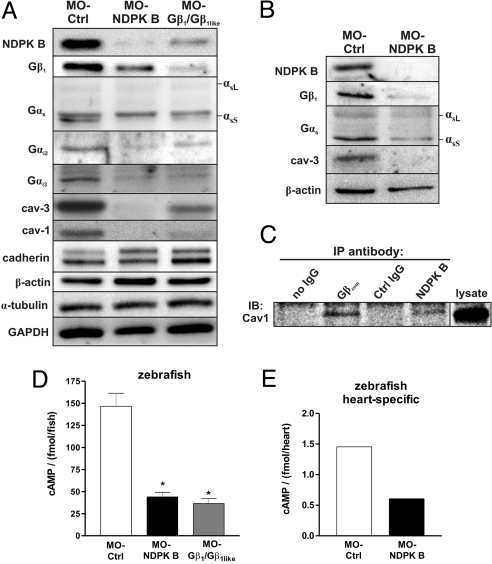
Increasing evidence (11, 12) indicates that Gβγ is required for the membrane insertion and stability of the associated Gα subunits. As heterotrimeric G proteins play a key role in the regulation of cardiac contractility by stimulating or inhibiting adenylyl cyclase activity, we studied the effects of either NDPK B or Gβ1 depletion on the expression of the Gα subunits in zebrafish embryos by immunoblot analysis. The protein content of the adenylyl cyclase-regulating Gα subfamilies, Gαs, and Gαi, was diminished in whole zebrafish lysates, following NDPK B as well as Gβ1/Gβ1like depletion (Fig. 5A). In NDPK B as well as Gβ1/Gβ1like morphants, the Gα protein levels were reduced by at least 50% compared to WT or MO-Ctrl embryos. Most importantly, the expression of several marker proteins from different cellular compartments, cadherin, α-tubulin, β-actin and GAPDH, was not affected by the depletion of either NDPK B or Gβ1/Gβ1like (Fig. 5A). Because of the marked cardiac phenotype of the morphant embryos, we additionally determined G protein levels in heart-specific tissue prepared from the transgenic zebrafish line Tg(cmlc2:gfp) (25) with heart-specific GFP expression. As shown in Fig. 5B, knockdown of NDPK B resulted in similar alterations of G protein α- and β-subunit content in tissue from zebrafish hearts. Interestingly, while the expression of the plasma membrane marker cadherin was not altered, the knockdown of NDPK B or Gβ1 resulted in a decrease in the caveolae forming membrane proteins caveolin-1 and -3 (Fig. 5A), which are known as important scaffold proteins for a large number of signaling molecules (13), including GPCRs, G proteins and effectors (14). The amount of muscle specific caveolin-3 was also strongly reduced in isolated fish hearts (Fig. 5B).
Fig. 5.
Alterations of G protein content and cAMP formation in NDPK B and Gβ knockdown zebrafish embryos. (A and B) Representative immunoblots of zebrafish embryos at 72 hpf following knockdown of either NDPK B or Gβ1/Gβ1like in total fish lysates (A) and heart-specific extracts (B) using specific antibodies against the indicated G protein subunit and caveolin isoform. Positions of the long (αsL) and short (αsS) Gαs splice variants are indicated. Note that GαsS is migrating below a nonspecific immunoreactive band. Marker proteins (cadherin, β-actin, α-tubulin, GAPDH) representing different cell compartments are shown as loading and specificity control. (C) Co-immunoprecipitation of caveolin-1 with NDPK B and Gβ. Endogenous Gβ or NDPK B was immunoprecipitated (IP) from zebrafish lysates (refer to Fig. 1C). Association of caveolin-1 was detected by immunoblotting (IB). A representative immunoblot is shown. (D) cAMP was measured in lysates from whole embryos at 72 hpf injected with the indicated MOs. Data are means ± SEM, n = 3, *, P < 0.05 vs. MO-Ctrl. (E) cAMP content of zebrafish hearts isolated from MO-Ctrl vs. MO-NDPK B-injected fishes with heart-specific GFP expression [Tg(cmlc2:gfp)] at 72 hpf. n = 365 hearts were pooled and lysed for each condition, respectively. The average of two independent experiments is shown.
As this coordinated loss of caveolins with NDPK B and Gβ1 pointed to a close association of these proteins, we analyzed the co-immunoprecipitations obtained from zebrafish lysates with the primary antibodies against Gβ or NDPK B (see Fig. 1C). Caveolin-1 was detected in both precipitates (Fig. 5C), indicating that caveolins are likely part of protein complexes including amongst others, Gβ and NDPK B.
NDPK/Gβγ complexes are involved in the basal cAMP production of cardiomyocytes (9). Thus, the reduced pump function of the hearts of the NDPK B and Gβ1 knockdown animals (see Figs. 2 and 4) suggested the analysis of basal cAMP levels in whole zebrafish and isolated hearts. Consistent with our results in rat cardiac myocytes (9), depletion of NDPK B or Gβ1 in zebrafish caused a significant decrease in basal cAMP production to 44 ± 5.3 fmol/fish and 36.5 ± 5.5 fmol/fish, respectively, compared to MO-Ctrl-injected fish with 146.7 ± 14.7 fmol/fish (Fig. 5D). Particularly, basal cAMP levels in isolated hearts from NDPK B morphants (0.6 fmol/heart) are markedly diminished compared to MO-Ctrl hearts (1.38 fmol/heart, Fig. 5E).
Our results indicate that NDPK B, caveolins, and heterotrimeric G proteins might depend on each other for maintenance of their protein expression levels. We therefore asked, whether this phenomenon occurs on the mRNA or on the protein level. We detected the relative content of mRNA encoding NDPK B and Gβ1 following MO-injection by quantitative real-time-PCR. The content of both mRNAs, encoding either NDPK B or Gβ1, showed no significant difference between morphants and control embryos (Fig. S7), arguing for post-translational regulation.
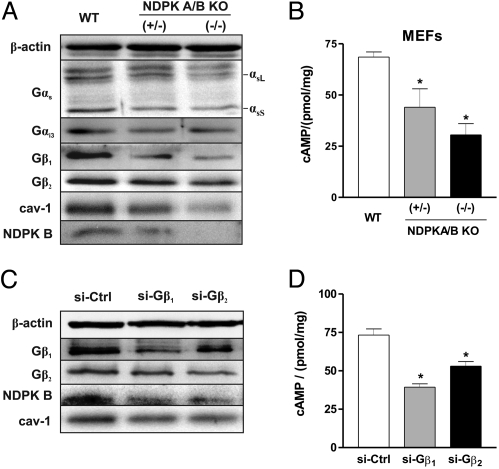
Alterations of G Protein Signaling in NDPK A/B Double-Knockout Mouse Embryonic Fibroblasts.
The regulation of G protein abundance and function by NDPK B in zebrafish embryos raised the question, whether this is a general phenomenon that also occurs in mammals. To address this question, we used mouse embryonic fibroblasts (MEFs) that have been derived from homozygous (−/−) and heterozygous (+/−) NDPK A and B double-knockout (KO) mice, respectively (15). As signal transduction by heterotrimeric G proteins as well as caveolae formation is located at the plasma membrane, we compared the protein amounts of caveolin-1 and different G protein subunits of WT MEFs, (+/−) and (−/−) NDPK A/B MEFs (Fig. 6A) in the membrane fraction. Similar to zebrafish morphants, loss of NDPK in MEFs was paralleled by diminished caveolin-1 content. Interestingly, the reduction of caveolin-1 was gene dose-dependent. The decrease in caveolin-1 paralleled the decreasing NDPK protein expression in heterozygous and homozygous NDPK KO MEFs (Fig. 6A). The reduction in NDPK B and caveolin-1 also resulted in decreased protein levels of the G protein subunits Gβ1, Gβ2, Gαs and Gαi3 in NDPK KO MEFs. Moreover, in line with our results in isolated rat cardiomyocytes (9) and zebrafish embryos, we found a significant reduction of the basal cAMP levels by 56% (44 ± 9 pmol/mg) in heterozygous and by 69% (30.5 ± 5.5 pmol/mg) in homozygous NDPK KO MEFs compared to WT MEFs (68.5 ± 2.5 pmol/mg) (Fig. 6B).
Fig. 6.
Alterations of G protein content and cAMP formation in NDPK A/B double-knockout and Gβ subunit knockdown MEFs. (A) Membrane fraction of mouse embryonic fibroblasts (MEFs) from wild-type (WT), heterozygous (+/−), and homozygous (−/−) NDPK A/B mice were subjected to immunoblot analysis with the indicated antibodies, showing the loss of G protein α and β subunits as well as caveolin-1 in the membrane fraction of NDPK A/B KO MEFs. Positions of Gαs splice variants (long, αsL, short, αsS) are indicated. (B) Basal cAMP production in WT, (+/−) and (−/−) NDPK A/B MEFs in the presence of 1 mM IBMX and 1 μM propranolol. Data are means ± SEM, n = 3, *, P < 0.05 vs. WT. (C) Immunoblot analysis of WT MEFs transfected with scrambled siRNA (si-Ctrl) and specific siRNA (si-) against Gβ1 and Gβ2 as indicated, demonstrating reduced expression of NDPK B and caveolin-1 in MEFs following Gβ1 or Gβ2 depletion. Expression levels of β-actin served as loading control. (D) Basal cAMP contents were measured 72 h after transfection with the indicated siRNA in the presence of 1 mM IBMX and 1 μM propranolol. Data are means ± SEM, n = 3, *, P < 0.05 vs. si-Ctrl.
To confirm the mutual dependence of NDPK B and Gβγ content in MEFs, we performed siRNA-mediated knockdown of Gβ1 and Gβ2 subunits in WT MEFs (16). Compared to control transfected cells, depletion of either Gβ1 or Gβ2 in MEFs resulted in a reduced NDPK B protein expression that is paralleled by a decrease in caveolin-1 (Fig. 6C) and a diminished basal cAMP production (69.2 ± 4 pmol/mg in si-Ctrl vs. 39.4 ± 2.2 pmol/mg in si-Gβ1 and 53.3 ± 2.2 pmol/mg in si-Gβ2) (Fig. 6D).
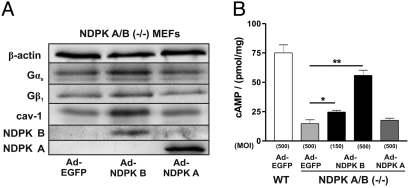
To test whether the alterations observed in the NDPK A/B KO MEFs can be attributed to the loss in NDPK B, we adenovirally re-expressed human NDPK B or NDPK A up to five-fold the WT level. Infection with an EGFP encoding adenovirus was used as a control. NDPK B expression (Ad-NDPK B) induced a significant increase in membranous Gαs, Gβ1 as well as caveolin-1 levels at 72 h post infection. In contrast, expression of NDPK A (Ad-NDPK A) was without effect (Fig. 7A). The basal cAMP content in the KO MEFs increased up to the WT levels by the expression of NDPK B, but not by NDPK A, in a dose-dependent manner [Fig. 7B, Ad-EGFP 14.7 ± 3.3, pmol/mg, Ad-NDPK A 17.6 ± 1.8 pmol/mg (MOI 500), Ad-NDPK B 24.6 ± 1.5 pmol/mg (MOI 150), and 55.9 ± 4.4 pmol/mg (MOI 500)]. Thus, re-expression of human NDPK B could at least partially rescue the reduction in G protein and cAMP content of NDPK KO MEFs.
Fig. 7.
Rescue of the phenotype of NDPK A/B double-knockout MEFs by adenoviral expression of human NDPK B. NDPK A/B (−/−) MEFs were infected with the adenoviruses Ad-EGFP, Ad-NDPK A and Ad-NDPK B at the multiplicity of infection (MOI) of 500. (A) Representative immunoblots of the membrane fraction of (−/−) MEFs infected as indicated confirmed the re-expression of NDPK A and B and showed elevated levels of caveolin-1, Gβ1 and Gαs compared with EGFP control infected cells. (B) cAMP production in (−/−) MEFs following infection with the adenoviruses at the indicated MOIs. Data are means ± SEM, n = 3, *, P < 0.05, **, P < 0.001 vs. Ad-EGFP infected (−/−) MEFs.
Discussion
As central regulators in signal transduction heterotrimeric G proteins are interacting with numerous proteins (1). We have previously complemented the classical concept of G protein activation by showing that a complex of NDPK B and Gβγ dimers can activate G proteins independently from GPCRs (7–9). To study the role of NDPK B and Gβγ in vivo, we used the zebrafish as model organism. After MO-mediated knockdown of NDPK B, as well as Gβ1, we detected the appearance of a phenotype with dramatically reduced cardiac contractility. Similar to injection of control morpholinos, the knockdown of NDPK A did not cause an obvious phenotype, although the total NDPK activity was reduced to a similar extent as by NDPK B depletion. Most likely, the remaining NDPK phosphate transfer activity is sufficient to maintain the housekeeping function of NDPK in nucleotide metabolism. The specificity in the requirement of the NDPK B isoform was further substantiated by the rescue experiments in which MO-resistant zebrafish NDPK B RNA was co-injected. Thus, the NDPK B morphant zebrafish is a living animal model with the loss of NDPK B gene function.
Our results further show that zebrafish Gβ1 is composed of two isoforms which apparently do not differ in their biological function and are obviously derived to a similar extent from two active genes encoding Gβ1 and Gβ1like. Hence, the double-knockdown of both Gβ1 and Gβ1like resulted in lesser Gβ1 protein expression which correlated to the severity of the cardiac phenotype. As for NDPK B, the Gβ1/Gβ1like morphants generated in this study represent a living vertebrate model that allows for the analysis of cardiovascular physiology with a specific depletion of a single Gβ subtype, while former genetic approaches were restricted to cell culture models, or lower organisms such as Dictyostelium discoideum and Caenorhabditis elegans (17–19).
We obtained several lines of evidence that the observed phenotypic alterations can be related to a lack of the interaction of NDPK B with Gβγ. First, with regard to the impaired cardiac contractility, the NDPK B and Gβ1/Gβ1like morphants developed a very similar phenotype and the MOs directed against NDPK B and Gβ1/Gβ1like act apparently synergistic (See Fig. S6). Second, the knockdown of NDPK B, but not of NDPK A, caused a loss in Gβ1γ2 paralleling the depletion of NDPK B protein expression. Reciprocally, the knockdown of Gβ1 and Gβ1like singly or in combination, caused a loss in NDPK B expression paralleling the reduction in Gβ1γ2 dimer content. Third, the NDPK B as well as the Gβ1/Gβ1like knockdown led to decreased abundance of the α-subunits of the adenylyl cyclase-regulating Gs and Gi protein subfamilies, whereas the expression of several marker proteins (cadherin, α-tubulin, β-actin, GAPDH, MHC) was not affected. Forth, co-immunoprecipitation experiments confirmed a close association of NDPK B with Gβγ in the zebrafish and thus argue for the existence of the previously purified NDPK B/Gβ1γ complex (7) also in the zebrafish. Our data therefore indicate that NDPK B and Gβ1γ2 are mutually dependent for the maintenance of their protein levels and both are essential for cardiac contractility in zebrafish embryos (7–9). In line with this interpretation, the NDPK A knockdown embryos reported herein as well as the published NDPK A KO mice (20) did not develop obvious defects.
Taking into account the pivotal role of heterotrimeric G proteins in the signaling of many cellular processes, we asked whether the mutual dependence of Gβγ and NDPK B expression levels is specific for the zebrafish or is a more general phenomenon. Using MEFs from non-viable NDPK A/B knockout mice, heterozygous and wild-type littermates, we could indeed confirm the findings in a mammalian genetic model. The gradual depletion and complete absence of NDPK A/B in membranes of MEFs from heterozygous and homozygous animals, respectively, was paralleled by a gradual loss of Gβ1 and Gβ2 as well as a reduction in basal cAMP levels. In addition, also in the MEF model, the reciprocal experiment, that is, the siRNA-mediated knockdown of Gβ1 or Gβ2 expression, resulted in a reduction of the NDPK B level and basal cAMP content. Similar to the zebrafish, re-expression of human NDPK B, but not NDPK A, in KO MEFs rescued the loss in G protein content and restored basal cAMP levels. Therefore, the data in mammalian cells are in complete agreement with the data obtained in the zebrafish and argue for an essential, evolutionarily conserved interaction of NDPK B with Gβγ which is required for G protein stabilization and function.
Interestingly, the depletion of NDPK B and Gβγ had a major impact on the abundance of caveolins and α-subunits of heterotrimeric G proteins in the zebrafish as well as in MEFs. It is well known that the individual members of the G protein heterotrimers confer to the stability of their binding partners (16, 21). In particular, by using RNAi-technology, recent studies showed (16, 17) that Gβ subunits are necessary for the stable accumulation of Gα proteins. Similarly, interfering with the membrane attachment of Gβγ dimers induced a loss of Gαs protein content in cardiac myocytes, and thus resulted in impaired contractility (12). On the other hand, caveolins are essential for the formation of caveolae, small plasma membrane invaginations, that are involved in compartmentalization of various signaling molecules, including components of GPCR-induced signal transduction cascades [for review see (14)]. Recent evidence suggests that in the heart, β-adrenoceptors, Gs, Gi and adenylyl cyclase type 5/6 are localized in caveolae which thereby locally confines cAMP generation (22). Our functional data together with the co-immunoprecipitation suggest that caveolins are together with NDPK B and Gβγ part of a larger protein complex. This complex is apparently required for the posttranslational stabilization and function of the participating proteins, particularly of heterotrimeric G proteins. Accordingly, the loss of NDPK B or Gβγ induces a depletion of caveolins, NDPK B and heterotrimeric G proteins at the plasma membrane as well as a reduction in basal cAMP production. In the heart, this is associated with a loss of contractile function as observed in NDPK B and Gβ morphants. In agreement with our hypothesis, the morpholino-induced knockdown of caveolin-3 in the zebrafish causes a cardiomyopathy-like phenotype (23). Mice with a genetic depletion of the muscle-specific caveolin-3 in the heart develop a severe cardiomyopathy (24).
In summary, our findings identify NDPK B as an essential complex partner of heterotrimeric G proteins regulating adenylyl cyclase activity. This complex is apparently associated with caveolin-enriched plasma membrane domains and is required to maintain stable levels of heterotrimeric Gs and Gi proteins at the plasma membrane. Thus, NDPK B contributes to G protein signaling not only by direct G protein activation via the previously described phosphorelay (7–9), but also by modulating the cellular amount and compartmentalization of heterotrimeric G proteins.
Materials and Methods
Zebrafish Maintenance and Manipulation.
Zebrafish Danio rerio (TE4/6, TüAB, Tg(cmlc2:gfp) strains) were maintained under standard conditions (25). Details on MOs, injections, histology and immunostaining are provided in the SI Text.
Measurement of Fractional Shortening.
Fractional shortening was assayed by measuring the systolic contractile function normalized to the diameters of the heart of immobilized embryos as described (26).
Immunoblot Analysis and Co-Immunoprecipition.
Embryos were treated with deyolking buffer (55 mM NaCl; 1.8 mM KCl; 1.25 mM NaHCO3) and washed twice with washing buffer (110 mM NaCl; 3.5 mM KCl; 2.7 mM CaCl2; 10 mM Tris-HCl, pH 8.5). Single zebrafish hearts were prepared from the zebrafish line Tg(cmlc2:gfp) with heart-specific GFP expression. Lysates were prepared in buffer H (10 mM Tris-HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM PMSF, and protease inhibitors) and homogenized with two 10-s bursts applied by a Polytron (Kinematica) at a setting of 20,000 rpm. Co-immunoprecipitation in these lysates was performed as described (9). Immunoblotting was performed according to standard procedures. Details on the used antibodies are provided in the SI Text.
NDPK Activity Assay.
NDPK phosphate transfer activity was measured as formation of 3H-GTP from 3H-GDP and ATP exactly as described previously (8) in whole zebrafish lysates prepared without detergents at 72 hpf.
Mouse Embryonic Fibroblasts.
Generation of NDPK A/B double-knockout mice in the genetic background of C57BL/6, preparation and culture of WT, (+/−) and (−/−) NDPK A/B KO MEFs have been described (15). Cell lysates were homogenizied as described above in buffer H without Triton X-100 and centrifuged at 100,000 × g to obtain membrane fractions (8). Details on recombinant adenoviruses and siRNAs are provided in the SI Text.
Measurement of cAMP Levels.
Generation of cAMP was assayed as previously described (8) using a cAMP immunoassay (Assay Designs).
Statistical Analysis.
All results were expressed as mean ± SEM. Statistical analysis was performed with Student's t test or ANOVA with Bonferroni post-hoc test as appropriate. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
This work was supported by Deutsche Forschungsgemeinschaft Grants Wi1373/9-1 (to T.W.), Wi1373/9–2 (to H.-J.H. and T.W.), and Ro2173/2-2, and Ro2173/3-1 (to W.R.); Bundesministerium für Bildung und Foschung Grants 01GS0108, 01GS0420, and 01GS0836 (to W.R.); and National Institute of Health/National Cancer Institute Grant CA76496 (to E.H.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901679106/DCSupplemental.
References
- 1.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera-Vera TM, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 3.Hippe HJ, Wieland T. High energy phosphate transfer by NDPK B/Gβγ complexes-an alternative signaling pathway involved in the regulation of basal cAMP production. J Bionenerg Biomembr. 2006;38:197–203. doi: 10.1007/s10863-006-9035-0. [DOI] [PubMed] [Google Scholar]
- 4.Janin J, et al. Three-dimensional structure of nucleoside diphosphate kinase. J Bioenerg Biomembr. 2000;32:215–225. doi: 10.1023/a:1005528811303. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury D, et al. The exonuclease TREX1 is in the SET complex and acts in concert with NM23–H1 to degrade DNA during granzyme A-mediated cell death. Mol Cell. 2006;23:133–142. doi: 10.1016/j.molcel.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell. 2006;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Cuello F, et al. Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gβ subunits. Complex formation of NDPK B with Gβγ dimers and phosphorylation of His-266 in Gβ. J Biol Chem. 2003;278:7220–7226. doi: 10.1074/jbc.M210304200. [DOI] [PubMed] [Google Scholar]
- 8.Hippe HJ, et al. Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gβ subunits. Specific activation of Gαs by an NDPK B/Gβγ complex in H10 cells. J Biol Chem. 2003;278:7227–7233. doi: 10.1074/jbc.M210305200. [DOI] [PubMed] [Google Scholar]
- 9.Hippe HJ, et al. Regulation of cardiac cAMP synthesis and contractility by nucleoside diphosphate kinase B/G protein βγ dimer complexes. Circ Res. 2007;100:1191–1199. doi: 10.1161/01.RES.0000264058.28808.cc. [DOI] [PubMed] [Google Scholar]
- 10.Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: A quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- 11.Lee YI, et al. Coordinate expression of the α and β subunits of heterotrimeric G proteins involves regulation of protein degradation in CHO cells. FEBS Lett. 2003;555:329–334. doi: 10.1016/s0014-5793(03)01267-5. [DOI] [PubMed] [Google Scholar]
- 12.Muhlhauser U, et al. Atorvastatin desensitizes β-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein γ-subunits. FASEB J. 2006;20:785–787. doi: 10.1096/fj.05-5067fje. [DOI] [PubMed] [Google Scholar]
- 13.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: Implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postel EH, et al. Targeted deletion of Nm23/nucleoside diphosphate kinase A and B reveals their requirement for definitive erythropoiesis in the mouse embryo. Dev Dyn. 2009;238:775–787. doi: 10.1002/dvdy.21887. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JI, Fraser ID, Choi S, Qin XF, Simon MI. Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc Natl Acad Sci USA. 2004;101:488–493. doi: 10.1073/pnas.0307549100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang JI, Choi S, Fraser ID, Chang MS, Simon MI. Silencing the expression of multiple Gβ-subunits eliminates signaling mediated by all four families of G proteins. Proc Natl Acad Sci USA. 2005;102:9493–9498. doi: 10.1073/pnas.0503503102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peracino B, et al. G protein β subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J Cell Biol. 1998;141:1529–1537. doi: 10.1083/jcb.141.7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwaal RR, et al. G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos. Cell. 1996;86:619–629. doi: 10.1016/s0092-8674(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 20.Arnaud-Dabernat S, Bourbon PM, Dierich A, Le Meur M, Daniel JY. Knockout mice as model systems for studying nm23/NDP kinase gene functions. Application to the nm23–M1 gene. J Bionenerg Biomembr. 2003;35:19–30. doi: 10.1023/a:1023561821551. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway. J Biol Chem. 1999;274:17365–17371. doi: 10.1074/jbc.274.24.17365. [DOI] [PubMed] [Google Scholar]
- 22.Calaghan S, Kozera L, White E. Compartmentalization of cAMP-dependent signaling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Nixon SJ, et al. Zebrafish as a model for caveolin-associated muscle disease; caveolin-3 is required for myofibril organization and muscle cell patterning. Hum Mol Genet. 2005;14:1727–1743. doi: 10.1093/hmg/ddi179. [DOI] [PubMed] [Google Scholar]
- 24.Woodman SE, et al. Caveolin-3 knockout mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 25.Rottbauer W, et al. Cardiac myosin light chain-2: A novel essential component of thick-myofilament assembly and contractility of the heart. Circ Res. 2006;99:323–331. doi: 10.1161/01.RES.0000234807.16034.fe. [DOI] [PubMed] [Google Scholar]
- 26.Rottbauer W, et al. VEGF-PLCγ1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–1634. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.