Once upon a time, plant development was all about hormones. Darwin (1) wrote nearly 130 years ago that “some influence,” later shown to be the phytohormone auxin, moved down the shoot to control the elongation of oat seedlings. In the 1950s, Skoog and Miller (2) showed that auxin and cytokinin control shoot regeneration in vitro, a technique that is extensively used to this day. With the rise of developmental genetics at the end of the 20th century, however, plant development became focused on transcription factors and their exquisite expression patterns. More recently, both views have converged to explain how plants develop intricate structures with precise expression patterns superimposed on cells that are constantly displaced by growth. This convergence was first exemplified by the role of auxin in patterning the growing root tip (3). In this issue of PNAS, Gordon et al. (4) shed light on the interplay of regulatory genes and cytokinin in the dynamic patterning of the opposite end of the plant, the shoot meristem.
The shoot meristem contains a small population of stem cells that constantly renews itself while providing precursor cells to build all aerial parts of the plant (5, 6). In Arabidopsis, maintenance of these stem cells requires a signal produced by a small group of underlying cells that express the WUS gene (7) (Fig. 1B). Because division and growth constantly displace cells in the meristem, the location of the WUS-expressing domain has to be frequently updated. The best-characterized signal that controls WUS expression is the CLV3 peptide, which is produced by the stem cells, diffuses to the underlying cell layers, and activates a receptor containing the CLV1 polypeptide to repress WUS. This negative signal limits the size of the stem cell population; however, it does not explain how WUS expression is maintained in a sharp domain in the center of the meristem, at a predictable distance from the epidermal layer. Many other genes regulate WUS directly or indirectly (5), but none of these inputs have explained how WUS expression can be transient in individual cells yet stable within the meristem.
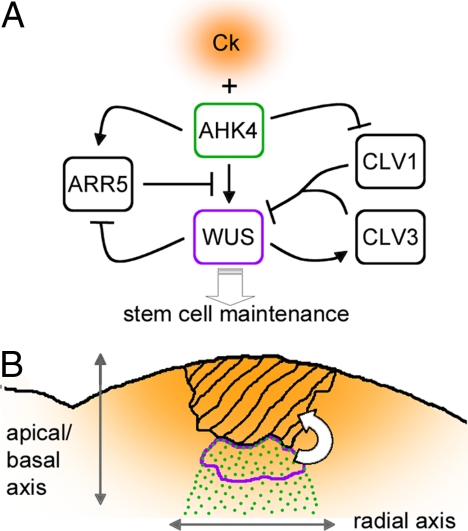
Fig. 1.
Role of cytokinin in patterning the shoot stem cell niche. (A) Regulatory network described by Gordon et al. (4). Ck is cytokinin; blunted lines and black arrows indicate repression and activation, respectively, and the white arrow represents the maintenance signal induced by WUS. (B) Speculative model of how cytokinin signaling establishes the spatial pattern of WUS expression. Graded orange represents an apical-basal gradient of cytokinin produced in the meristem region where stem cells are located (hatched region); green dots mark the region where AHK4 is expressed in response to cues along the radial axis of the shoot. Where a minumum level of cytokinin reaches the AHK4 receptor, the network represented in A translates the graded cytokinin signal into the sharp boundaries of the WUS-expressing domain (surrounded by the purple line). This region then produces the signal (white arrow) that maintains the shoot stem cells.
Gordon et al. (4) now implicate cytokinin in the dynamic pattern of WUS expression. Cytokinin is an adenine-like phytohormone that is sensed by a family of Arabidopsis histidine kinase (AHK) receptors, which pass on the signal to two types of transcription factors: type B Arabidopsis response regulators (ARRs) activate cytokinin-induced genes, and type A ARRs function in a negative feedback loop to dampen cytokinin responses (8). Cytokinin had previously been implicated in shoot meristem function. In maize, the abnormal phylotaxy 1 (abphyl1) mutation disrupts a type A ARR and increases meristem size (9). Consistent with type A ARRs inhibiting meristem function, WUS directly represses their expression (10). In rice, the cytokinin biosynthesis gene LONELY GUY (LOG) is expressed in the apical region of the shoot meristem and is required for meristem maintenance (11). All of these studies implicated cytokinin in controlling meristem size, but did not place cytokinin at the core of the mechanism that patterns the meristem. Gordon et al. (4) took advantage of a recently developed marker gene that reports cytokinin signaling (12) to show that cytokinin responses are not uniform within the meristem: the reporter was expressed most strongly in the center of the meristem, in the same region as WUS. They also found that the cytokinin signal and WUS reinforce each other through multiple feedback loops. External cytokinin activated WUS directly and independently of the CLV pathway; at the same time, repression of CLV1 by cytokinin (13) further facilitated WUS expression. Considering that WUS represses type A ARRs that normally act as a break on cytokinin signaling (10), cytokinin signaling and WUS should reinforce each other in a positive feedback loop (Fig. 1A).
The behavior of a system with multiple feedback loops (Fig. 1A) is not easy to understand intuitively, but the importance of these results was made clear by computer simulations of the meristem regulatory network. In a network where cytokinin simultaneously activated WUS and repressed CLV1, WUS expression increased very steeply above a critical cytokinin concentration. No sharp increase was seen in simulations where cytokinin controlled only WUS or CLV1. Spatially, this steep increase in WUS expression could be translated into a sharp expression boundary within a field of cells with increasing cytokinin signaling (Fig. 1B). This binary-like behavior would also be a mechanism to increase robustness of WUS expression to fluctuations in the underlying cytokinin signal.
Because of the positive feedback loop between cytokinin signaling and WUS, the cytokinin reporter alone could not tell whether the higher levels of signaling in the center of the meristem were a cause or a consequence of WUS expression. Here, a crucial piece of information was that the cytokinin input into WUS and CLV1 regulation was mediated by the AHK2 and AHK4 receptors and that at least the latter was spatially restricted, with higher expression in the center of the meristem. According to published expression array data, AHK2 and AHK4 are not activated by WUS (10). Therefore, the feedback loops that activate and stabilize WUS expression seem to take spatial cues from an underlying, independently established expression pattern of cytokinin receptors (Fig. 1B). What sets up this pattern? Throughout Arabidopsis development, AHK4 (also known as WOODEN LEG) is expressed in the vascular cylinder (14), which is the innermost region of the concentric arrangement of tissues seen in plant organs. So AHK4 might link the mechanism that establishes the radial pattern of the plant to the mechanism that specifies the shoot stem cell niche. The radial pattern of the shoot is controlled by a family of HD-ZIP transcription factors (15); combined mutation of three of these HD-ZIP factors (CORONA, PHABULOSA, and PHAVOLUTA) caused not only an enlargement of the vascular cylinder of the stem, but also an increase in meristem size very similar to that seen in clv mutants (16). It will be interesting to see whether this occurs at least in part through misregulation of the AHK4 receptor leading to an increased WUS expression domain.
How does all of this explain the dynamic pattern of WUS expression? A compelling model emerges in the light of recent evidence suggesting that the stem cells overlying the WUS domain are a source of cytokinin (11, 17, 18) (Fig. 1B). So in addition to the (still unknown) signal from WUS-expressing cells that specifies stem cells, a signal may be sent back by the stem cells to specify the WUS domain. Distance from the source of cytokinin would determine how deep the WUS domain is; overlap with the preexisting radial pattern of the AHK4 receptor would locate expression to the center of the meristem. The multiple feedback loops connecting cytokinin and WUS would translate a graded cytokinin signal into the sharp expression boundaries of WUS. The specification of the stem cell niche by the overlap of radial and apical-basal patterns would be reminiscent of what happens in the root meristem, where an apical-basal auxin gradient overlaps with the radial expression pattern of SCARECROW and SHORT ROOT to specify the position of the root stem cell niche (3).
The cytokinin signal and WUS reinforce each other through multiple feedback loops.
Another important implication of Gordon et al. (4) relates back to the classical experiments on shoot regeneration in vitro. Shoot formation from root explants in vitro is triggered by high levels of cytokinin through ectopic activation of WUS, which is sufficient to specify new shoot stem cell niches (19, 20). Gordon et al. (4) show that activation of WUS during in vitro regeneration also correlates with AHK4 expression; pretreatment with auxin, which facilitates subsequent shoot regeneration, also causes broad activation of AHK4. So the study of WUS regulation looped back to classic in vitro culture experiments; the auxin-AHK4-cytokinin-WUS connection clarifies the link between the long-known role of hormones during shoot regeneration in vitro and their normal role in the shoot meristem. The circular interactions between regulatory genes and plant hormones do not make it easier to understand regulatory networks, but studies like that of Gordon et al. (4) show that these loops exist for a good reason.
Acknowledgments.
My work is supported by the Biotechnology and Biological Sciences Research Council and the European Union.
Footnotes
The author declares no conflict of interest.
See companion article on page 16529.
References
- 1.Darwin C. The Power of Movement in Plants. London: John Murray; 1880. [Google Scholar]
- 2.Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–131. [PubMed] [Google Scholar]
- 3.Aida M, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S, Chickarmane V, Ohno C, Meyerowitz E. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci USA. 2009;106:16529–16534. doi: 10.1073/pnas.0908122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams L, Fletcher JC. Stem cell regulation in the Arabidopsis shoot apical meristem. Curr Opin Plant Biol. 2005;8:582–586. doi: 10.1016/j.pbi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Sablowski R. The dynamic plant stem cell niches. Curr Opin Plant Biol. 2007;10:639–644. doi: 10.1016/j.pbi.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Mayer KF, et al. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 8.Muller B, Sheen J. Advances in cytokinin signaling. Science. 2007;318:68–69. doi: 10.1126/science.1145461. [DOI] [PubMed] [Google Scholar]
- 9.Giulini A, Wang J, Jackson D. Control of phyllotaxy by the cytokinin-inducible response regulator ABPHYL1. Nature. 2004;430:1031–1034. doi: 10.1038/nature02778. [DOI] [PubMed] [Google Scholar]
- 10.Leibfried A, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- 11.Kurakawa T, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- 12.Muller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–1097. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsay DL, Sawhney VK, Bonham-Smith PC. Cytokinin-induced changes in CLAVATA1 and WUSCHEL expression temporally coincide with altered floral development in Arabidopsis. Plant Sci. 2006;170:1111–1117. [Google Scholar]
- 14.Mahonen AP, et al. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 2000;14:2938–2943. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emery JF, et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Prigge MJ, et al. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasinski S, et al. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol. 2005;15:1560–1565. doi: 10.1016/j.cub.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Yanai O, et al. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol. 2005;15:1566–1571. doi: 10.1016/j.cub.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SP, et al. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–3548. doi: 10.1242/dev.010298. [DOI] [PubMed] [Google Scholar]
- 20.Gallois J-L, Nora FR, Mizukami Y, Sablowski R. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev. 2004;18:375–380. doi: 10.1101/gad.291204. [DOI] [PMC free article] [PubMed] [Google Scholar]