Abstract
Intake of an unconditionally preferred taste stimulus (e.g., saccharin) is reduced by contingent administration of a drug of abuse (e.g., morphine). We examined the influence of insular cortex (IC) lesions on morphine-induced suppression of an olfactory cue and two taste stimuli with different levels of perceived innate reward value. Two major findings emerged from this study. First, morphine suppressed intake of an aqueous odor as well as each taste stimulus in neurologically intact rats. Second, IC lesions disrupted morphine-induced suppression of the taste stimuli but not the aqueous odor cue. These results indicate that the perceived innate reward value of the CS is not a factor that governs drug-induced intake suppression.
Keywords: Insular cortex, Taste, Odor, Morphine, Rat
1. Introduction
The present article is concerned with two issues: the nature of the intake suppression that occurs when an orally-consumed conditioned stimulus (CS) is paired with a drug of abuse unconditioned stimulus (US) and the neural substrates of that phenomenon. It is well established that aversive USs such as the states induced by toxins, vestibular disorientation and x-rays support conditioned taste aversions (CTAs) that result in a change in palatability and the avoidance/rejection of the gustatory CS (for reviews see edited volumes by Barker, Best & Domjan, 1977; Braveman & Bronstein, 1985; Milgram, Krames, & Alloway, 1977; Reilly & Schachtman, 2009). Because they also suppress CS intake, drugs of abuse were for many years viewed as aversive USs that induce CTAs (e.g., Berger, 1972; Cappell & LeBlanc, 1971; Carey, 1973; Le Magnen, 1969; Lester, Nachman & Le Magnen, 1970; Vogel & Nathan, 1975). This position may be considered paradoxical because drugs of abuse are, of course, self-administered by humans and other animals, which would seem to suggest that these stimuli are possessed of rewarding rather than aversive properties. Moreover, in an elegant series of studies that examined stereotypical orofacial responses using taste reactivity methodology, Parker (e.g., 1982 e.g., 1988 e.g., 1991 e.g., 1993 e.g., 1996 e.g., 2003; for reviews see Parker 1995; Parker, Limebeer & Rana, 2009) established that unlike the quintessential CTA-inducing US (lithium chloride, LiCl), drugs of abuse (e.g., amphetamine, cocaine, methamphetamine, methylphenidate, LSD, morphine, phencyclidine) do not support conditioned disgust reactions when the associated CS is infused into the mouth. So, what, then, is the nature of the intake suppression induced by drugs of abuse?
Grigson (1997) re-framed the issue by proposing that drugs of abuse induce CS suppression because of their known rewarding, not their hypothetical aversive, properties in much the same way that the orally self-administered sucrose US suppresses intake of the saccharin CS in the anticipatory negative contrast (ANC) paradigm (e.g., Flaherty & Checke, 1982; Flaherty & Grigson, 1988; for a review see Flaherty, 1996). According to this analysis, the reward value of the saccharin CS is compared, unfavorably, with the anticipated higher reward value of the drug US which causes the CS to be devalued and, consequently, intake to be suppressed. By definition, then, this “reward comparison” hypothesis stipulates that a psychoactive drug US can suppress intake of a CS only if that CS possesses some perceived innate reward value. Taken as support for this analysis, Grigson (1997) demonstrated that morphine and cocaine each suppress intake of an innately rewarding stimulus (0.15% saccharin) but not a more neutral stimulus (0.1 M sodium chloride, NaCl) whereas an LiCl US induced a CTA to both gustatory stimuli1. Similarly, Grigson demonstrated that the suppressive effect of a drug US (morphine) increases as the concentration of the saccharin CS increases (i.e., no suppression at 0.015% and more rapid suppression at 0.15% than 0.075% saccharin), a pattern that parallels the influence of the sucrose US in the ANC paradigm (Flaherty, Turovsky & Krauss, 1994). Together, these data were presented as the initial empirical foundation for the reward comparison hypothesis and against the CTA account of drug-induced suppression of gustatory stimulus ingestion. As recently reviewed by Grigson, Twining, Freet, Wheeler and Geddes (2009), data are accumulating that are consistent with the reward comparison hypothesis.
There is some evidence that the neural substrates of CTA differ from that responsible for drug-induced CS suppression. Specifically, lesions of the gustatory thalamus (GT; the functional name for the parvicellular region at the medial extension of the ventral posteromedial nucleus), which have little if any influence on CTA acquisition (Flynn, Grill, Schulkin & Norgren, 1991; Mungarndee, Lundy & Norgren, 2006: Reilly, Bornovalova, Dengler & Trifunovic, 2003; Reilly & Pritchard, 1996), eliminate the CS suppression induced with either a drug of abuse (Grigson, Lyuboslavsky & Tanase, 2000; Reilly & Trifunovic, 1999a) or a sucrose US (Reilly, Bornovalova & Trifunovic, 2004; Reilly & Pritchard, 1996; Schroy, Wheeler, Davidson, Scalera, Twining & Grigson, 2005). Together with other results (e.g., Reilly & Trifunovic 1999b, 2003), the pattern of impaired and spared gustatory functions in rats with GT lesions suggests that this nucleus is critical for the operation of the comparison mechanism that allows the relative reward value of gustatory stimuli to be determined over time (e.g., Reilly, 2005a, 2005b).
The insular cortex (IC), the cortical component of the central gustatory system (for reviews see Lundy & Norgren, 2004; Travers, 1993) is reciprocally connected with the GT (Cechetto & Saper, 1987; Kosar, Grill & Norgren, 1986; Krettek & Price, 1977; Norgren & Wolf; 1975; Wolf, 1968). There is, therefore, reason to expect that the IC, like the GT, may be involved in drug-induced CS suppression. Indeed, we have recently reported that excitotoxic lesions of the IC disrupt morphine-induced suppression of a saccharin CS (Roman & Reilly, 2009; see also Mackey, Keller & van der Kooy, 1986; Zito, Bechera, Greenwood & van der Kooy, 1988). Although this result might be explained in the same way as the same deficit found in rats with GT lesions (see preceding paragraph), we favored an interpretation in terms of a lesion-induced impairment in the perception of taste novelty. This analysis was derived from work on the role of the IC in taste-guided behaviors. Specifically, IC lesions disrupt the initial neophobic response to a novel taste stimulus (but not to a novel aqueous odor stimulus or a novel oral trigeminal stimulus) while having no influence on the asymptotic intake of the taste when it became familiar (Lin, Roman, St. Andre & Reilly, 2009). Furthermore, IC lesions retard the acquisition of a CTA when the taste is novel but not when the taste is familiar (Kiefer & Braun, 1977; Roman, Lin & Reilly, 2009; Roman & Reilly, 2007). Finally, Roman, Nebieridze, Sastre and Reilly (2006) found that the same IC lesions that impaired CTA acquisition to a novel taste stimulus (0.15% saccharin) had no influence on the acquisition of a conditioned odor aversion (COA) to a novel olfactory stimulus (0.02% aqueous orange extract). This pattern of impaired and spared functions encouraged the view that the primary deficit consequent to IC lesions concerns the detection of taste novelty (for a review see Reilly, 2009).
The goal of the present study was to further investigate the role of the IC in drug-induced suppression of orally consumed stimuli using procedures that test the reward comparison hypothesis. Experiment 1 examined the effects of IC lesions on the suppression of an odor stimulus (0.02% solution of orange extract). This aqueous odor stimulus, which primarily stimulates the olfactory epithelium retronasally, is used at this low concentration to ensure that it has no taste component. Since the olfactory stimulus possesses no calories and does not taste sweet (unlike the non-caloric saccharin, which is an effective stimulus in this procedure), the aqueous orange odor presumably has little innate reward value for the rat. Thus, like the NaCl solution used by Grigson (1997), the reward comparison hypothesis predicts that morphine should not suppress consumption of the odor stimulus. Rather than use the 5-min CS access per trial that was employed by Grigson (1997), the odor stimulus was available for 15 each trial to match the CS duration used in our earlier CTA/COA studies. The same procedure was used in Experiments 2 and 3 which examined, respectively, morphine-induced suppression of 0.1 M NaCl and 0.01 M malic acid. As defined by Grigson (1997), 0.1 M NaCl is less preferred, and therefore more neutral, than 0.15% saccharin. Malic acid has, to humans, a sour taste and is an aversive taste stimulus for rats at the concentration used in the present study (Coldwell & Tordoff, 1996). Accordingly, the reward comparison hypothesis predicts that morphine will suppress intake of neither of these two taste stimuli. With regard to lesion effects, if, as suggested by Roman et al. (2006), the IC is involved in learned taste-related but not learned odor-related behaviors then lesion-induced disruptions in the suppression of NaCl and malic acid, but not orange odor, are to be expected if morphine suppresses intake of these stimuli in neurologically intact rats. Furthermore, because the IC and GT are interconnected, the IC may be a component of the reward comparison mechanism that seemingly involves the GT. If so, we predict that IC lesions, like GT lesions, will disrupt drug-induced CS suppression. Alternatively, given the evidence that IC lesions impair the perception of taste novelty, we would expect the lesions to delay (because of latent inhibition), but not prevent, the acquisition of drug-induced CS suppression.
2. Results
2.1. Anatomical
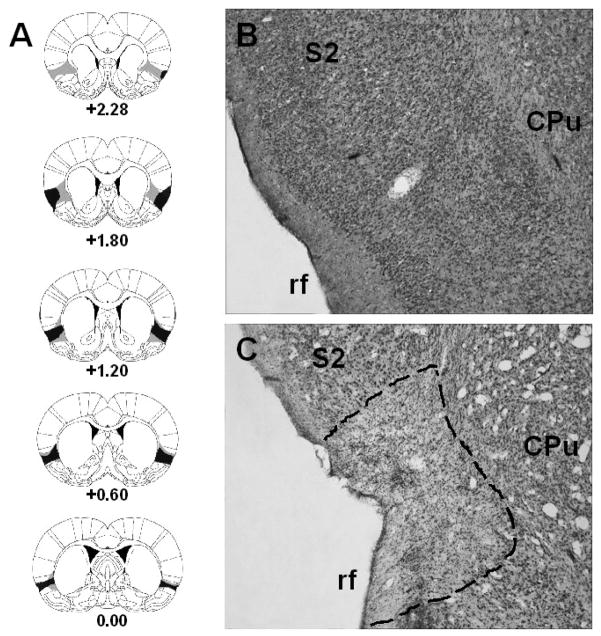
Defined by Kosar et al. (1986), the gustatory portion of the IC is located along the dorsal bank of the rhinal fissure, extending ~0.5 mm dorsoventrally and ~2.5 mm anteroposteriorly. The presence of gliosis and the lack of cell bodies were used to define the extent of the excitatory lesions. Histological reconstructions of the ICX rats used in Experiment 1–3 are shown in Figure 1. As shown in this figure, in addition to most of the IC, some lesions extended into surrounding areas including the claustrum, piriform and somatosensory cortex. These encroachments, which varied across animals, were minor in extent. Rats with unilateral or subtotal lesions were excluded from the statistical analyses. Furthermore, in the downtime between Experiment 1 and Experiment 2, two ICX rats were euthanized for health reason unrelated to the study. After these exclusions, the final number of rats in each experiment was: Experiment 1: SHAM-Saline = 10, SHAM-Morphine = 10, ICX-Saline = 8, ICX-Morphine = 7; Experiment 2: SHAM-Saline = 10, SHAM-Morphine = 10, ICX-Saline = 7, ICX-Morphine = 6; Experiment 3: SHAM-Saline = 10, SHAM-Morphine = 10, ICX-Saline = 9, ICX-Morphine = 7.
Fig. 1.
Serial reconstructions (Panel A) of the smallest (black) and largest (grey) lesions of the insular cortex at five levels (0.00, +0.60, +1.20, +1.80, +2.28) anterior to bregma shown on diagrams adapted with permission from Paxinos and Watson (2005). Representative digital photomicrograph of the IC in the left hemisphere of a neurologically intact subject (Panel B) and a rat with an excitotoxic lesion (Panel C). The dashed line in Panel C shows the perimeter of the lesion. Abbreviations: CPu = caudate putamen; rf = rhinal fissure; S2 = secondary somatosensory cortex.
2.2. Behavioral
2.2.1. Experiment 1: Orange Odor CS
The average baseline water intake for each group is summarized in Table 1. An ANOVA revealed that there were no significant main effects of Lesion or Drug and no Lesion X Drug interaction (Fs < 1). Thus, prior to Experiment 1, SHAM and ICX rats were drinking comparable amounts of water each day.
Table 1.
Baseline 15-min water intake (ml) from Experiments 1–3. For each experiment, the data (mean ±SE) were collapsed across the last 3 water trials prior to the first conditioning trial for the neurologically intact (SHAM) and insular cortex-lesioned (ICX) rats injected with either saline or morphine.
| SHAM | ICX | |||
|---|---|---|---|---|
| Saline | Morphine | Saline | Morphine | |
| Experiment 1 | 17.73 (0.98) | 17.87 (0.62) | 18.02 (0.96) | 16.98 (1.34) |
| Experiment 2 | 16.98 (0.64) | 17.20 (0.79) | 18.79 (1.19) | 18.56 (1.40) |
| Experiment 3 | 18.58 (0.97) | 19.07 (0.92) | 20.67 (1.13) | 18.55 (0.79) |
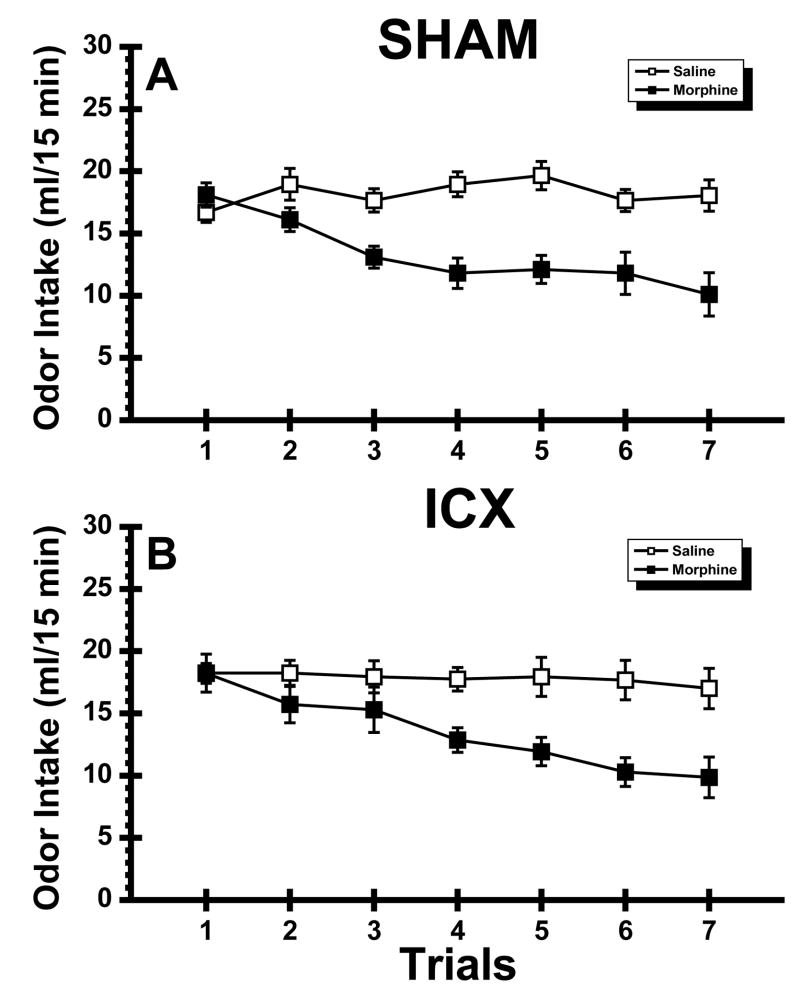
Figure 2 shows mean fluid intake in SHAM and ICX rats across the seven, 15-min orange odor trials of Experiment 1. Inspection of the figure indicates that both the SHAM-Morphine subjects and the ICX-Morphine rats reduced their intake of the olfactory CS relative to their respective saline-injected control groups. An ANOVA conducted on the data summarized in Figure 2 found highly significant main effects of Drug, F(1,31) = 30.98, p < .001, Trial, F(6,186) = 7.95, p < .001, and a significant Drug X Trial interaction, F(6,186) = 8.09, p < .001. No other main effects or interaction were significant (Fs < 1). Post hoc comparisons (simple main effect) of the Drug X Trial interaction revealed that morphine-injected rats drank significantly less of the CS solution than the saline-injected control subjects on Trials 2–7 (ps < .05). These analyses clearly show that morphine suppressed intake of the olfactory stimulus when the CS was available for 15 min each trial and that IC lesions had no influence on this behavior.
Fig. 2.
Experiment 1: Mean (±SE) 15 min intake (ml) of aqueous orange odor across 7 trials in normal (SHAM; Panel A) subjects and rats with neurotoxic lesions of the insular cortex (ICX; Panel B) that were injected with saline or morphine.
2.2.2. Experiment 2: NaCl CS
Confirming normal water consumption in ICX rats, statistical analysis conducted on preconditioning water intake data (see Table 1) found no significant main effects of Lesion (p > .05) or Drug (F <1), and no Lesion X Drug interaction (F < 1).
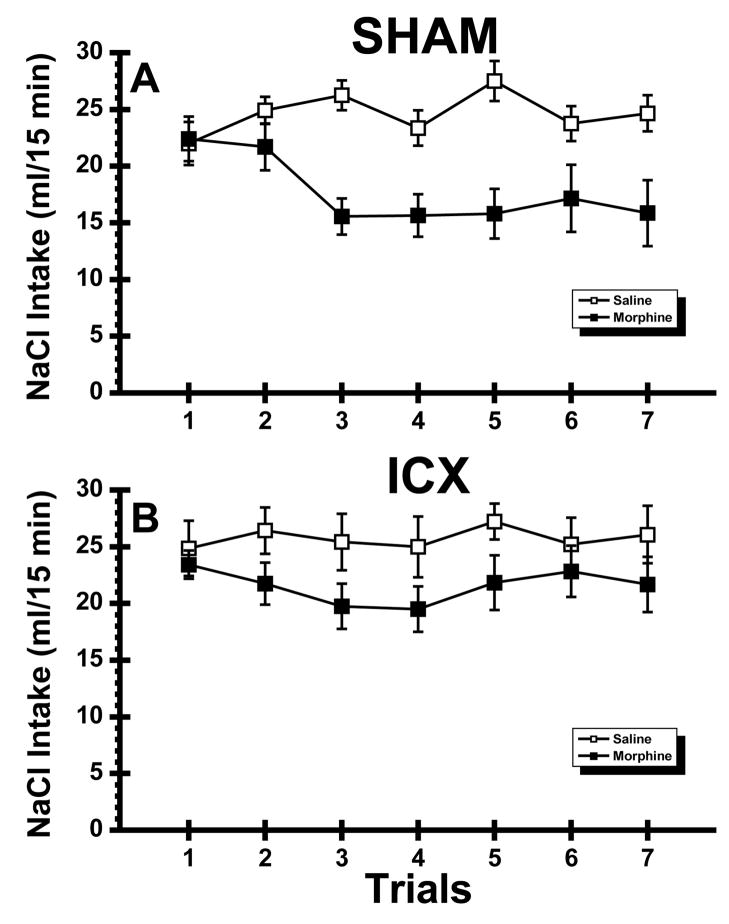
Mean NaCl consumption is shown in Figure 3 for the saline and morphine treated SHAM and ICX subjects. As shown in the figure, SHAM rats injected with morphine clearly suppressed NaCl intake relative to their saline-injected control subjects. The data from the ICX rats show that the morphine-injected rats drank about the same amount of NaCl on the first and final trial of the experiment, suggesting that these rats failed to learn the task. Thus, it was surprising that the overall ANOVA found only a significant main effect of Drug, F(1,29) = 10.58, p < .01 and a significant Drug X Trial interaction, F(6,174) = 4.06, p < .001. To aid interpretation of the results, separate two-way ANOVAs were conducted on the SHAM and ICX data from Trials 1–7. These analyses revealed that SHAM-Morphine rats drank lower volumes of NaCl than the SHAM-Saline rats (Drug X Trial interaction, F(6,108) = 5.64, p < .01), an effect that emerged on Trial 3 and was sustained until the end of the experiment on Trial 7 (ps < . 05). On the other hand, the NaCl intake of the two ICX groups was not significantly different at anytime during the experiment (p >.05).
Fig. 3.
Experiment 2: Mean (±SE) 15 min intake (ml) of sodium chloride (NaCl) across 7 trials in normal (SHAM; Panel A) subjects and rats with neurotoxic lesions of the insular cortex (ICX; Panel B) that were injected with saline or morphine.
2.2.3. Experiment 3: Malic Acid CS
An ANOVA conducted on the Experiment 3 pre-conditioning water intake data summarized in Table 1 found no significant main effect of either Lesion or Drug (Fs < 1), nor a Lesion X Drug interaction (p > .05). Thus, ICX rats drank normal amounts of water prior to the conditioning trials of Experiment 3.
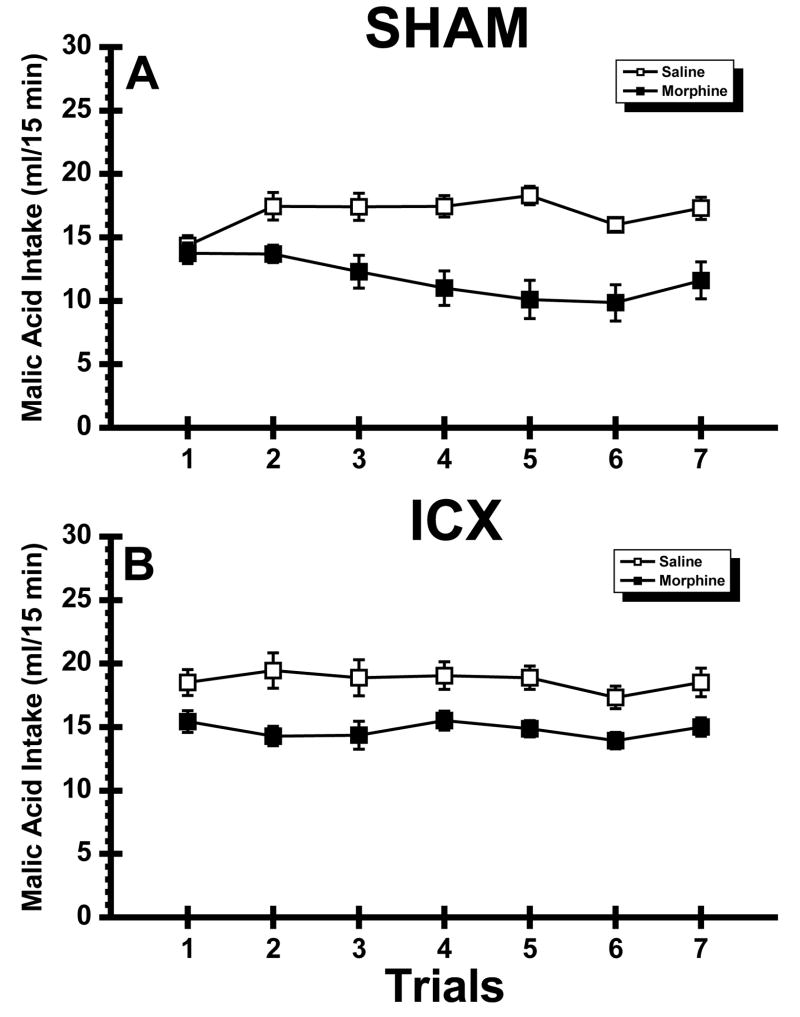
Figure 4 shows mean malic acid intake over the seven trials of Experiment 3. Inspection of the figure suggests that while contingent injections of morphine reduced malic acid consumption over trials in SHAM rats, no such suppression occurred in the ICX-Morphine rats which unexpectedly maintained a constant, presumably unlearned, difference of ~4 ml of malic acid relative to the ICX-Saline rats throughout the experiment. This view of the data was supported by the statistical analysis which found significant main effects of Lesion, F(1,32) = 9.00, p < .01, Drug, F(1,32) = 32.20, p < . 001, and Trial, F(6,192) = 2.31, p < .05, as well as a significant Lesion X Drug X Trial interaction, F(6,192) = 2.41, p < .05. Post hoc comparisons that examined the source of the triple interaction revealed a significant Drug X Trial interaction in SHAM rats, F(6,192) = 5.34, p < .001 but not in the ICX rats (F < 1). Furthermore, these additional statistics revealed that the SHAM-Morphine rats consumed significantly less malic acid on Trials 2–7 (ps < .05) than the SHAM-Saline subjects.
Fig. 4.
Experiment 3: Mean (±SE) 15 min intake (ml) of malic acid across 7 trials in normal (SHAM; Panel A) subjects and rats with neurotoxic lesions of the insular cortex (ICX; Panel B) that were injected with saline or morphine.
As shown in Table 1, baseline water intake of the two ICX groups differed by 2.1 ml, which, though not significant, may be related to the unexpected performance of these groups during the conditioning trials. Accordingly, malic acid intake was normalized by expressing intake on Trials 2–7 as a percentage of Trial 1 intake for each of the four groups2. The same pattern of significance was obtained as described above for the raw data from this experiment. That is, morphine suppressed intake of the malic acid CS in the SHAM subjects and there were no significant differences in the CS intake of the two groups of ICX rats (which had overlapping means with intake fairly constant across all trials for each group).
3. Discussion
A series of experiments were conducted to investigate the role of the IC in drug-induced CS suppression. Unlike the brief (5 min) CS duration that is often favored in studies of this type, the present experiments each utilized 15 min access per trial in order to afford comparability with our prior CTA/COA research. In Experiment 1, morphine was found to be effective at suppressing intake of the odor stimulus. This finding casts doubt on the explanatory power of the reward comparison hypothesis which predicts that stimuli lacking reward value should not be suppressed by drugs of abuse. Similarly, the results from Experiment 2 indicate that the previous failure of morphine to suppress intake of NaCl (Grigson, 1997) represents a ceiling effect in consumption when access was limited to 5 min per trial and is not due to the relatively low innate reward value of that sapid stimulus. The expression “relatively low” is used because 0.1 M NaCl is less preferred than 0.15% saccharin in a two-bottle preference test. On this basis Grigson (1997) asserted if “…suppression of CS intake is due to reward comparison, then drugs of abuse should suppress intake when a saccharin, but not when an NaCl, solution serves as the CS” (page 130). Indeed, the finding that morphine suppressed intake of saccharin but not NaCl in that study was seen as compelling evidence in support of the reward comparison hypothesis. Given that the prediction about the nature of the CS was seemingly verified, the notion that the CS must possess some level of innate reward value gained traction and is, of course, the centerpiece of the reward comparison hypothesis. It should be remembered, however, that although 0.1 M NaCl is less preferred than 0.15% saccharin, it is nonetheless highly preferred relative to water in a two-bottle test (e.g., Coldwell & Tordoff, 1996; Tordoff, Alarcon & Lawler, 2008). Further evidence against the notion that morphine only influences consumption of stimuli that have perceived innate reward value that surpasses some threshold level was obtained in Experiment 3 where the drug of abuse suppressed intake of a taste stimulus, malic acid, that is innately aversive to rats (Coldwell & Tordoff, 1996).
It might be argued that in the absence of parallel experiments involving aqueous orange odor, NaCl and malic acid CSs paired with a sucrose US in an ANC procedure that the present results cannot be meaningfully interpreted as evidence against a reward comparison mechanism of intake suppression. However, such a claim misses the essential significance of the present results. That is, even if sucrose was found to suppress intake of these CSs, it could hardly be claimed that the underlying mechanism involved a comparison between the relative reward value of the CS and the sucrose US because two of these three CSs (orange odor and malic acid) do not possess innate reward value and certainly would, like 0.1 M NaCl, be less preferred than 0.15% saccharin in a two-bottle preferences test. This analysis also serves to bring into focus a significant difference between drug- and sucrose-induced suppression of CS intake. It is often claimed (e.g., Grigson & Twining, 2002) that a comparison between the otherwise rewarding CS and the drug US devalues, and suppresses intake of, the CS. However, Flaherty and colleagues (e.g., Flaherty, Coppotelli, Grigson, Mitchell & Flaherty, 1995) argued against the view that devaluation of the CS is a necessary condition for the occurrence of sucrose-induced ANC. Thus, it would appear that the mechanisms underlying drug- and sucrose-induced CS intake suppression are different. If this is the case, then comparisons between the two behavioral phenomena have limited theoretical value.
That morphine has been found to suppress intake of stimuli with perceived positive (e.g., Roman & Reilly, 2009), neutral (Experiment 1), and aversive (Experiment 3) innate values contradicts the basic idea of the reward comparison hypothesis that “Rats decrease intake of a gustatory CS following taste-drug pairings because the rewarding properties of the gustatory stimulus pale in comparison to those of the impending drug of abuse” (Grigson, 1997, page 134). The present results have at least two important ramifications for our understanding of drug-induced suppression of orally consumed stimuli. First, the principle of CS equipotentiality (i.e., all detectable taste stimuli are equal) cannot be used to differentiate the suppressive effects of CTA-inducing USs such as LiCl from drugs of abuse. Second, drug-induced intake suppression is not dependent upon the perceived innate reward value of the CS.
Can the reward comparison hypothesis be revised to accommodate the present results? One scenario involves an appeal to the notion that, because they were presented in water to water deprived subjects, the orange odor, NaCl and malic acid CSs acquired conditioned reward value. By this revision, stimuli with either perceived innate reward value (e.g., saccharin) or conditioned reward value are compared with, and suppressed by, the drug US. An obvious way to test this view would be to examine whether the aforementioned odor and taste stimuli are suppressed by morphine in rats that are water replete rather than water deprived. But, of course, it is not clear that non-deprived rats would be motivated to drink these neutral/aversive stimuli presented in water. Thus, the prediction that morphine should fail to suppress intake of these stimuli, may be difficult to test. Beyond this practical problem, an issue arises concerning the explanatory power of an account that includes stimuli with conditioned reward value. As the original nomenclature indicates, the reward comparison hypothesis asserts that the reward value of the saccharin CS is compared with that of the drug US, a computation that is predicated on the prior acquisition of a Pavlovian association between the two stimuli. This comparison results in the devaluation of the saccharin CS and the consequent reduction in consumption of that stimulus. However, given that saccharin possesses perceived innate reward value it is, perhaps, more appropriate to view drug-induced saccharin suppression as US-US learning rather than CS-US learning. Furthermore, this Pavlovian association is between two appetitive (or rewarding) USs. From this perspective, malic acid supports an aversive cue-appetitive US association whereas orange odor, which is presumed to have little or no innate reward value, will involve a CS-appetitive US association. And, herein lies a problem for any account of drug-induced intake suppression that includes stimuli with conditioned as well as innate reward value. Specifically, a priori one would expect that the acquisition of conditioned reward value would proceed more slowly for stimuli that are innately aversive than for those that are neutral or stimuli that possess innate reward value. However, the data show that, relative to saline-injected control subjects, consumption of saccharin (Roman & Reilly, 2009), orange odor, NaCl and malic acid (Experiments 1, 2 and 3, respectively, of the present study) are all suppressed after a single pairing with the same dose of morphine in neurologically intact rats. Thus, the speed with which drugs of abuse can suppress intake of neutral/aversive aqueous CSs would seem to argue against the idea that conditioned reward value has a role in this phenomenon. Furthermore, even if all these types of stimuli could acquire conditioned reward value equally rapidly, another problem emerges. That is, on trials where intake is suppressed (which can be as early as trial 2) the ingested stimulus is required to be both valued (by water) and devalued (by morphine) at the same time. In order for sustained intake suppression, this dynamic must be maintained across trials. But, it is not clear how these opposite properties can co-exist simultaneously. Thus, we are unconvinced that an appeal to conditioned reward value is a workable revision to the reward comparison hypothesis.
IC lesions have no influence on the acquisition of COAs but disrupt the acquisition of CTAs (Roman et al., 2006; see also Bertrand, Yannick, Mathilde, Frederic, Nadine & Guillaume, 2009). Furthermore, IC lesions disrupt CTA acquisition when the taste is novel but not when it is familiar and safe (Kiefer & Braun, 1977; Roman, Lin & Reilly, 2009; Roman & Reilly, 2007). This pattern of CTA results indicates that IC lesions disrupt processing of the taste stimulus that will, when paired with the US, become the CS and rules out interpretations based on lesion deficits in the detection/processing of the US and in the mechanism that associatively links the CS with the US. More specifically, we propose that IC lesions disrupt the perception of taste novelty and that the deficits in CTA learning in ICX rats occur because taste aversions are acquired more slowly by taste stimuli that are perceived as familiar relative to taste stimuli that are perceived as novel (e.g., Lubow, 1989, 2009). Thus, as noted in the Introduction, we expected IC lesions to selectively disrupt learned taste-guided behaviors while having no influence on learned odor-guided behaviors. Consistent with this view, the IC lesions in the present study impaired morphine-induced intake suppression when a taste stimulus but not an olfactory stimulus was used as the CS.
We propose that IC lesions disrupt drug-induced suppression of a taste CS for the same reason that they disrupt CTA acquisition: impairment in the perception of taste novelty. This analysis anticipates that IC lesions should delay but not prevent the acquisition of drug-induced CS suppression. Interestingly, Geddes, Han, Baldwin, Norgren and Grigson (2008; Experiment 1) recently reported that ICX rats required twice as many CS-US pairings as SHAM subjects (6 versus 3 trials, respectively) for a cocaine US (10 mg/kg) to suppress intake of a Polycose CS3. Furthermore, Roman and Reilly (2009), in a study showing that IC lesions disrupt morphine-induced saccharin suppression, provided some evidence, albeit between-phase rather than across-groups, that latent inhibition influences drug-induced CS suppression in neurologically intact rats. As discussed earlier, anatomical connectivity between the GT and IC gives reason to expect that the IC, like the GT, may be involved in drug-induced CS suppression. Given that GT lesions produce a different pattern of behavioral impairments than IC lesions, it is unlikely that the two areas are performing identical functions. For example, irrespective of the familiarity of the taste CS, GT lesions have little influence on CTA acquisition (Flynn, Grill, Schulkin & Norgren, 1991; Mungarndee, Lundy & Norgren, 2006: Reilly, Bornovalova, Dengler & Trifunovic, 2003; Reilly & Pritchard, 1996). There is, then, no suggestion that GT lesions influence the perception of taste novelty. Finally, if the GT and IC were performing identical functions with regard to drug-induced CS suppression then the presumably intact GT should compensate for the absent IC and no behavioral deficit would be found in ICX rats. That behavioral deficits are obtained in ICX rats suggests that the IC and the GT are performing interdependent not identical functions. To reiterate, we propose that instead of a failure in the reward comparison mechanism the disruption of drug-induced suppression of a taste CS in ICX rats is a secondary consequence of a failure to recognize the novelty of the taste stimulus, a parsimonious interpretation that also account for the influence of IC lesions on LiCl-induced CTA acquisition. By this analysis, ICX rats would be expected to suppress intake of a drug-paired CS if sufficient additional trials were given, as is the case for CTA learning in such animals.
In conclusion, the non-differential suppression effects of morphine on stimuli with different perceived innate reward values raises doubts about the explanatory power of the reward comparison hypothesis account of drug-induced CS suppression. In turn, this prompts questions concerning whether the nature of the CS is a factor that governs intake suppression when a highly rewarding natural reward (i.e., sucrose) serves as the US, a stimulus which presumably has no aversive value. Resolution of the latter issue, which does not affect our argument against the role of reward comparison in drug-induced CS suppression, will provide a more comprehensive understanding of the role of rewarding USs in CS suppression. Empirical examination of these issues will expand our knowledge concerning how artificial (e.g., drugs of abuse) and natural (e.g., sucrose) rewards differentially influence feeding behavior and the neural substrates of such activities.
4. Experimental procedures
4.1 Subjects
Male CD-IGS rats, purchased from Charles River Laboratories (Portage, MI), served as subjects. The rats were individually housed in hanging stainless steel cages in a vivarium kept on a 12-hour light-dark cycle (lights on from 7:00 am until 7:00 pm), with food and water available ad libitum unless different conditions are noted. The rats weighed 290–320 g when they underwent surgery, and at all times were treated according to the guidelines recommended by the National Institutes of Health (1986) and the American Psychological Association (1996); the Institutional Animal Care and Use Committee at the University of Illinois at Chicago approved the experimental protocols.
4.2 Surgery
Each rat that received excitotoxic lesions of the IC (Group ICX) was anesthetized with 50-mg/kg i.p. injection of sodium pentobarbital and fixed into a stereotaxic apparatus with blunt earbars after the head had been shaved. Body temperature was monitored with a rectal thermometer, and maintained at approximately 37°C with a heating pad (Harvard Apparatus, Holliston, MA). The scalp was sterilized with betadine, and a midline incision was made to expose the skull surface. Trephine holes were drilled over the IC in both hemispheres, and a micropipette filled with 0.15 M N-methyl-D-aspartic acid (NMDA; Sigma, St. Louis, MO) was lowered to the injection site. Two iontophoretic infusions were made in each hemisphere with a Midgard precision current source (Stoelting, Wood Dale, IL), the first 10-min infusion was targeted at the following coordinates: AP +1.2 mm, ML ±5.0 mm, DV −5.0 mm. The second, 6-min infusion was aimed at AP +1.2 mm, ML ±5.0 mm, DV −4.3 mm. After the second infusion, a second micropipette was filled with NMDA, and the process was repeated in the opposite hemisphere. Following the final infusion, the incision was closed with wound clips, and the rat was allowed to recover from anesthesia under a heat lamp. Two types of control preparations were used. In the first, all surgical treatments were identical to those given to the ICX rats, except no NMDA infusions were delivered. Rats in the second control preparation were anesthetized but given no further treatments. As expected, the behavior of these two control groups was indistinguishable and therefore the data were collapsed into as a single control group (Group SHAM). Following anesthesia/surgery, all rats were given at least one week to recover before any experimentation began. The number of subjects in each experiment was: Experiments 1 and 2, SHAM = 20 and ICX = 21; Experiment 3, SHAM = 20 and ICX = 24.
4.3 Apparatus
All the experimental sessions occurred in the home cages. Fluids were available from inverted Nalgene graduated cylinders that were attached to the front of the cages. Monitored to the nearest 0.5 ml, fluid intake served as the dependent measure in these experiments.
4.4 Procedure
4.4.1. Experiment 1
Following recovery from surgery, the animals were acclimated to, and maintained on, a water deprivation schedule that allowed 15 min access each morning. The conditioning trials occurred every third day and were initiated after water intake stabilized. The SHAM and ICX rats were randomly assigned into one of two drug conditions: 0.9% saline or 15 mg/kg morphine sulfate (Henry Schein, Indianapolis, IN). A total of 6 conditioning trials and one CS only test trial were given. On each conditioning trial, the rats were given 15 min access to 0.02% aqueous orange extract (Flavorganics, Newark, NJ) and, 5 min later, an i.p. injection of saline or morphine according to the designated drug condition.
4.4.2. Experiment 2
The rats used in Experiment 1 were employed as subjects in Experiment 2. The two experiments were separated by 37 days during which animals were given free access to food and water. Thereafter, the rats were returned to the same water deprivation schedule and conditioning procedures as those described in Experiment 1, except that 0.1 M NaCl served as the CS.
4.4.3. Experiment 3
The rats in this experiment had prior experience lever pressing for food in an operant chamber. They were, however, naïve with respect to the water deprivation schedule, taste stimulus and morphine that were used in the present experiment. The procedure was identical to that described in Experiment 1, except that 0.01 M malic acid served as the CS.
4.5. Histology
After the behavioral testing was completed, the rats were deeply anesthetized with sodium pentobarbital (100 mg/ml) and perfused transcardially with physiological saline and 4% buffered formalin. The brains were extracted and stored in 4% buffered formalin followed by 20% sucrose for at least 2 days each. The brains were frozen and sliced in a cryostat at 50 μm, stained with cresyl violet, and evaluated under a light microscope.
4.6. Data analysis
All analyses were conducted with the help of Statistica software (6.0; StatSoft, Tulsa, OK). For each experiment, baseline water intake was analyzed using a two-way (Lesion X Drug) analysis of variance (ANOVA) whereas CS intake was analyzed with a three-factor (Lesion X Drug X Trial) ANOVA. Post hoc comparisons, if needed, were conducted using F-tests with an adjusted error term (from the overall ANOVA), which considers the variance from the whole experiment and is not restricted to variance from the data subset that is being analyzed. For all analyses, the significance value was set at a α = .05.
4.7. Abbreviations
ANOVA, analysis of variance; CS, conditioned stimulus; COA, conditioned odor aversion; CTA, conditioned taste aversion; GT, gustatory thalamus; IC, insular cortex; ICX, insular cortex-lesioned; LiCl, lithium chloride; NMDA, N-methyl-D-aspartic acid; NaCl. Sodium chloride; SHAM, non-lesioned control; US, unconditioned stimulus.
Acknowledgments
This research was supported by grants DC04341 and DC06456 from the National Institute of Deafness and Other Communication Disorders. Portions of the data in this article were presented at the 38th annual meeting of the Society for Neuroscience in Washington, DC in November 2008.
Footnotes
Citing data from her laboratory that rats prefer 0.15% saccharin over 0.1 M NaCl in a two-bottle test, Grigson (1997) predicted that morphine and cocaine would not suppress intake of 0.1 M NaCl because that solution has less innate reward value than saccharin and therefore could not engage the reward comparison mechanism that she argues is responsible for drug-induced intake suppression.
We thank an anonymous reviewer for suggesting this analysis.
The results of a second experiment by Geddes et al. (2008) are difficult to interpret because saline- and morphine-injected ICX rats each suppressed intake of the saccharin CS, a pattern of results that suggests some form of carry-over effect in the ICX rats from the Polycose-cocaine trials they received in Experiment 1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Guidelines for ethical conduct in the care and use of animals. Washington, DC: American Psychological Association; 1996. [Google Scholar]
- Barker LM, Best MR, Domjan M. Learning Mechanisms in Food Selection. Baylor University Press; Waco, Texas: 1977. [Google Scholar]
- Berger B. Conditioning of food aversions by injections of psychoactive drugs. J Comp Physiol Psychol. 1972;81:21–26. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Yannick S, Mathilde B, Frederic L, Nadine R, Guillaume F. Critical role of insular cortex in taste but not odour aversion memory. Eur J Neurosci. 2009;29:1654–1662. doi: 10.1111/j.1460-9568.2009.06711.x. [DOI] [PubMed] [Google Scholar]
- Braveman NS, Bronstein P, editors. Ann New York Acad Sci. Vol. 443. 1985. Experimental Assessment and Clinical Applications of Conditioned Food Aversions; pp. 1–441. [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22:352–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Long-term aversions to a saccharin solution induced by repeated amphetamine injections. Pharmacol Biochem Behav. 1973;1:265–270. doi: 10.1016/0091-3057(73)90115-9. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representations in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am J Physiol. 1996;271:R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive Relativity. Cambridge University Press; Cambridge, England: 1996. [Google Scholar]
- Flaherty CF, Checke S. Anticipation of incentive gain. Anim Learn Behav. 1982;10:177–182. [Google Scholar]
- Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J Exp Psychol Anim Behav Proc. 1995;21:229–247. doi: 10.1037//0097-7403.21.3.229. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Grigson PS. From contrast to reinforcement: Role of response contingency in anticipatory contrast. J Exp Psychol Anim Behav Proc. 1988;14:165–176. [PubMed] [Google Scholar]
- Flaherty CF, Turovsky J, Krauss KL. Relative hedonic value modulates anticipatory contrast. Physiol Behav. 1994;55:1047–1054. doi: 10.1016/0031-9384(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions. II Effects on sodium appetite, taste aversion learning, and feeding behavior. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Geddes IR, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122:1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behav Neurosci. 1997;111:129–136. [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine-, but not LiCl-induced conditioned taste aversions in rats: Evidence for the reward comparison hypothesis. Brain Res. 2000;858:327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin in take: A model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 74–91. [Google Scholar]
- Kiefer SW, Braun JJ. Absence of differential associative responses to novel and familiar taste stimuli in rats lacking gustatory neocortex. J Comp Physiol Psychol. 1977;91:498–507. doi: 10.1037/h0077347. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Peripheral and systemic actions of food in the caloric regulation of intake. Ann New York Acad Sci. 1969;157:1126–1157. doi: 10.1111/j.1749-6632.1969.tb12940.x. [DOI] [PubMed] [Google Scholar]
- Lester D, Nachman M, Le Magnen J. Aversive conditioning by ethanol in the rat. Quart J Stud Alcohol. 1970;31:578–586. [PubMed] [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent Inhibition and Conditioned Attention Theory. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 37–57. [Google Scholar]
- Lundy RF, Jr, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. 3. Academic Press; San Diego: 2004. pp. 891–921. [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24:71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Krames L, Alloway TM, editors. Food Aversion Learning. Plenum Press; New York: 1977. [Google Scholar]
- Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol Behav. 2006;87:542–551. doi: 10.1016/j.physbeh.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Government Printing Office; Washington, DC: U.S: 1986. (DHEW Publication No. 86–23) [Google Scholar]
- Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92:123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- Parker LA. Nonconsummatory and consummatory behavioral CRs elicited by lithium- and amphetamine-paired flavors. Learn Motiv. 1982;13:281–303. [Google Scholar]
- Parker LA. Positively reinforcing drugs may produce a different kind of CTA than drugs which are not positively reinforcing. Learn Motiv. 1988;19:207–220. [Google Scholar]
- Parker LA. Taste reactivity responses elicited by reinforcing drugs: A dose-response analysis. Behav Neurosci. 1991;105:955–964. doi: 10.1037//0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107:188–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Reviews. 1995;19:143–51. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA. LSD produces a place preference and taste avoidance, but does not produce taste aversion. Behav Neurosci. 1996;109:503–508. doi: 10.1037//0735-7044.110.3.503. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: Evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 92–113. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Academic Press; San Diego, CA: 2005. [Google Scholar]
- Reilly S. Invited talk at the Eastern Psychological Association. Boston, MA: 2005a. Gustatory thalamus and consummatory contrast effects. [Google Scholar]
- Reilly S. Invited address at the American Psychological Association. Washington, DC: 2005b. Gustatory thalamus and incentive relativity. [Google Scholar]
- Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 309–327. [Google Scholar]
- Reilly S, Bornovalova M, Dengler C, Trifunovic R. Effects of excitotoxic lesions of the gustatory thalamus on latent inhibition and blocking of conditioned taste aversion in rats. Brain Res Bull. 2003;62:117–128. doi: 10.1016/j.brainresbull.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova M, Trifunovic R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: Evidence against a memory deficit. Behav Neurosci. 2004;118:365–376. doi: 10.1037/0735-7044.118.2.365. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav Neurosci. 1996;110:746–759. [PubMed] [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. [Google Scholar]
- Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamus lesions. Behav Neurosci. 1999a;113:1008–1019. doi: 10.1037//0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in the rat. Behav Neurosci. 1999b;113:1242–1248. [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Gustatory thalamus lesions disrupt successive negative contrast: Evidence against a memory deficit. Behav Neurosci. 2003;117:606–615. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- Roman C, Lin JY, Reilly S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Res. 2009;1259:68–73. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition. Eur J Neurosci. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Insular cortex lesions and morphine-induced suppression of conditioned stimulus intake in the rat. Behav Neurosci. 2009;123:206–211. doi: 10.1037/a0014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twinning RC, Grigson PS. The role of the gustatory thalamus in the anticipation and comparison of rewards over time in rats. Am J Physiol. 2005;288:R966–R980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP. Orosensory processing in neural systems of the nucleus of the solitary tract. In: Simon SA, Roper SD, editors. Mechanisms of Taste Transduction. CRC Press; Boca Raton: 1993. pp. 339–394. [Google Scholar]
- Vogel JR, Nathan BA. Learned taste aversions induced by hypnotic drugs. Pharmacol Biochem Behav. 1975;3:189–194. doi: 10.1016/0091-3057(75)90147-1. [DOI] [PubMed] [Google Scholar]
- Wolf G. Projections of thalamic and cortical gustatory areas in the rat. J Comp Neurol. 1968;132:519–530. doi: 10.1002/cne.901320403. [DOI] [PubMed] [Google Scholar]
- Zito KA, Bechera A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30:693–699. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]