Abstract
We obtained brain MRIs, plasma homocysteine levels and apolipoprotein E genotyping for 11 American Indian Alzheimer disease (AD) subjects and 10 Indian controls. We calculated white matter hyperintensity volume (WMHV), whole brain volume (WBV), and ratio of white matter hyperintensity volume to whole brain volume (WMHV/WBV). There were no significant differences between AD subjects and controls in gender, history of hypertension, diabetes, or history of high cholesterol, but hypertension and diabetes were more common among AD subjects. There was no difference between AD and control groups in age (range for all subjects was 61–89 years), % Indian heritage, waist size or body mass index. Median Indian heritage was 50% or greater in both groups. Range of education was 5–13 years in the AD group and 12–16 years in controls. Median plasma homocysteine concentration was higher in AD subjects (11 μmol/L vs. 9.8 μmol/L), but did not achieve statistical significance. Significantly more AD subjects had apolipoprotein Eε4 alleles than did controls (63% vs.10%). Neuroimaging findings were not significantly different between the 2 groups, but AD subjects had greater WMHV (median 15.64 vs. 5.52 cc) and greater WMHV/WBV ratio (median 1.63 vs. 0.65 %) and a far greater range of WMHV. In combined AD subjects and controls, WBV correlated with BMI and age. WMHV and WMHV/WBV correlated inversely with MMSE scores (p = 0.001, 0.002, respectively). In addition, WMHV correlated positively with % Indian heritage (p = 0.047).
Keywords: Alzheimer disease, American Indian, white matter hyperintensities, homocysteine, apolipoprotein E
INTRODUCTION
The findings of many investigators suggest that cerebral vascular pathology may synergize with Alzheimer disease (AD) pathology, leading to earlier onset of clinical dementia symptoms and more severe dementia than would have been produced by the AD pathology alone. For example, [1] found that among 61 prospectively followed persons with autopsy confirmed AD, individuals with brain infarcts had poorer cognitive function and a higher prevalence of dementia than those without infarcts. In a comparison of 72 subjects with autopsy proven AD with 32 subjects who evidenced AD pathology and cerebral infarcts or lacunes, the subjects with vascular lesions had more severe dementia despite essentially no difference in semiquantitative measures of plaques and tangles [2,3] found that persons diagnosed clinically with AD who also met NINDS-AIREN neuroimaging criteria for vascular dementia [4] had more rapid clinical progression than did subjects diagnosed with AD alone.
There is also evidence that lesser degrees of vascular pathology may also be important. In a histological examination of 7 brains following postmortem MRI, subcortical WMH were associated with arteriosclerosis, dilated perivascular spaces and vascular ectasia with occasional gliosis and small areas of infarction [5]. A pathological study of WMH in 11 elderly subjects suggested that punctate, early confluent and confluent WMH reflected ischemic damage ranging from mild perivascular alterations to large areas with variable loss of fibers, multiple small cavitations and marked arteriolosclerosis and that irregular periventricular WMH were characterized by microcystic infarcts and patchy myelin rarefication. Hyperintense periventricular caps and a smooth halo were areas of demyelination with subependymal gliosis and breaks in the ependymal lining [6]. Other pathological studies confirm the association of WMH with cerebrovascular disease but also find WMH attributable to non-vascular causes [7, 8].
Clinical evidence for a vascular origin of WMH includes a population-based study of 111 subjects ranging in age from 65–84 years in which the prevalence and severity of lesions increased with age and were associated independently with a history of stroke or myocardial infarction, factor VIIc activity and fibrinogen level. There was an association with hypertension and high cholesterol only in subjects from 65–74 years of age [9]. In a random sample of 1077 community-dwelling persons between 60–90 years of age, the prevalence and degree of WMH increased with age, and to a higher degree in women than in men [10, 11] found that age-adjusted WMH load in subjects 55–72 years of age was significantly higher in persons diagnosed clinically as vascular dementia than in persons diagnosed clinically as cognitively normal, mild cognitive impairment, or AD. In another study showing an association between hypertension and WMH, there was reduced risk of progression following successful reduction of blood pressure [12]. There is also evidence that elevated plasma homocysteine levels may be related to vascular disease [13], cognitive impairment [14], and volume of WMH [15].
In sum, the evidence from pathological and clinical studies suggests that WMH are at least partially due to the effects of cerebrovascular disease and aging [15], and may be increased by elevated homocysteine level, as indicated in Fig. (1).
Fig. 1.
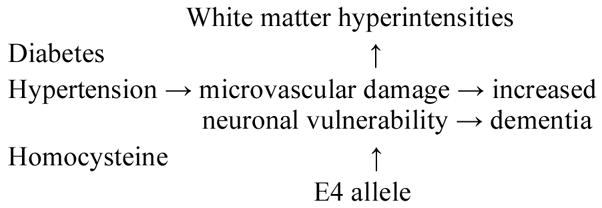
Possible Relationship of Risk Factors to White Matter Hyperintensities, Neuronal Vulnerability, and Dementia.
In a small study of American Indians, we found an association between diabetes and hypertension and the clinical diagnosis of probable AD [17]. The present report compares findings in American Indians with a diagnosis of probable AD with age-matched, cognitively intact American Indians and assesses the relationship of plasma homocysteine to WMH volume (WMHV), whole brain volume (WBV), and WMHV/WBV and the frequency of the apolipoprotein E4 allele in both groups.
METHODS
The Alzheimer’s Disease Center at the University of Texas Southwestern Medical Center has since 1991 maintained an American Indian Satellite, presently at the Choctaw Nation Healthcare Center, located in Talihina, Oklahoma and its outlying clinics. The mandate of this satellite is to provide clinical care to American Indians and to perform clinical research. The Choctaw Nation in Oklahoma consists of approximately 60,000 persons scattered over 10 ½ counties (15,000 square miles) in rural southeastern Oklahoma. The population density in this part of the state is approximately 20–30/sq.mi [18].
Subjects
Subjects were community-dwelling Indians with AD and age-matched Indian controls living in southeastern Oklahoma who were willing to undergo non-contrast MRI study at a private open MRI facility in Fort Smith, Arkansas. Controls signed informed consent for themselves; AD subjects and their legal representatives both signed informed consents using forms approved by the Choctaw Nation IRB and the UT Southwestern Medical Center IRB. Each subject was given a $50 Wal-Mart gift certificate for participation; cost of transportation provided by the MRI facility was paid by the investigators. Subject identifiers were kept in a locked file in the office of M.W.
Diagnosis
Subjects with AD were diagnosed by experienced neurologists (R.N.R., K.B.W.) and geriatric psychiatrists (M.F.W.) by [19] criteria. Control subjects were individuals recruited at health fairs or from among spouses of AD subjects. Controls had no memory complaints, intact memory as judged by an informant, and Mini-mental State Examination (MMSE [20] scores within normal limits for age and education [21]. History of diabetes, hypertension, myocardial infarction, stroke, high cholesterol (or current use of lipid-lowering drug) was obtained in addition to measurement of height, weight, waist, and plasma homocysteine concentration.
MRI Methods
MRIs were performed on a Hitachi AIRIS II® 0.3 Tesla open MRI scanner. FLAIR images (7000/2200/130 [repetition time/inversion time/echo time]) were acquired in the axial plane with 5-mm slice thickness and interslice gap of 0.5 mm. The field of view (FOV) was 220 mm, and matrix size was 128 ×128. FLAIR sequence allows better differentiation of brain lesions by suppressing the effect of cerebro-spinal fluid, which appears as high-intensity signal in conventional T2-weighted images.
Image Processing and Lesion Measurement
Images were converted from DICOM to ANALYZE format and were then analyzed by a semi-automatic software tool developed on MATLAB (Math Works, Inc., Natick MA) as described in [22] to quantify total WMH volume and whole brain volume. Because periventricular WMH and deep white matter WMH correlate highly with each other (r2 > 0.87) and with total WMH (r2 > 0.95) [23], we calculated total WMH volume instead of quantifying periventricular WMH and deep WMH separately. Two raters were trained in this method and an interrater reliability analysis was conducted on MRIs from an independent sample of nine subjects with diffuse axonal injury; intraclass correlation coefficients were 0.982, 0.954, and 0.981 for WMHV, WBV, and WMHV/WBV. The two raters, who were blinded to subject diagnosis, followed the protocol to obtain volume of WMH, WBV, and WMH/WBV ratio in the Indian AD subjects and controls.
Homocysteine Methods
Blood for plasma homocysteine determination was collected in EDTA tubes and immediately placed on ice. It was centrifuged cold within 2 hours at the Clinical Laboratory of the Choctaw Nation Healthcare Center in Talihina, Oklahoma. Plasma was shipped in refrigerated transport tubes to a commercial laboratory (LabCorp) at which the first samples were analyzed using an HPLC method with fluorescence detection [24]. Subsequent samples were sent by Lab-Corp to Cambridge Biomedical Research Group where homocysteine concentration was determined by a competitive immunoassay technique [25] using a Siemens DPC immuno-assay kit with an analytic sensitivity of 0.5 μmol/L. Comparison of this method with an HPLC method revealed comparable values (r = 0.925; mean = 13.4 μmol/L for the immunoassay vs. 13.2 μmol/L for HPLC vs. 13.4 μmol/L for the immunoassay) [26]. We repeated homocysteine determinations for 15 subjects and found equally acceptable agreement between values for the 2 methods (r = .915; mean = 13.84 μmol/L for HPLC and 13.17 μmol/L for the immunoassay). We therefore used the results of these assays interchangeably.
Genotyping Method
Single drops of blood were placed on FTA® Microcards (Whatman International). DNA was later extracted in the UT Southwestern Molecular Diagnostics Laboratory using a QIAmp DNA Mini Kit (Quiagen). Exon 4 of the ApoE gene was amplified by PCR in a DNA Thermal Cycler using primers: sense 5′-GAGACCATGAAGGAGTTGAAGGCC-3′ and antisense 5′-ACATGGTCCGGCCCCGGGCGCTCCC-3′. In addition to the buffer and nucleotide components described by the supplier (Invitrogen, Carlsbad, CA), each amplification reaction contained 100–500ng of genomic DNA, 0.15 ÿM of each primer, 1 U/ÿl of Taq polymerase, 50 uM of dNTP’s, 10% DMSO in a final volume of 40 ÿl. Each reaction mixture was heated at 95°C for 2 minutes for denaturation, and subjected to 35 cycles of amplification by denaturation (94°C for 45 seconds), primer annealing (65°C for 45 seconds), extension (72°C for 80 seconds), and final extension (72°C for 10 minutes). The PCR products (20 μL) were further treated by adding 1 μL shrimp alkaline phosphatase (1U/μL) (Roche) and 1 μL exonuclease I (20U/μL) (New England Biolabs), 37°C for 30 minutes, to remove the excessive primers and nucleotides, and then 95°C for 5 minutes to inactivate the enzymes. Then, 5 μL of treated PCR products were given to the Sequence Core for sequencing using the sense primer 5′-GAGACCATGAAGGAGTTGA AGGCC-3′. The sequenced result of each sample was translated to protein sequence using MacVector software and genotyping at the APOE locus was determined manually with C112/C158 as E2, C/R as E3 and R/R as E4.
Statistical Methods
Interclass Correlation Coefficients (ICC) were used to determine the inter-rater reliability among the measures (WMHV, WBV, and WMHV/WBV ratio) for the two independent raters. The mean values for the two raters were used in subsequent analyses. Groups were compared using Mann-Whitney U tests, categorical measures were compared using Fisher’s Exact tests, and correlations between continuous measures was performed using Spearman Rank order correlations. All statistical tests were performed using SPSS for Windows V15.0 and p < 0.05 was considered significant.
RESULTS
Data were available for 11 AD subjects and 10 controls. As indicated in Table 1, there were no significant differences between AD subjects and controls in gender, history of hypertension, diabetes, or high cholesterol. No subject reported having had a myocardial infarction or stroke. Table 2 indicates no difference between AD control groups in age, % Indian heritage, waist size or BMI. Despite lack of statistical significance, education was lower in the AD group and homocysteine values were higher. Apolipoprotein E genotyping was available for 8 AD subjects and 10 controls. There were 5 AD subjects (63%) with an apolipoprotein ε4 (apoE4) allele and 1 control (10%) (Fisher’s Exact 2-sided p = 0.043), in line with findings from other groups of late-onset AD [27]. There was no significant relationship between the presence of an ApoEε4 allele and Indian heritage in the AD group or in the combined AD and control groups (p = 0.5466). There were no significant differences in neuroimaging findings between the 2 groups, but AD subjects clearly had greater volume of WMH (median 15.64 vs. 5.52 cc) and greater WMHV/WBV ratio (median 1.63 vs. 0.65 %) and a far greater range of WMHV (See Table 3). While women had significantly higher WMHV and WMHV/WBV ratios than men (p = 0.003 for both), the presence or absence of hypertension, diabetes or depressions were not significantly different (see Tables 4 and 5). When the 2 groups were combined, WBV correlated with BMI (r = 0.54, p = 0.015) and age (r = −0.60, p = 0.005). Percentage of Indian heritage was positively correlated with WMHV (r = 0.56, p = 0.011 and education was inversely correlated with WMVH/WBV (r = −0.47, p = 0.035). Both WMHV and WMHV/WBV correlated inversely with MMSE scores (r = −0.69, p = 0.001; r = −0.64, p = 0.004, respectively), supporting the impact of WMH on cognition.
Table 1.
Gender and Medical History Characteristics of American Indian AD Subjects and Controls*
| Variable | AD N (%) | Control N (%) | Total N (%) | Fisher’s Exact Test p-value |
|---|---|---|---|---|
| Female | 8 (72.7) | 7 (70.0) | 15 (71.4) | >0.999 |
| Hypertension | 10 (90.9) | 8 (80.0) | 18 (85.7) | 0.586 |
| Diabetes | 6 (54.5) | 4 (40.0) | 10 (47.6) | 0.670 |
| High cholesterol or hypolipidemic agent | 3 (27.3) | 6 (60.0) | 9 (42.9) | 0.198 |
N and (%) are provided in this table.
Table 2.
Quantitative Characteristics of American Indian AD Subjects and Controls
| AD | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean Rank | Median | Range | N | Mean Rank | Median | Range | Mann-Whitney p-value |
| Age | 11 | 11.41 | 71.00 | 61–89 | 10 | 10.55 | 75.50 | 64–82 | 0.756 |
| Education | 11 | 8.55 | 12.00 | 5–13 | 10 | 13.70 | 12.00 | 12–16 | 0.061 |
| % Native American | 11 | 10.68 | 50.00 | 3–100 | 10 | 11.35 | 68.75 | 13–100 | 0.809 |
| Mini-mental State Exam Score | 9 | 5.28 | 15.00 | 1–28 | 10 | 14.25 | 29.50 | 25–30 | <0.001 |
| Plasma homocysteine (μmol/l) | 11 | 12.50 | 11.00 | 8.6–32.8 | 10 | 9.35 | 9.80 | 6.2–20.1 | 0.251 |
| Waist Size (Inches) | 11 | 12.36 | 34.00 | 32–47 | 10 | 9.50 | 33.50 | 32–38 | 0.314 |
| BMI (kg/m2) | 11 | 12.14 | 27.34 | 19.84–38.3 | 10 | 9.75 | 26.69 | 21.35–32.62 | 0.387 |
Table 3.
Relationship of White Matter Hyperintensity Volume (WMHV) to Whole Brain Volume (WBV)
| Choctaw AD | Choctaw Controls | Mann-Whitney | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean Rank | Median | Range | N | Mean Rank | Median | Range | p-value |
| WMHV(cc.) | 11 | 13.36 | 15.64 | 1.55 – 65.57 | 10 | 8.40 | 5.52 | 2.26 – 15.39 | 0.072 |
| WBV(cc.) | 11 | 12.27 | 899 | 855 – 1115 | 10 | 9.60 | 879 | 812 – 1014 | 0.349 |
| WMHV/WBV(%) | 11 | 13.27 | 1.63 | 0.14 – 7.64 | 10 | 8.50 | 0.65 | 0.22 – 1.78 | 0.085 |
Table 4.
Comparisons of White Matter Hyperintensity Measures by Gender
| Variable | N | Mean Rank | Median | Range | N | Mean Rank | Median | Range | Mann-Whitney p-value |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | |||||||
| WMHV (cc.) | 15 | 13.40 | 14.31 | 2.60 – 65.57 | 6 | 5.00 | 3.18 | 1.55 – 7.63 | 0.003 |
| WBV (cc.) | 15 | 9.60 | 884.6 | 811.5 – 1017.7 | 6 | 14.50 | 947.1 | 865.77 – 114.60 | 0.112 |
| WMHV/WBV (%) | 15 | 13.40 | 1.58 | 0.29 – 7.64 | 6 | 5.00 | 0.35 | 0.14 – 0.88 | 0.003 |
Table 5.
White Matter Hyperintensities and Hypertension, Diabetes, and High Cholesterol/Hypolipidemic Agent
| Variable | N | Mean Rank | Median | Range | N | Mean Rank | Median | Range | Mann-Whitney p-value |
|---|---|---|---|---|---|---|---|---|---|
| Hypertension | No | Yes | |||||||
| WMHV (cc.) | 3 | 14.33 | 15.39 | 5.68 – 53.85 | 18 | 10.440 | 6.12 | 1.55 – 65.57 | 0.356 |
| WBV (cc.) | 3 | 10.33 | 883.2 | 862.3 – 956.1 | 18 | 11.11 | 894.1 | 811.5 – 1114.6 | 0.887 |
| WMHV/WBV (%) | 3 | 14.33 | 1.78 | 0.65 – 5.63 | 18 | 10.44 | 0.69 | 0.14 – 7.64 | 0.356 |
| Diabetes | No | Yes | |||||||
| WMHV (cc.) | 11 | 11.36 | 10.21 | 1.55 – 65.57 | 10 | 10.60 | 5.81 | 2.83 – 60.13 | 0.809 |
| WBV (cc.) | 11 | 10.45 | 874.0 | 854.89 – 1114.60 | 10 | 11.60 | 895.9 | 811.5 – 964.1 | 0.705 |
| WMHV/WBV (%) | 11 | 11.36 | 1.18 | 0.135 – 7.64 | 10 | 10.60 | 0.66 | 0.30 – 6.74 | 0.809 |
| High cholesterol/hypolipidemic agent | No | Yes | |||||||
| WMHV (cc.) | 12 | 12.58 | 14.85 | 2.60 – 65.57 | 9 | 8.89 | 5.68 | 1.55 – 60.13 | 0.193 |
| WBV (cc.) | 12 | 10.50 | 886.4 | 854.9 – 1017.71 | 9 | 11.67 | 893.1 | 811.5 – 1114.6 | 0.702 |
| WMHV/WBV (%) | 12 | 12.58 | 1.60 | 0.29 – 7.64 | 9 | 8.89 | 0.66 | 0.14 – 6.74 | 0.193 |
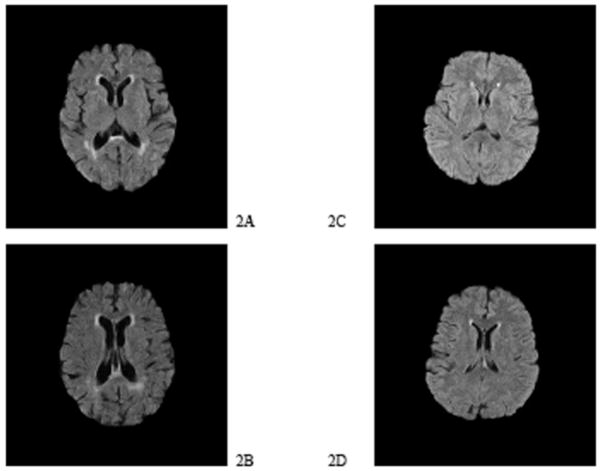
Fig. (1) illustrates the difference between AD subjects and controls in WMHV and WMHV/WBV. MRIs were selected from the median AD subject and the median control subject in terms of WMHV. The AD subject (Figs. 2A and 2B) had much smaller WBV than the control subject (Figures 2C and 2D) as shown by the degree of gyral atrophy and increase in ventricular volume, greater absolute WMHV and greater WMHV/WBV ratio.
Fig. 2.
Comparison of Median AD Subject with Median Control Subject.
2A. AD subject at level of head of caudate.
2B. AD subject at level of lateral ventricles.
2C. Control subject at level of head of caudate.
2D. Control subject at level of lateral ventricles.
DISCUSSION
The correlation of WBV with BMI was expected, as brain weight varies with body height and weight. [28] The correlation of WMHV and WMHV/WBV ratio with increasing age is also in agreement with other studies [29]. The inverse relationship between MMSE score and WMHV and WMHV/WBV ratio also agrees with previous findings concerning the impact of WMH on cognition [30] and supports that notion that WMH partially reflect vascular brain disease [6]. The significant relationship between WMHV and WMHV/WBV and Indian heritage fits with the increase of hypertension and diabetes with greater Indian heritage; however the significantly greater WMHV and WMHV/WBV ratio in women appeared to differ from earlier findings. For example, [31], in a study of 243 healthy subjects ranging from 16–64 years of age, found no gender difference in WMH, nor did [32] in a study of 477 healthy subjects aged 60–64 years. A study by [33] found in elders that deep WMH volume increased twice as rapidly in women than men, but not progression of periventricular WMH. Thus, it is possible that Indian women may be more susceptible to the brain effects of cardiovascular comorbidities and risk factors, or may have more risk factors. In our sample, 7/10 women had a history of diabetes as compared with 3/10 men. We found no evidence of an association of inheritance of apoE4 alleles and degree of Indian heritage.
An important confound in studies of relationship of ethnicity to biological measures is the inexactness of ethnicity, which is usually based on self designation rather than an objective criterion. American Indians have the advantage of a semi-objective measure, the Certificate of Degree of American Indian Blood (CDIB), which certifies that an individual has a specific percentage of Indian blood of a federally recognized tribe or tribes [34]. CDIB validity is supported by ascertainment of the au H2 genotype, found only in populations with European ancestry [35] showed 29% History of tau H2 frequency in Caucasians, 28% in persons claiming < 50% Indian heritage, and only 8% in those claiming > 50% Indian heritage. In this series, subjects averaged 50% or more Indian heritage.
The generalizability of our findings is limited because of the small number of subjects, and sample bias due to the non-randomness of subject selection, which was based on the willingness of subjects to undergo MRI study and be transported approximately 60 miles to the MRI facility. It is nevertheless a first look at aspects of an understudied population. We are completing a study of plasma homocysteine and apoE genotype in a larger sample.
Acknowledgments
This research was supported by Alzheimer’s Association Grant IIRG-04-1410, NIA AG12300, the Wallace, Barbara and Kelly King Foundation, and Forest Laboratories. Mr. Carey Fuller coordinated the recruiting effort in Oklahoma.
References
- 1.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 2.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer’s disease: CERAD, par XVIII. Consortium to Establish a Registry for Alzheimer’s Disease. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- 3.Sheng B, Cheng LF, Law CB, Li HL, Yeung KM, Lau KK. Coexisting cerebral infarction in Alzheimer’s disease is associated with fast dementia progression: applying the National Institute for Neurological Disorders and Stroke/Association Internationale pour la Recherche en l′Enseignement en Neurosciences neuroimaging criteria in Alzheimer’s disease with concomitant cerebral infarction. J Am Geriatr Soc. 2007;55:918–922. doi: 10.1111/j.1532-5415.2007.01171.x. [DOI] [PubMed] [Google Scholar]
- 4.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 5.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlation. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Estes ML, Furlan AJ, Awad IA. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Arch Neurol. 1992;49:747–752. doi: 10.1001/archneur.1992.00530310095018. [DOI] [PubMed] [Google Scholar]
- 8.Braffman BH, Zimmerman RA, Tojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. Am J Roentgenol. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 9.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 10.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hentschel F, Damian M, Krumm B, Froelich L. White matter lesions – age-adjusted values for cognitively healthy and demented subjects. Acta Neurol Scand. 2007;115:174–180. doi: 10.1111/j.1600-0404.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 12.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity changes in older adults and relationship to blood pressure: brain atrophy, WMH change and blood pressure. J Neurol. 2007;254:713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 13.Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999;354:407–413. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- 14.Dufoil C, Alperovitch A, Ducros V, Tzurio C. Homocysteine, white matter hyperintensities, and cogntion in healthy elderly people. Ann Neurol. 2002;53:214–221. doi: 10.1002/ana.10440. [DOI] [PubMed] [Google Scholar]
- 15.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperin-tensity volume: The Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 17.Weiner MF, Rosenberg RN, Womack KB, Svetlik DA, Fuller C, Klink A, et al. Atherosclerosis risk factors in american indians with Alzheimer’s disease: preliminary findings. Alz Dis Assoc Disord. doi: 10.1097/WAD.0b013e318169d701. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.http://quickfacts.census.gov/qfd/states/40/40079.html
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 22.Marquez de la Plata C, Ardelean A, Koovakkattu D, Srinivasan P, Miller A, Phoung V, et al. Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. J Neurotrauma. 2007;24:591–598. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki A, Sato Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluoresecence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 25.Zappacosta B, Persichilli S, Minucci A, Scribano D, Antenucci M, Fasanella S, et al. Evaluation of a new enzymatic method for homocysteine measurement. Clin Biochem. 2006;39:62–66. doi: 10.1016/j.clinbiochem.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Siemens DPC Diagnostics. Immunolite Manual. Los Angeles, CA: 2000. [Google Scholar]
- 27.Weiner MF, Vega G, Risser RC, Honig LS, Cullum CM, Crum-packer D, et al. Apolipoprotein E4, other risk factors, and course of Alzheimer’s disease. Biol Psychiatry. 1999;45:633–638. doi: 10.1016/s0006-3223(98)00222-4. [DOI] [PubMed] [Google Scholar]
- 28.Ho KC, Roessmann U, Straumfjord JV, Monroe G. Analysis of brain weight. II Adult brain weight in relation to body height, weight, and surface area. Arch Pathol Lab Med. 1980;104:640–645. [PubMed] [Google Scholar]
- 29.Taylor WD, MacFall JR, Provenzale JM, Payne ME, McQuoid DR, Steffens DC, et al. Serial MR imaging of hyperintense white matter lesions in elderly patients: correlation with vascular risk factors. Am J Roentgenol. 2003;181:571–576. doi: 10.2214/ajr.181.2.1810571. [DOI] [PubMed] [Google Scholar]
- 30.De Groot JC, de Leeuw F-E, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: The Rotterdam Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging. 2006;16:243–251. doi: 10.1111/j.1552-6569.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 32.Wen W, Sachdev P. The topography of white matter hyperin-tensities on brain MRI in healthy 60- to 84-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Van den Heuvel DM, Admiraal-Behloul F, ten Dam VH, Olofsen H, Bollen EL, Murray HM, et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63:1699–1701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- 34.Department of the Interior: Bureau of Indian Affairs 25 CFR Part 70 RIN 1076-AD98.
- 35.Henderson JN, Crook R, Crook J, Hardy J, Onstead L, Carson-Henderson L, et al. Apolipoprotein E4 and tau allele frequencie-samong Choctaw Indians. Neurosci Lett. 2002;324:77–79. doi: 10.1016/s0304-3940(02)00150-7. [DOI] [PubMed] [Google Scholar]