Abstract
Understanding mechanisms fostering coexistence between invasive and resident species is important in predicting ecological, economic, or health impacts of invasive species. The mosquito Aedes aegypti coexists at some urban sites in southeastern United States with invasive Aedes albopictus, which is often superior in interspecific competition. We tested predictions for three hypotheses of species coexistence: seasonal condition-specific competition, aggregation among individual water-filled containers, and colonization–competition tradeoff across spatially partitioned habitat patches (cemeteries) that have high densities of containers. We measured spatial and temporal patterns of abundance for both species among water-filled resident cemetery vases and experimentally positioned standard cemetery vases and ovitraps in metropolitan Tampa, Florida. Consistent with the seasonal condition-specific competition hypothesis, abundances of both species in resident and standard cemetery vases were higher early in the wet season (June) versus late in the wet season (September), but the proportional increase of A. albopictus was greater than that of A. aegypti, presumably due to higher dry-season egg mortality and strong wet-season competitive superiority of larval A. albopictus. Spatial partitioning was not evident among cemeteries, a result inconsistent with the colonization-competition tradeoff hypothesis, but both species were highly independently aggregated among standard cemetery vases and ovitraps, which is consistent with the aggregation hypothesis. Densities of A. aegypti but not A. albopictus differed among land use categories, with A. aegypti more abundant in ovitraps in residential areas compared to industrial and commercial areas. Spatial partitioning among land use types probably results from effects of land use on conditions in both terrestrial and aquatic-container environments. These results suggest that both temporal and spatial variation may contribute to local coexistence between these Aedes in urban areas.
Keywords: Aggregation, Coexistence, Condition-specific competition, Spatial partitioning
Introduction
Ecological theory and empirical work indicate that competition should result in competitive exclusion when resources are limited. However, competitive exclusion may be avoided via a number of mechanisms, including differential resource use (e.g., Tilman 1982), temporally varying condition-specific competition (e.g., Chesson 2000a), and colonization-competition tradeoff across a patchy environment (e.g., Calcagno et al. 2006). Understanding mechanisms of coexistence is particularly interesting in the context of biological invasions. Competitively superior invasive species may impact resident species without causing extinction of residents over their entire introduced range (e.g., Juliano and Lounibos 2005). Testing hypotheses for mechanisms contributing to local or spatially patterned coexistence of invasive and resident species is important for predicting future ecological, economic, or health impacts of invasive species, and tests our understanding of the general mechanisms by which competing species coexist.
The Asian Tiger mosquito, Aedes albopictus, is native to Asia and has invaded North and South America, Europe, and Africa in the past two decades (see Juliano and Lounibos 2005, and references therein). This species is well studied (Lounibos 2002) and provides us with an opportunity to determine whether patterns of coexistence with natives in its new range are consistent with any of these coexistence mechanisms. Aedes albopictus, along with many of its competitors, are vectors of arboviruses, including dengue, La Crosse encephalitis, and West Nile virus (Gerhardt et al. 2001; Ibañez-Berñal et al. 1997; Turell et al. 2005), and understanding mechanisms of coexistence of these vectors is of some human health importance.
Most laboratory (e.g., Barrera 1996; Daugherty et al. 2000) and field (Juliano 1998) studies show convincingly that larval A. albopictus are superior competitors for resources compared to A. aegypti, the resident species it encounters most frequently in the Americas. This competitive superiority is the likely mechanism for displacement of A. aegypti in some locations in the field (Juliano 1998; Juliano et al. 2004). However, despite this frequently observed competitive advantage for A. albopictus, A. aegypti manages to coexist with invading A. albopictus at some sites in the southeastern United States (Juliano et al. 2004; O’Meara et al. 1995).
A number of hypotheses could explain the persistence of A. aegypti after the invasion of A. albopictus at some sites. Here we focus on three alternative (but not mutually exclusive) hypotheses that seem likely to be testable using field data. One hypothesis is condition-specific competition, wherein the outcome of competition is altered or reversed under different biotic conditions. When there is temporal or spatial variation in abiotic environments, and when species respond differently to those environments, the competitive outcome can be altered and coexistence can result (Chesson 2000a). A second hypothesis is a colonization-competition tradeoff, wherein species coexist in patchy environments when the superior ability to disperse among patches prevents local and regional exclusion of competitively inferior species (Amarasekare and Nesbit 2001; Calcagno et al. 2006). A third hypothesis is aggregation, in which species coexist because of spatial separation. Such spatial separation due to independent aggregation is most likely to arise due to attraction of competitors to different environmental conditions that have some spatial independence (Ives 1988; Chesson 2000b; Hartley and Shorrocks 2002). Some of the conditions necessary for the coexistence mechanisms postulated by these hypotheses are present in this system.
Condition-specific competition between these Aedes mosquitoes has been observed in the laboratory (Costanzo et al. 2005). Experimental manipulation of container drying regime in laboratory colonies shows that, in dry conditions, A. albopictus suffers a greater interspecific density effect than does A. aegypti, whereas under wetter conditions, A. aegypti suffers a greater interspecific density effect than does A. albopictus (Costanzo et al. 2005). Under dry conditions, Aedes albopictus eggs suffer greater mortality than do A. aegypti eggs (Costanzo et al. 2005). This paradoxical effect of differential mortality of eggs likely arises because mortality affects R*, the level of resources (in this case, for larvae) necessary to produce zero net population growth (Tilman 1982). For resource competition, advantage is determined by R* (Tilman 1982). Abiotic conditions that differentially affect mortality, even in non-competing life-stages like eggs, can alter the outcome of interspecific competition by differentially changing species’ R*s (Chase and Leibold 2002; Costanzo et al. 2005). The southern part of the Florida peninsula experiences a distinct cycle of rainy and dry seasons (Juliano et al. 2004), and field data show that A. albopictus and A. aegypti coexist most commonly in warmer, seasonally dry sites in South Florida (Juliano et al. 2002). For coexistence to occur under this circumstance, environments must fluctuate between conditions favoring the different species, and there must be resistant life stages (e.g., dormant eggs) that persist through times when a species is at a disadvantage (Chesson 2000a). Together, these field and laboratory results suggest that condition-specific competition due to temporally varying drying regime, along with storage of resistant eggs is a potential mechanism for coexistence in southern Florida.
Some of the conditions required for a colonization-competition tradeoff are met by the environment in which A. albopictus and A. aegypti interact. These species are more likely to coexist in urban areas of Florida (O’Meara et al. 1995; Rey et al. 2006), and in urban areas high densities of water-filled containers appear to be patchy and associated with certain local land uses, such as cemeteries containing flower vases (O’Meara et al. 1995) that may act as discrete patches of habitat. A poorer competitor in a metapopulation may escape local and regional exclusion if it has a superior ability to colonize newly vacant, sparsely populated, or even well-occupied individual containers or patches (Amarasekare and Nesbit 2001; Calcagno et al. 2006).
A similar mechanism for coexistence on patchy resources is invoked in the aggregation hypothesis (Chesson 2000b; Hartley and Shorrocks 2002), which predicts coexistence if the competing stage of the competitively superior species is aggregated over divided, ephemeral resources, independently of the competitively inferior species. Aggregation is most likely to be effective in this way when species are attracted to different, spatially independent environmental cues, which is a form of spatial resource partitioning (Chesson 2000b), though some (Hartley and Shorrocks 2002) have argued that if random aggregation is sufficiently strong, competitors could be more influenced by intraspecific competition than inter-specific competition, resulting in coexistence (see Chesson 2000b; Hartley and Shorrocks 2002, for caveats about the conditions necessary to produce coexistence via this mechanism).
In this paper, we test whether temporal and spatial distributions of immature A. albopictus and A. aegypti in metropolitan Tampa, Florida, are consistent or inconsistent with these three hypotheses for coexistence of A. aegypti with invading A. albopictus. Metropolitan Tampa is ideal for such tests because it has numerous sites with water-filled containers (cemeteries) within a few kilometers of one another, and because these species have coexisted at some sites in metropolitan Tampa for over 15 years (G.F. O’Meara, unpublished data), suggesting stability. Because our investigation is observational, it is but the first step in evaluating these hypotheses; however, our hypotheses lead to testable predictions about the distribution and abundance of these Aedes in Tampa for each coexistence hypothesis:
Condition-specific competition predicts strong seasonal patterns of coexistence with both species predicted to be more abundant late in the wet season (May–October—see http://www.dnr.state.sc.us/climate/sercc/climateinfo/historical/historical_fl.html), after a lengthy period of wet conditions, but with the proportional increase of A. albopictus from early to late wet season expected to be greater than that of A. aegypti due to high dry-season (November–April) egg mortality and strong wet-season competitive superiority of A. albopictus.
Spatial partitioning and a colonization-competition trade-off across cemeteries predicts highest abundances of A. albopictus and A. aegypti in cemeteries compared to non-cemetery urban sites.
The aggregation hypothesis of coexistence among individual containers predicts that A. albopictus and A. aegypti distributions will be aggregated among containers and independently associated with one another.
Materials and methods
Three discrete areas in metropolitan Tampa (Tampa, St. Petersburg, and Bradenton), all separated by Tampa Bay, were selected for study. These areas have a number of cemeteries within an 8 km radius (Tampa, n = 10; St. Petersburg, n = 5; and Bradenton, n = 4). Other sites with high densities of container habitats, such as tire yards, were present in each area but preliminary observations suggested that few had long-standing piles of tires outside that would fill with water and provide larval mosquito habitat. Thus, we decided not to survey tire yards in this study. Habitat between cemeteries (hereafter ‘urban matrix’) is characterized by a high density of human-created structures, but also includes diverse vegetation, and residential, commercial, and industrial land use.
In the early rainy season (June) in 2006, we placed five standard green cemetery vases within each cemetery and three other randomly chosen parts of the urban matrix, to obtain estimates of oviposition activity at each site. Each cemetery and urban site was divided into five equally sized blocks, and one vase was placed in the shade as close to a randomly selected point within each block as possible. Most occupied vases at cemeteries are partially shaded (O’Meara et al. 1995). Vases were provisioned with 0.70 g oak (Quercus virginiana) leaves and filled 400 ml deionized water, providing roughly natural amounts of leaves, which form the resource base in resident vases. A Masonite paddle was clipped to the inside of each vase as an oviposition substrate. Holes were drilled in vases just above the waterline to prevent vase flooding.
After sufficient time for mosquitoes to oviposit (10 days), we collected the vases and removed and counted all larvae and pupae. Paddles from all vases were stored under humid conditions for 10 days, and then eggs were synchronously hatched in a solution of 0.44 g nutrient broth/l deionized water. Numbers of field-collected larvae and pupae and laboratory-hatched larvae were summed by species to quantify the relative abundances of each species at these sites.
In addition to vase ovitrapping, which measures pre-interaction habitat choice, we also sampled the presence/absence of larvae and pupae in resident vases at each cemetery in Tampa to examine the distribution of Aedes among containers after community interactions. Three sample points within each block at a cemetery were randomly chosen and the closest shaded wet vase to each point sampled (3 vases × 5 blocks = 15 total vases). No samples were taken from bronze vases or vases lined with copper because of their toxicity to Aedes, or from vases with cut flower arrangements, as the arrangements usually decay rapidly, fouling the water, and rarely do such vases support Aedes larvae (O’Meara et al. 1995). Mosquitoes were collected from vases by either extracting the water with a kitchen baster, or, when possible, by dumping the contents (Juliano et al. 2002; O’Meara et al. 1995). The contents of vases were sieved and examined for the occupancy of A. albopictus and A. aegypti.
During the late rainy season (September) in 2006, we repeated our sampling of standard green vases and resident vases in cemeteries and standard vases in non-cemetery urban sites in Tampa only. Because June data indicated substantial oviposition of Aedes in urban sites (see Results), we undertook a new sampling approach to relate urban land use to Aedes oviposition. A 6 × 10 km area encompassing the ten cemeteries in Tampa was divided into 60 1 km2 grid cells. Three oviposition traps were placed in shade near the center of each cell. Ovitraps were placed at ground level and within 20 m of one another (180 total traps). Sample sites were categorized as residential, commercial, or industrial according to the Florida Land Use Cover Classification System (FLUCCS, Geographic Mapping Section 1999) of the South West Florida Water Management District (http://www.swfwmd.state.fl.us/data/gis/layer_library/category/physical_dense).
Ovitraps consisted of black plastic cups (400 ml), with holes drilled 4 cm from the base to prevent flooding and hatching of Aedes eggs. Ovitraps were lined with seed germination paper (Nasco Science, Fort Atkinson, WI), filled with 225 ml deionized water, and baited with an additional 25 ml grass/oak leaves (72 g senescent live oak leaves and 36 g Zoisa grass in 5.4 l deionized water for 3 days). Ovitraps at alternating sampling sites were set out over 3 days, with 20, 18, and 22 sites being provisioned with traps on each successive day. The relatively small size and inconspicuous color of these ovitraps was expected to minimize chances of them being disturbed in areas of relatively high human activity, and has been used in previous oviposition surveys in urban landscapes (Rey et al. 2006). After 10 days, ovitraps were collected and all larvae identified. Germination paper from each ovitrap was stored in humid conditions for 10 days, then immersed in nutrient broth solution to hatch Aedes eggs. Numbers of field-collected larvae and pupae and laboratory-hatched larvae were summed by species. All larvae and pupae from standard green vases, resident vases, and black ovitraps were reared to 4th instar to facilitate identification and stored in 70% ethanol for later examination in the laboratory.
Aggregation at the level of the competing stages (measured as eggs and early instar larvae) could result from two processes: aggregation of female oviposition visits and deposition of eggs aggregated in clutches (Hartley and Shorrocks 2002). Considerable debate has occurred on which of these two processes is most important in facilitating coexistence (see Heard and Remer 1997; Hartley and Shorrocks 2002 for a discussion). Recent models indicate that a model incorporating both processes allows coexistence, and that a model in which ovipositing females are randomly distributed and clutches are the only source of aggregation can produce coexistence if interspecific competition is partially reduced by some form of within-patch resource partitioning (Hartley and Shorrocks 2002). We, like most prior studies on Diptera in patchy and ephemeral systems (see Chesson 2000b, Hartley and Shorrocks 2002), test only whether competing stages are aggregated, and do not attempt to separate aggregation of ovipositing females from aggregation due to multi-egg clutches.
Statistical analyses
Numbers of resident cemetery vases occupied vs unoccupied in early and late season were analyzed using paired t-tests. Abundances of each species in standard green vases and ovitraps were analyzed using four nested ANOVAs. In the first ANOVA on vase data from Tampa, Season (June, September), Site Type (cemetery, urban site) and Interaction were fixed effects, and Site nested within Site Type and Season × Site nested within Site Type were random effects because we want to draw inferences about the population of sites (cemetery or urban) where Aedes might oviposit. In the second and third ANOVAs on St Petersburg and Bradenton vase data, respectively, Site Type (cemetery, urban site) was a fixed factor and Site nested within Site Type was a random factor. In the fourth ANOVA on Tampa ovitrap data, Land Use (residential, commercial, and industrial) and Date were fixed factors and Site nested within Land Use was a random factor. A preliminary analysis revealed that Date was not significant, and it was removed from the model. Significant differences between individual Land Uses were tested a posteriori by Tukey’s test. All ANOVAs were unbalanced because of trap loss, hence denominator mean squares were calculated using Satterthwaite’s formulas (SAS Institute 2003), resulting in non-integer degrees of freedom. For all analyses of abundances, proportions were arcsine-square-root transformed and abundance data were square-root transformed to meet assumptions of normality and homogeneity of variances.
Associations between A. albopictus and A. aegypti among resident cemetery vases and green vases were tested using Mantel-Haenszel tests on multiple 2 × 2 tables (Sokal and Rohlf 1995). Mantel-Haenszel tests control for site effects even when individual sites contain few observations as long as the overall sample size is adequate. Fisher’s Exact tests were used to test species associations among black ovitraps. Low sample sizes of each species prevented controlling for site when testing for the aggregation of each species, thus departures of randomness were investigated by pooling data from all sites and using χ2 goodness-of-fit tests for Poisson distribution. The ratio of variance to mean (s2/Ȳ) was used to indicate the nature of any departure from Poisson expectation. ANOVAs on data from standard green vases tested for significant differences between sites using pairwise contrasts, with sequential Bonferroni correction for all possible comparisons (19) within each analysis. The results of these ANOVAs identified sites with significantly greater abundances, which were then removed from χ2 goodness-of-fit tests to control for site density differences. These modified tests gave similar conclusions and are not reported. Frequency classes were grouped until all frequencies were greater than 2. Other grouping criteria (>5 and >0.05) were also tested, but yielded similar conclusions and are not reported.
Results
Aedes albopictus and A. aegypti were the most frequent species sampled in resident cemetery vases, being collected from 31.9% (84/263) and 24.3% (64/263) vases, respectively (Table 1). The third most common taxon was Culex spp., collected from only 15 vases (5.7%). Aedes albopictus and A. aegypti also were the most common species collected in our experimentally placed standard vases (pooled data across sites, A. albopictus: 65.5%, 1650/2518; A. aegypti: 18.1%, 455/2518) and ovitraps (A. albopictus: 66.7%, 3131/4695; A. aegypti: 32.2%, 1513/4695). All other mosquito species (Aedes triseriatus, Toxorhynchites rutilus, Anopheles sp., Uranotaenia sp.) collected from resident vases, standard vases, and ovitraps constituted less than 1% of the total number of individuals.
Table 1.
Numbers of resident vases and experimentally positioned standard vases (in parentheses), and proportions of vases, occupied by immatures of Aedes albopictus and Aedes aegypti at each cemetery in Tampa
| Number of vases sampled | A. albopictus only | A. aegypti only | A. albopictus and A. aegypti | Neither | Proportion A. albopictus | Proportion A. aegypti | |
|---|---|---|---|---|---|---|---|
| Early rainy season (June) | |||||||
| American Legion | 9 (5) | 0 (0) | 2 (1) | 0 (0) | 7 (4) | 0 (0) | 0.222 (0.200) |
| Centro Asturiano | 15 (5) | 1 (1) | 0 (0) | 0 (0) | 14 (4) | 0.067 (0.200) | 0 (0) |
| Marti Cemetery | 15 (5) | 0 (0) | 3 (1) | 1 (0) | 11 (4) | 0.067 (0) | 0.267 (0.200) |
| Myrtle Hill | 15 (5) | 1 (2) | 1 (0) | 1 (0) | 12 (3) | 0.133 (0.400) | 0.167 (0) |
| N25 | 15 (4) | 1 (2) | 0 (0) | 1 (0) | 13 (2) | 0.133 (0.500) | 0.067 (0) |
| Oaklawn | 11 (4) | 0 (0) | 2 (1) | 0 (0) | 9 (3) | 0 (0) | 0.222 (0.250) |
| Orange Hill | 15 (4) | 1 (1) | 0 (1) | 0 (1) | 14 (1) | 0.067 (0.500) | 0 (0.500) |
| Rose Hill | 15 (4) | 4 (0) | 1 (0) | 1 (0) | 9 (4) | 0.333 (0) | 0.111 (0) |
| Shady Grove | 15 (5) | 0 (0) | 0 (0) | 1 (1) | 14 (4) | 0.067 (0) | 0.067 (0.200) |
| Woodlawn | 15 (5) | 1 (0) | 2 (3) | 2 (1) | 10 (1) | 0.200 (0.200) | 0.133 (0.600) |
| Early total | 140 (46) | 9 (6) | 11 (7) | 7 (3) | 113 (30) | 0.114 (0.196) | 0.129 (0.217) |
| Late rainy season (Sept) | |||||||
| American Legion | 9 (4) | 0 (1) | 5 (1) | 2 (0) | 2 (2) | 0.222 (0.250) | 0.778 (0.250) |
| Centro Asturiano | 13 (4) | 5 (3) | 0 (0) | 2 (1) | 6 (0) | 0.538 (1.000) | 0.154 (0.250) |
| Marti Cemetery | 15 (5) | 0 (0) | 3 (1) | 1 (0) | 11 (4) | 0.067 (0) | 0.067 (0.200) |
| Myrtle Hill | 13 (5) | 6 (3) | 0 (0) | 5 (2) | 2 (0) | 0.846 (1.000) | 0.385 (0.400) |
| N25 | 15 (5) | 5 (3) | 0 (0) | 0 (2) | 10 (0) | 0.333 (1.000) | 0 (0.400) |
| Oaklawn | 10 (5) | 1 (3) | 1 (0) | 7 (2) | 1 (0) | 0.800 (1.000) | 0.800 (0.400) |
| Orange Hill | 11 (4) | 4 (1) | 0 (0) | 1 (2) | 6 (1) | 0.455 (0.750) | 0.091 (0.500) |
| Rose Hill | 12 (5) | 5 (1) | 1 (0) | 6 (3) | 0 (1) | 0.917 (0.800) | 0.583 (0.600) |
| Shady Grove | 12 (5) | 6 (2) | 0 (0) | 2 (2) | 4 (1) | 0.667 (0.800) | 0.167 (0.400) |
| Woodlawn | 13 (5) | 1 (0) | 1 (0) | 9 (3) | 2 (2) | 0.769 (0.600) | 0.769 (0600) |
| Late total | 123 (47) | 33 (17) | 11 (2) | 35 (17) | 44 (11) | 0.553 (0.723) | 0.374 (0.404) |
A total of 17, 3, and 1 standard green vases from Tampa (both seasons), St. Petersburg, and Bradenton, respectively, were either lost or damaged. Lack of wet vases sometimes yielded samples of fewer than 15 resident vases in a cemetery
Seasonal patterns relating to condition-specific competition
As predicted, the proportions of occupied resident vases in Tampa cemeteries were significantly greater in the late rainy season compared to the early rainy season for both A. aegypti (t = −2.76, P < 0.022) and A. albopictus (t = −5.73, P < 0.0001) (Table 1). In the early rainy season, more vases were occupied by A. aegypti than A. albopictus, but in the late season A. albopictus was more frequent (Table 1). When pooling data across all cemeteries, the proportion increase in vase occupancy from early to late season was clearly greater for A. albopictus (485%, 0.553/0.114) than A. aegypti (290%, 0.374/0.129).
Compared with resident vases, proportions of standard green vases in Tampa that were occupied by A. albopictus and A. aegypti showed similar seasonal trends, although proportions for some individual cemeteries (e.g., June at Orange Hill) differed considerably (Table 1). When pooling data from resident and standard vases, A. albopictus was collected from two cemeteries and A. aegypti from one cemetery during September at which they were not collected during June (Table 1). Neither species was sampled at a cemetery in June and then not found in either resident or standard vases in September.
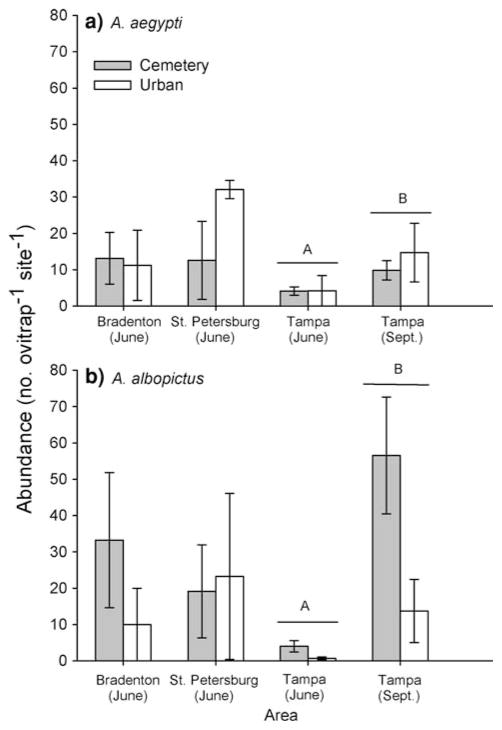
ANOVAs on Tampa data showed significantly higher densities of both species in standard vases in the late rainy season compared to early in the rainy season (Table 2, Fig. 1). There was no significant interaction between season and site type, indicating that the season effect was consistent between cemeteries and urban sites.
Table 2.
Nested ANOVAs on abundance of A. aegypti and A. albopictus in standard green vases from Tampa, St. Petersburg, and Bradenton and black ovitraps from Tampa
|
A. aegypti |
A. albopictus |
|||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Standard green vases | ||||||
| Tampa | ||||||
| Season | 1, 12.8 | 17.94 | 0.0010* | 1, 11.7 | 13.65 | 0.0032* |
| Site type | 1, 12.5 | 1.01 | 0.3344 | 1, 11.6 | 1.95 | 0.1886 |
| Season × site type | 1, 13.1 | 0.97 | 0.3432 | 1, 11.8 | 1.23 | 0.2891 |
| Site (site type) | 11, 11 | 1.38 | 0.3019 | 11, 11 | 1.33 | 0.3238 |
| Season* site (site type) | 1, 13.1 | 0.92 | 0.3432 | 1, 11.8 | 1.23 | 0.2891 |
| St. Petersburg | ||||||
| Site type | 1, 5.9 | 0.15 | 0.7147 | 1, 5.9 | 0.51 | 0.5012 |
| Site (site type) | 6, 29 | 4.47 | 0.0025* | 6, 26 | 4.31 | 0.0032* |
| Bradenton | ||||||
| Site type | 1, 5.0 | 0.92 | 0.3806 | 1, 5.0 | 0.17 | 0.7003 |
| Site (site type) | 5, 27 | 2.54 | 0.0521* | 5, 27 | 3.29 | 0.0190* |
| Black ovitraps in Tampa | ||||||
| Land use | 2, 69.3 | 12.64 | <0.0001* | 2, 64.4 | 1.96 | 0.1495 |
| Site (land use) | 54, 75 | 1.51 | 0.0499* | 54, 75 | 2.22 | 0.0007* |
A total of 48 black ovitraps were lost (26.7%) during the Tampa-wide study in September, including all three traps at four grid cells, giving a total of 56 replicated sample areas
Significant effect
Fig. 1.
Abundance (mean ± SE) of a native Aedes aegypti and b invasive Aedes albopictus collected in standard green vases in cemeteries (grey bars) or urban areas (white bars) from metropolitan Tampa, Florida in 2006: Bradenton and St. Petersburg in June and Tampa in June and September. Significant (P < 0.05) differences between June and September was found for both species in Tampa, denoted by different letters above bars
Spatial patterns among habitat patches
ANOVAs on Tampa data showed no difference in abundances for both species between cemeteries vs urban sites (Table 2, Fig. 1). Non significant differences between cemeteries and urban sites in the abundances of both species were also seen in June in both St. Petersburg and Bradenton (Table 2).
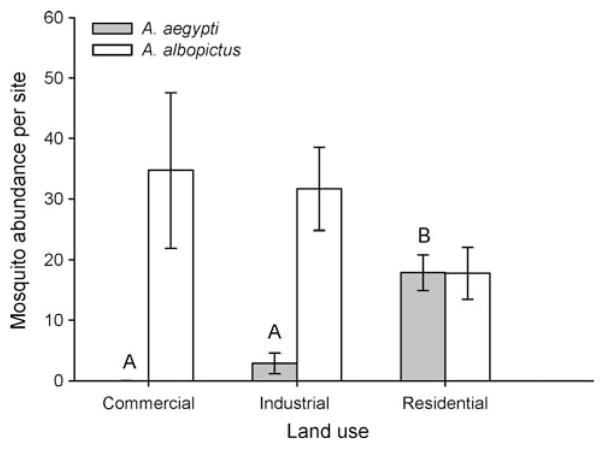
Consistent with results from standard green vases, most individual ovitraps from Tampa in the late rainy season were colonized by Aedes (73.5%, 97/132). When data were grouped by sample site, only 12.5% (7/56) of sites had no Aedes. Aedes albopictus was collected in relatively similar numbers in different types of land use, whereas A. aegypti was collected predominantly from residential sites and never collected from commercial sites (Fig. 2). Abundance of A. aegypti but not A. albopictus differed significantly among land use types (Table 2). Multiple pairwise comparisons showed that A. aegypti oviposition was greater in residential sites compared with commercial and industrial sites (P = 0.0234), but not different between commercial and industrial sites (P = 0.2344–0.5678) (Fig. 2).
Fig. 2.
Abundance (mean ± SE) of A. aegypti and A. albopictus collected in ovitraps from commercial, industrial or residential land use sites in Tampa. Significant (P < 0.05) differences between types of land use was found only for A. aegypti, determined a posteriori by Tukey’s test and denoted by different letters above bars
Spatial patterns among individual containers
Aedes albopictus and A. aegypti were significantly negatively associated in resident cemetery vases in both June and September, but not green cemetery vases or black ovitraps (Table 3). Both species were highly significantly aggregated among green vases and black ovitraps (Table 4).
Table 3.
Association of A. albopictus and A. aegypti using Mantel-Haentzel tests for resident cemetery vases and standard green cemetery vases, and Fishers Exact tests for black ovitraps
| Data set | χ2 | P |
|---|---|---|
| Resident vases in Tampa | ||
| June | 12.30 | 0.0005* |
| September | 8.55 | 0.0001* |
| Standard green vases | ||
| Tampa, June | 0.188 | 0.665 |
| Tampa, Sept | 3.140 | 0.076 |
| St. Petersburg | 0.042 | 0.838 |
| Bradenton | 1.561 | 0.212 |
| Black ovitraps in Tampa | ||
| Commercial | – | – |
| Industrial | – | 0.202 |
| Residential | – | 0.109 |
For Mantel-Haentzel tests df = 1. There were insufficient data for a test of ovitraps in commercial sites
Significant effect
Table 4.
Aggregation of A. albopictus and A. aegypti among resident cemetery vases, standard green vases, and black ovitraps using the criteria of s2/Y and goodness-of-fit for the Poisson distribution
| Data set |
A. albopictus |
A. aegypti |
||||||
|---|---|---|---|---|---|---|---|---|
| s2/m | χ2 | df | P | s2/m | χ2 | df | P | |
| Standard green vases | ||||||||
| Tampa, June | 22.81 | 646.74 | 6 | <0.0001* | 24.32 | 432.13 | 4 | <0.0001* |
| Tampa, Sept | 65.37 | 340.84 | 7 | <0.0001* | 20.94 | 306.81 | 10 | <0.0001* |
| St. Petersburg | 102.25 | 40.22 | 4 | <0.0001* | 39.84 | 42.33 | 1 | <0.0001* |
| Bradenton | 70.18 | 95.36 | 4 | <0.0001* | 41.12 | 157.59 | 6 | <0.0001* |
| Black ovitraps | ||||||||
| Commercial | 129.62 | 70.07 | 7 | <0.0001* | – | – | – | – |
| Industrial | 36.28 | 13.40 | 2 | <0.0001* | – | – | – | – |
| Residential | 43.03 | 577.24 | 7 | <0.0001* | 30.81 | 763.39 | 14 | <0.0001* |
There were insufficient data for a test of A. aegypti among ovitraps in commercial and industrial sites
All effects were significant
Discussion
Although A. albopictus is usually superior in competition to A. aegypti, these species coexist at some sites in the southeastern United States, including metropolitan Tampa. The results of this field study in metropolitan Tampa show clear patterns in temporal and spatial abundances of A. aegypti and A. albopictus that are consistent with seasonal condition-specific competition and local aggregation as potential mechanisms of coexistence.
The proportion of resident cemetery vases that were occupied by Aedes and numbers of larvae in standard green vases were significantly greater late in the rainy season compared to early in the rainy season for both species. This result is consistent with numerous studies showing that the seasonal patterns of abundance of both A. albopictus and A. aegypti are linked with local rainfall (e.g., Barrera et al. 2006; Serpa et al. 2006). Consistent with the condition-specific competition hypothesis, the increase in proportion occupancy and larval abundance per vase between early vs late rainy season was clearly greater for A. albopictus than for A. aegypti. Based on laboratory evidence (Costanzo et al. 2005), this result suggests low numbers of A. albopictus in the early rainy season are a result of higher egg mortality in the dry season leading up to that sample, and the more limited increase of A. aegypti during the wet season is consistent with strong negative effects of interspecific competition on A. aegypti during the wet season. Our results, combined with the findings of Costanzo et al. (2005) provide evidence that condition-specific competition is a mechanism that may contribute to persistence of A. aegypti in south Florida. These results are consistent with theoretical work (e.g., Chesson and Huntly 1997) indicating that simple population reductions caused by environmental harshness are insufficient for species coexistence, but rather that differential response of competitors to environmental variation is required.
Aedes aegypti and Aedes albopictus were independently aggregated among experimental containers, a result consistent with the spatial independence mechanism of the aggregation hypothesis. Such aggregation is likely to result in high intraspecific competition among hatching larvae, and spatially low interspecific competition if the aggregation is greater within each species than across species (Ives 1988; Chesson 2000b; Hartley and Shorrocks 2002). Consistent with low aggregation between species, Aedes aegypti and A. albopictus were negatively associated in resident cemetery vases, but showed no significant negative association in experimental containers. These results suggest that although these Aedes species may oviposit independently of one another, the conditions present in resident vases (including competitive interactions) affect Aedes larvae over time so that developing larvae become negatively associated. Even within one cemetery, resident vases vary considerably in their biotic and abiotic conditions (O’Meara et al. 1995), and such environmental variation among vases may favor either A. aegypti or A. albopictus. It is also likely that these Aedes species choose to oviposit in containers with particular conditions, and that observed aggregation of each species is partly caused by attraction environmental cues that have some independence (Chesson 2000b). Aggregation caused both by random processes and cuing on the environment may reduce the competitive impact of A. albopictus on A. aegypti and facilitate the coexistences of these species. Although we did not measure larval abundances in resident vases, it is likely that A. albopictus larvae were aggregated, at least upon hatching and before larval interactions occur, in resident vases as A. albopictus eggs were in experimental vases and ovitraps.
We found no difference in abundances of A. aegypti and A. albopictus between cemeteries and randomly selected areas in the intervening urban matrix, a result inconsistent with the colonization-competition tradeoff and suggesting that these species do not form metapopulations within habitat patches in urban areas, but rather form a more contiguous population with varying abundances across a mosaic of urban habitats of varying suitability.
Standard ovitrapping across Tampa in the late rainy season indicated spatial partitioning between Aedes species by land use. Aedes aegypti was more abundant in residential areas, compared to sites in industrial and commercial areas. Aedes albopictus had the lowest mean abundance at residential sites compared to industrial and commercial sites, although this trend was not significant. Higher abundances of A. aegypti in ovitraps placed in residential areas may arise if A. aegypti avoids oviposition in commercial and industrial areas dominated by A. albopictus already inhabiting containers. Some mosquitoes alter their oviposition behavior in response to conspecifics or to controphic non-mosquito larvae (e.g., Mokany and Shine 2003) and conspecific eggs (Chadee et al. 2002) already present in the habitat. However, we are unaware of any evidence for oviposition deterrence by other competing mosquito species, and this is an area for future research. Another explanation for higher A. aegypti oviposition in residential areas is that females may choose to oviposit where conditions in the aquatic habitat are more suited to the physiological tolerances of A. aegypti larvae, implying that the environmental conditions of domestic containers are closer to optimal than those in industrial and commercial areas. Our ovitraps likely gave an accurate representation of species distributions in resident containers among the different land uses.
A third and probably most plausible explanation for species-specific differences in oviposition ecology may be the attraction of female A. aegypti to higher densities of humans, the preferred bloodmeal hosts of this highly anthrophilic species (Harrington et al. 2001). Aedes albopictus takes its bloodmeals from a broader range of hosts (Gomes et al. 2003) and is likely to derive a greater proportion of its energy requirements from vegetation-based sugar (Braks et al. 2006). Total vegetation and host number may not be concentrated in any particular land use, thus explaining their generalist oviposition patterns.
Local habitat segregation in oviposition between A. albopictus and A. aegypti has been well documented along rural to urban gradients (Braks et al. 2003; Rey et al. 2006). Braks et al. (2003) showed that in zones of sympatry, A. albopictus is more common in rural areas, A. aegypti predominates in urban areas and the two species overlap in suburban areas (Braks et al. 2003). Relative to other land uses, urban areas sampled by Braks et al. (2003) were characterized by high human densities that likely provided numerous human bloodmeals and immature habitats; two shared factors in residential areas in our study. Rey et al. (2006) related Aedes abundance to a range of habitat variables in three counties in South Florida and found that A. aegypti was only positively correlated with the urban-related variable, buildings, and negatively related to variables related to more rural or open settings (bare ground, canopy vegetation, mixed vegetation, and unpaved road). In contrast, A. albopictus abundance was significantly negatively related to urbanization-related variables (building, paved road, and open building) but positively related to rural variables, such as ground vegetation, unpaved road, and bare ground. Carbajo et al. (2006) analyzed the spatial pattern of A. aegypti oviposition in Buenos Aries and its relationship with demographic and environmental variables, showing A. aegypti oviposition was higher in areas that had higher densities of houses and were closer to industrial sites, and lower in areas with higher human populations or higher densities of flats. To our knowledge, our study is the first to compare the spatial patterns of competing mosquitoes at the within-city scale and relate them across different land uses in an entirely urban environment.
Although A. albopictus and A. aegypti densities do not appear specifically associated with cemeteries, the patchiness of urban landscapes still may contribute to the coexistence of these competitors. Such a scenario would likely foster the coexistence of the inferior competitor A. aegypti with A. albopictus given that A. aegypti starts the rainy season with the numerical advantage. Aedes aegypti may be able to escape competitive exclusion at the city scale despite extinctions in localized areas as long as it persists until the end of the rainy season when it regains competitive advantage. These findings suggest that the roles of specific habitat variables, including vegetative, topographic, and demographic factors, in driving Aedes oviposition (and species coexistence) in purely urban environments merits further investigation, and will be needed to assess accurately local disease risk in densely populated cities.
The results of this field study suggest seasonal condition-specific competition, aggregation among individual containers, and spatial partitioning by land use may all foster the coexistence of A. albopictus and A. aegypti in Tampa, Florida. Identifying habitat distribution patterns and environmental variation associated with land uses within cities may help the management of key vector species and influence the spread of disease in many regions.
Acknowledgments
We thank G.F. O’Meara, S.S. Loew, K. Damal, M. Reiskind, L.P. Lounibos, and two anonymous referees for useful comments, and the Mosquito and Aquatic Weed Control Center of Hillsborough County, FL for their use of facilities and resources. This experiment was funded by NIAID grant R01-AI-44793 and by a grant to SAJ from Illinois State University. Experiments comply with the laws of the United States.
Footnotes
Communicated by Craig Osenberg.
Contributor Information
Paul T. Leisnham, Department of Environmental Science and Technology, University of Maryland, College Park, MD 20742-2315, USA, e-mail: leisnham@umd.edu
S. A. Juliano, School of Biological Sciences, Behavior, Ecology, Evolution, and Systematics Section, Illinois State University, Normal, IL 61790-4120, USA
References
- Amarasekare P, Nisbet RM. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am Nat. 2001;158:572–584. doi: 10.1086/323586. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthrophilic mosquito species. Med Vet Entomol. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno V, Mouquet N, Jarne P, David P. Coexistence in a metacommunity: the competition-colonization trade-off is not dead. Ecol Lett. 2006;9:897–907. doi: 10.1111/j.1461-0248.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- Carbajo AE, Curto SI, Schweigman NJ. Spatial distribution pattern of oviposition in the mosquito Aedes aegypti in relation to urbanization in Buenos Aires: southern fringe bionomics of an introduced vector. Med Vet Entomol. 2006;20:209–218. doi: 10.1111/j.1365-2915.2006.00625.x. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Corbet PS, Greenwood JJD. Egg-laying yellow fever mosquitoes avoid sites containing eggs laid by themselves or by conspecifics. Entomol Exp Appl. 2002;57:1990. [Google Scholar]
- Chase JM, Leibold MA. Spatial scale dictates the productivity-biodiversity relationship. Nature. 2002;416:427–429. doi: 10.1038/416427a. [DOI] [PubMed] [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000a;31:343–366. [Google Scholar]
- Chesson P. General theory of competitive coexistence in spatially-varying environments. Theor Popul Biol. 2000b;58:211–237. doi: 10.1006/tpbi.2000.1486. [DOI] [PubMed] [Google Scholar]
- Chesson PL, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geographic Mapping Section. Florida land use, cover and forms classification system handbook. 3. Florida Department of Transportation Surveying and Mapping Office; Tallahassee, FL: 1999. [Google Scholar]
- Gerhardt RR, et al. The first isolation of La Crosse virus from naturally occurring infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AC, Silva NN, Marques GRAM, Brito M. Host-feeding patterns of potential human disease vectors in the Paraiba Valley Region, State of Sao Paulo. Brazil J Vector Biol. 2003;28:74–78. [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Hartley S, Shorrocks B. A general framework for the aggregation model of coexistence. J Anim Ecol. 2002;71:651–662. [Google Scholar]
- Heard SB, Remer LC. Clutch-size behaviour and coexistence in ephemeral-patch competition models. Am Nat. 1997;150:744–770. doi: 10.1086/286092. [DOI] [PubMed] [Google Scholar]
- Ibañez-Berñal SB, Briseño JP, Mutebi EA, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Ives AR. Covariance, coexistence and the population dynamics of two competitors using a patchy resource. J Theor Biol. 1988;133:345–361. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition. Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion. Oecologia. 2004;194:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Mokany A, Shine R. Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Aust Ecol. 2003;28:33–37. [Google Scholar]
- O’Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. J Med Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS user’s guide: statistics. Version 9.1. SAS Institute Inc; Cary, NC: 2003. [Google Scholar]
- Serpa LLN, Costa K, Voltolini JC, Kakitani I. Seasonal variation of Aedes aegypti and Aedes albopictus in a city of Southeastern Brazil. Rev Saude Publica. 2006;40:1101–1105. [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3. Freeman; New York: 1995. [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton, NJ: 1982. [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]