Abstract
Both glutamate and nitric oxide (NO) may play an important role in cardiovascular reflex and respiratory signal transmission in the nucleus tractus solitarii (NTS). Pharmacological and physiological data have shown that glutamate and NO may be linked in mediating cardiovascular regulation by the NTS. Through tract tracing, multiple-label immunofluorescent staining, confocal microscopic, and electronic microscopic methods, we and other investigators have provided anatomical evidence that supports a role for glutamate and NO as well as an interaction between glutamate and NO in cardiovascular regulation in the NTS. This review article focuses on summarizing and discussing these anatomical findings. We utilized antibodies to markers of glutamatergic neurons and to neuronal NO synthase (nNOS), the enzyme that synthesizes NO in NTS neurons, to study the anatomical relationship between glutamate and NO in rats. Not only were glutamatergic markers and nNOS both found in similar subregions of the NTS and in vagal afferents, they were also frequently colocalized in the same neurons and fibers in the NTS. In addition, glutamatergic markers and nNOS were often present in fibers that were in close apposition to each other. Furthermore, N-methyl-D-aspartate (NMDA) type glutamate receptors and nNOS were often found on the same NTS neurons. Similarly, alpha-amino-3-hydroxy-5-methylisoxozole-proprionic acid (AMPA) type glutamate receptors also frequently colocalized with nNOS in NTS neurons. These findings support the suggestion that the interaction between glutamate and NO may be mediated both through NMDA and AMPA receptors. Finally, by applying tracer to the cut aortic depressor nerve (ADN) to identify nodose ganglion (NG) neurons that transmit cardiovascular signals to the NTS, we observed colocalization of vesicular glutamate transporters (VGluT) and nNOS in the ADN neurons. Thus, taken together, these neuroanatomical data support the hypothesis that glutamate and NO may interact with each other to regulate cardiovascular and likely other visceral functions through the NTS.
Keywords: aortic depressor nerve, glutamate, nodose ganglion, neuronal nitric oxide synthase, vesicular glutamate transporters, N-methyl-D-aspartate, alpha-amino-3-hydroxy-5-methylisoxozole-proprionic acid
1. Introduction
Numerous neurotransmitters and modulators are present in the nucleus tractus solitarii (NTS), which plays a major role in regulation of many physiological functions that include cardiovascular, gustatory, respiratory, hepatic and renal function in several mammalian species (Lawrence and Jarrott, 1996; Bradley et al., 1996). Among neurotransmitters and modulators, the excitatory amino acid glutamate is considered to be a neurotransmitter at cardiovascular, respiratory and gustatory afferent terminals in the NTS (Talman, 1994; Lawrence and Jarrott, 1996; Bradley et al., 1996). Some studies also suggest that nitric oxide (NO), which produces depressor and bradycardia responses similar to those elicited by glutamate, may also participate in cardiovascular and respiratory signal transduction in the NTS (Machado and Bonagamba, 1992; Ogawa et al., 1995). Furthermore, these two neurotransmitters may interact with each other to modulate cardiovascular function and gastrointestinal functions in the NTS, as suggested by many pharmacological studies (Di Paola et al., 1991; Krowicki et al., 1997; Lo et al., 1997; Lin et al., 2000a; Talman et al., 2001; Matsuo et al., 2001; Yamanashi et al., 2002; Dias et al., 2003; Talman and Lin, 2006). The suggestion that NO and glutamate may interact in the NTS is also supported by some neuroanatomical studies. This article will focus on reviewing these chemical neuroanatomical data from our own studies as well as from other laboratories that support the role of glutamate and NO and their interaction in signal transmission in the NTS. Most of these studies were performed in anesthetized rats, unless indicated otherwise.
2. Physiological and pharmacological support for actions and interactions of NO and glutamate in the NTS
Many studies have focused on identification of neurotransmitters involved in mediating physiological functions controlled by the NTS and on interactions between numerous putative transmitters found in the NTS of several mammalian species (Lawrence and Jarrott, 1996; Bradley et al., 1996; Jordan, 2005; Potts, 2006). Studies of the excitatory amino acid glutamate and its role in cardiovascular regulation through the NTS have demonstrated that glutamate is present in the NTS and the NG, the site of neurons that give rise to vagal afferents that terminate in the NTS of rats, cats and lambs (Takayama and Miura, 1992; Sweazey, 1995; Sykes et al., 1997). Electrical or natural stimulation of baroreceptor afferents may cause release of glutamate from transmitter stores in the NTS of rats and cats (Granata and Reis, 1983; Meeley et al., 1989; Gordon and Talman, 1992; Lawrence and Jarrott, 1994; Allchin et al., 1994; Ohta and Talman, 1996) where it may bind to glutamate receptors and evoke cardiovascular responses like those produced by activation of cardiovascular reflexes (Talman et al., 1980; Gordon and Talman, 1992). Furthermore, glutamate receptor antagonists block cardiovascular reflexes after their injection into the rat NTS (Ohta and Talman, 1994). Therefore, it is apparent that glutamate plays a critical role in cardiovascular reflex transmission as it may for other visceral reflex transmission within the NTS.
Nitric oxide (NO), like glutamate, may also participate in cardiovascular and respiratory signal transduction in the rat NTS (Machado and Bonagamba, 1992; Ogawa et al., 1995). Microinjection of S-nitrosocysteine, an NO donor, or L-arginine, an NO-precursor, into the NTS produces hypotension and bradycardia in both anaesthetized and conscious rats (Lewis et al., 1991; Machado and Bonagamba, 1992). L-nitro-arginine methyl ester (L-NAME), an NO synthase (NOS) inhibitor, evokes opposite responses (Harada et al., 1993). Thus, NO or NO donors may play a role in transmission of cardiovascular reflex signals in the NTS. The role of NO in the NTS is likely not restricted to cardiovascular control but may include other regulatory functions as well. For example, NO may also participate in gastrointestinal control given that microinjection of L-arginine into the NTS decreases intragastric pressure and microinjection of L-NAME produces an opposite response (Krowicki et al., 1997).
Reports suggest a relationship between actions of glutamate and NO in some regions of brain. For example, activation of glutamate receptors in the cerebellum leads to synthesis and release of NO (Garthwaite et al., 1988; Garthwaite et al., 1989) that in turn activates soluble guanylate cyclase (sGC) to increase cGMP in rats (Garthwaite and Garthwaite, 1987). L-NG-monomethylarginine (L-NMMA), which blocks NO synthesis from L-arginine, inhibits increases of cGMP induced by N-methyl-D-aspartic acid (NMDA), a glutamate receptor agonist (Garthwaite et al., 1989). Through these and other interactions glutamate and NO may jointly play a role in such critical activities as long term potentiation in the hippocampus (Izumi et al., 1992) and long term depression in the cerebellum (Shibuki and Okada, 1991). Similar to these areas of the brain, interactions between glutamate and NO may also exist in the NTS. It was reported that NO release may be linked to glutamate receptor activation in the NTS (Di Paola et al., 1991; Wang et al., 2007) and that NO in the NTS may lead to release of glutamate (Lawrence and Jarrott, 1993). Depressor and bradycardia effects of glutamate or NMDA microinjected into the NTS were blocked by prior administration of NO synthase (NOS) inhibitors, while NMDA and non-NMDA receptor antagonists attenuated depressor and bradycardic effect of NO precursor L-arginine (Lo et al., 1997). Furthermore, an increase in NO production, indexed by the levels of nitrite and nitrate in dialysate, has been observed after injection of NMDA into the NTS (Matsuo et al., 2001) and release of glutamate was inhibited by a NOS inhibitor introduced into the NTS (Matsuo et al., 2001). Thus, these pharmacological data indicate that NO and glutamate may interact with each other in the NTS, as was found in the hippocampus and the cerebellum, in modulating cardiovascular regulation.
3. Anatomical support for interaction between NO and glutamate in the NTS
3.1. Distribution of glutamate in the NTS
We have used immunofluorescent staining to study the distribution of glutamate in the rat NTS (Lin et al., 2000b). Although the antibody we used has been demonstrated to be very specific by immunoblot analysis and immunoadsorption studies (Hepler et al., 1988), we recognized that it may stain both metabolic pools, as well as neurotransmitter pools of glutamate (Fonnum, 1984). However, while it is not possible to identify conclusively the metabolic pool of glutamate in the tissue, it has been assumed that neurons and fibers that are enriched with glutamate when compared with background labeling represent those utilizing glutamate as a neurotransmitter (Saha et al., 1995b). Consistent with other reports in rats (Dun et al., 1994; Krowicki et al., 1997), our study showed that glutamate is present in many fibers and neurons in all NTS subnuclei throughout the NTS. The central subnucleus contained a very high density of glutamate-containing neurons while the dorsolateral, medial and interstitial subnuclei contained a moderate density of glutamate-containing neurons.
3.2. Distribution of nNOS in the NTS
Nitric oxide (NO) is formed via the oxygenation of L-arginine by the catalytic enzyme NOS. There are three isoforms of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) (Moncada and Higgs, 1991; Knowles and Moncada, 1994). While iNOS is scarcely present in the rat NTS (Lin et al., 2007), the two constitutive forms of NOS, nNOS and eNOS, have been documented to be present in the rat NTS (Dun et al., 1994; Krowicki et al., 1997; Lin et al., 1998; Lin et al., 2007). Using antibodies generated against nNOS, immunofluorescent staining of the rat NTS has shown that nNOS is present in neurons and fibers of all NTS subnuclei (Dun et al., 1994; Krowicki et al., 1997; Lin et al., 1998). Consistent with immunofluorescent staining findings, nNOS mRNA is also observed in the NTS via in situ hybridization (Lin et al., 1997). Variations in the intensity of nNOS immunoreactivity and density of stained neurons among different NTS subnuclei suggest that the amount of nNOS varies from one subnucleus to another. In general, more nNOS containing neurons and fibers are observed in the rostral rat NTS than in the caudal rat NTS (Dun et al., 1994; Krowicki et al., 1997; Lin et al., 1998). The highest density of nNOS containing fibers and neurons is found in the central subnucleus, an area that receives visceral afferents from the stomach, mouth and esophagus in several mammalian species that include rat, cat and monkey (Hamilton and Norgren, 1984; Gwyn et al., 1985; Altschuler et al., 1989). This finding may suggest a role for NO in gustatory regulation through the central subnucleus. Other subnuclei, for example the dorsolateral, commissural, medial and interstitial subnuclei, demonstrate a moderate density of nNOS containing neurons and fibers. These subnuclei receive visceral afferents from baroreceptors and the carotid body (Ciriello, 1983; Housley et al., 1987). Finding nNOS in neurons in these areas supports pharmacological findings that NO may be involved in regulation of blood pressure and heart rate (Lewis et al., 1991; Machado and Bonagamba, 1992).
3.3. Colocalization of nNOS and glutamate in the NTS
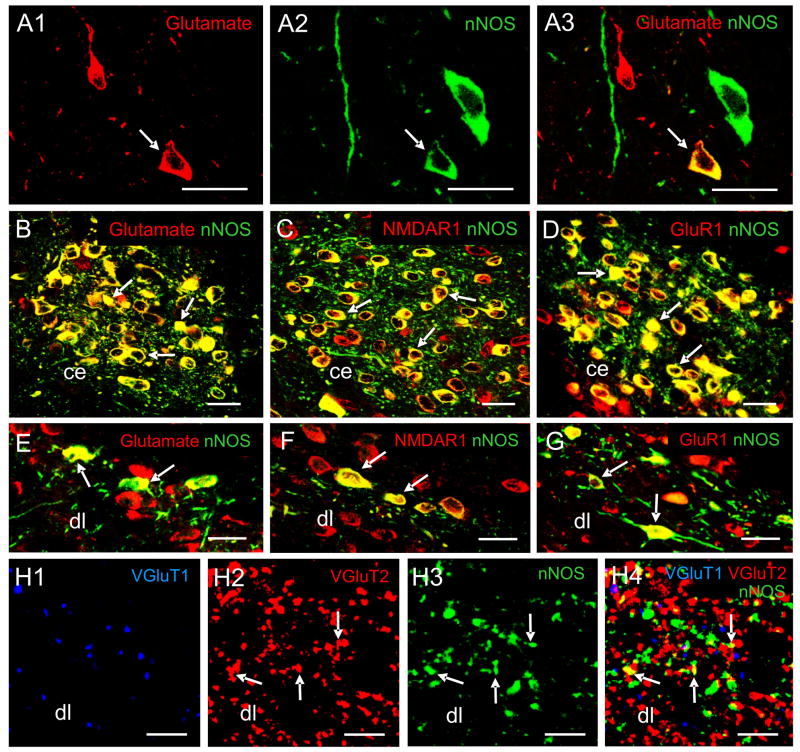
We used confocal laser scanning microscopy to examine the distribution of glutamate and nNOS in the rat NTS after brain stem sections had undergone immunofluorescent labeling for glutamate and nNOS (Lin et al., 2000b). Glutamate-immunoreactive (IR) and nNOS-IR cells and fibers were distributed in homologous regions of the NTS and proximate to each other. In addition, many neurons and fibers throughout all subnuclei of the NTS were both glutamate-IR and nNOS-IR (representative subnuclei are shown in Fig. 1, panels A1-3, B and E). Similar results have been reported in the gustatory region of the NTS when nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase was used as a histochemical marker of NOS and was combined with glutamate immunohistochemistry (Maqbool et al., 1995).
Fig. 1.
Pseudo-colored confocal images showing multiple-label immunofluorescent staining of NTS cells, fibers, and subnuclei. A1–A3: A merged image (A3) of NTS cells demonstrates glutamate-IR cells (red, A1) and nNOS-IR neurons (green, A2). The arrow in A1–A3 indicates an NTS neuron that is double-labeled for glutamate and nNOS and appears yellow. B–D: The central subnucleus (ce) contains many neurons that are double-labeled (examples are indicated by arrows) for glutamate (red) and nNOS (green) (B), for NMDAR1 (red) and nNOS (green) (C), and for GluR1 (red) and nNOS (green) (D). E–G: The dorsolateral subnucleus (dl) contains neurons that are double-labeled (examples are indicated by arrows) for glutamate (red) and nNOS (green) (E), for NMDAR1 (red) and nNOS (green) (F), and for GluR1 (red) and nNOS (green) (G). H1–H4: A merged image (H4) of the dl at a high magnification demonstrates fibers labeled for VGluT1 (blue, H1), VGluT2 (red, H2), nNOS (green, H3). Fibers that are double-labeled with nNOS (red) and VGluT2 (green) appear yellow (indicated by arrows). Scale bar = 5 μm in H1–H4, 20 μm in other panels. Data were extracted from previous publications (Lin et al., 1998; Lin and Talman, 2000; Lin et al., 2000b; Lin and Talman, 2001; Lin and Talman, 2002; Lin et al., 2004) with permission.
We further analyzed the percentage of glutamate-IR cells that were additionally stained for nNOS in rat NTS subnuclei (Lin et al., 2000b). We observed that 86 ± 7% of glutamate-IR cells also nNOS-IR in the central subnucleus (Fig. 1B). This subnucleus not only receives projections from the stomach, mouth and esophagus, as mentioned earlier, but also nNOS neurons in this subnucleus may act as interneurons in a central pathway connecting esophageal afferents and efferents (Gai et al., 1995). Based on the observation that a high percentage of neurons in the central subnucleus contain both nNOS and glutamate, we conjecture that this subnucleus may mediate gustatory functions through an intracellular interaction between glutamate and NO. However, to date, there are no pharmacological data available regarding an interaction between NO and glutamate in mediating gustatory functions by the NTS.
The percentages of glutamate-IR cells that were also nNOS-IR were 19% to 28% in other rat NTS subnuclei except the medial subnucleus where a lower percentage (10 ± 7%) was found. We also analyzed the percentage of nNOS-IR cells that were additionally stained for glutamate in NTS subnuclei. The central nucleus again had the highest percentage (83 ± 13%) of nNOS-IR neurons that were also glutamate-IR. The percentages of nNOS-IR neurons that were also glutamate-IR were 20% to 64% in the other subnuclei except the commissural subnucleus where a lower percentage (9 ± 8%) was found. Although lower percentages of double-labeled neurons were present in the dorsolateral and commissural subnuclei potential contacts between glutamate-IR fibers/perikarya and nNOS-IR fibers/perikarya were often observed. Therefore, interaction between glutamate and NO may exist in subregions of the NTS even when colocalization of nNOS and glutamate may be infrequent.
4. Additional anatomical support: Colocalization of nNOS and vesicular glutamate transporters (VGluT) in the NTS
Because glutamate immunohistochemistry cannot distinguish the metabolic pool of glutamate from the neurotransmitter pool; a better marker for glutamatergic neurotransmission was desired. Vesicular glutamate transporters (VGluT) have been identified and have been recognized as markers (Fremeau et al., 2001; Fujiyama et al., 2001; Stornetta et al., 2002) for glutamatergic neurons and their axon terminals (Takamori et al., 2000). They are considered better markers of glutamatergic processes than glutamate, glutaminase, or plasma membrane excitatory amino acid transporters, which have also been used to identify glutamatergic neurons (Kaneko et al., 2002). Currently 3 types of VGluT are recognized: VGluT1, VGluT2 and VGluT3 (Takamori et al., 2000; Fremeau et al., 2001; Fujiyama et al., 2001; Gras et al., 2001; Stornetta et al., 2002; Fremeau et al., 2002). Immunohistochemical studies revealed that all three forms of VGluT are present in the rat NTS (Kaneko et al., 2002; Stornetta et al., 2002; Lin et al., 2004; Lin and Talman, 2005a) and that some of them colocalize with nNOS in the rat NTS as detailed below (Lin et al., 2004; Corbett et al., 2005; Lin and Talman, 2005a).
4.1. Distribution of VGluT1 and VGluT2 in the NTS
Our study showed that the rat NTS contained lower levels of VGluT1-IR in neuronal processes, but VGluT1-IR was not present in the cell bodies or primary dendrites. Finding VGluT1 in neuronal processes and not in the cell bodies in the NTS is consistent with results from other areas of rat brain (Fremeau et al., 2001; Fujiyama et al., 2001). A very high level of VGluT1 immunoreactivity was present in dense fibers in the gracilis and cuneatus nuclei, especially at the level of and caudal to the area postrema (Lin et al., 2004). VGluT1-IR fibers were present in moderate density in the lateral and interstitial subnuclei of the NTS. A low density of VGluT1-IR fibers was found in the rest of the subnuclei that included dorsolateral, medial and central subnuclei. As was seen with VGluT1-IR, VGluT2-IR structures also exhibited a punctate pattern of fluorescein staining in fibers. That observation replicates results from studies in other areas of the rat brain (Fremeau et al., 2001; Fujiyama et al., 2001). While VGluT1-IR was absent from some subnuclei, VGluT2-IR was present in all subnuclei. VGluT2-IR was found in subnuclei that receive cardiovascular afferents, such as the dorsolateral, commissural and subpostremal subnuclei, as well as those that receive gustatory afferents, such as the central, lateral and interstitial subnuclei of rat and cat (Kalia and Mesulam, 1980; Kalia et al., 1980; Kalia and Sullivan, 1982). These results provide immunohistochemical support for the suggestion that glutamate is one of the neurotransmitters that participate in regulation of numerous physiological functions mediated by the NTS (Talman et al., 1980; Lawrence and Jarrott, 1996; Bradley et al., 1996).
Because both VGluT1-IR and VGluT2-IR were present in some subnuclei of the NTS, we performed double-label immunofluorescent staining for VGluT1 and VGluT2 to examine their relationship in the NTS. Subnuclei of the NTS that contained both VGluT1-IR and VGluT2-IR included the dorsolateral, lateral, ventral, intermediate and interstitial subnuclei. Although both VGluT1 staining and VGluT2 staining were observed in these subnuclei, VGluT1 staining and VGluT2 staining rarely colocalized in any fiber in these subnuclei. For example, the dorsolateral subnucleus contained both VGluT1-IR fibers and VGluT2-IR fibers, but only very rare fibers were positive for both VGluT1 and VGluT2. Therefore, it seems that there is a population of glutamatergic fibers that contain only VGluT2 and another population that contains only VGluT1. The physiologic relevance of this finding requires further investigation, but it is noteworthy that VGluT1 and VGluT2 are often distributed in a complementary fashion in the central nervous system in rat (Herzog et al., 2001; Ziegler et al., 2001; Kaneko and Fujiyama, 2002). This complementary localization of VGluT1 and VGluT2 is found at the axon terminal level in several rat brain regions where both VGluT1 and VGluT2 are present (Kaneko and Fujiyama, 2002). Contrary to these results, VGluT1-IR terminals located in the lateral half of the rat NTS have been reported to display VGluT2-IR (Lachamp et al., 2006). The explanation for this discrepancy is not clear, but it may be due to differences in tissue fixation or antibody dilution between our study and that of Lachamp et al. (Lachamp et al., 2006).
4.2. Colocalization of VGluT2 and nNOS in the NTS
Our double-label immunofluorescent staining of VGluT2 and nNOS revealed that that the density of VGluT2-IR fibers in a rat NTS subnucleus often matched that of nNOS-IR fibers (Lin et al., 2004). For example, the subpostremal and gelatinosus subnuclei, which contained a high density of nNOS-IR fibers, also contained a high density of VGluT2-IR fibers. The ventral, intermediate, interstitial and commissural subnuclei, which contained a low density of nNOS-IR fibers, also contained a low density of VGluT2-IR fibers. On the other hand, there was no such relationship between nNOS-IR fibers and VGluT1-IR fibers in these NTS subnuclei. At high magnification, we found that VGluT2-IR, but not VGluT1-IR, and nNOS-IR colocalized in some fibers in NTS subnuclei (Fig. 1, panels H1-H4). In addition, VGluT2-IR fibers were seen apposed to nNOS-IR fibers. These observations provide additional anatomical support for the hypothesis that glutamate and NO may interact in different subregions of the NTS that are implicated in mediating various gastrointestinal or cardiorespiratory functions.
Finding nNOS as well as vesicular glutamate transporters in presynaptic fibers in the NTS would support potential simultaneous release of NO and glutamate from those same fibers. It would also support potential modulation of glutamate receptors or glutamate-mediated signal transduction by NO acting at the postsynaptic neuron. NO formed in the presynaptic neuron could diffuse into the target (postsynaptic) cell and act directly on sGC to increase levels of cyclic GMP (Southam and Garthwaite, 1993). The finding that nNOS and sGC colocalize in cells and fibers in the rat NTS (Lin and Talman, 2005b) supports such possible interaction between nNOS and sGC in the NTS. Similar modulation of signal transduction through actions of NO could also be effected through actions of glutamate alone released from terminals (VGluT2 positive) in the NTS. Glutamate, thus released, could then act on postsynaptic neurons that express nNOS. On the other hand, NO could also feed back to the presynaptic site to inhibit glutamate release from terminals. Such an intercellular interaction between NO and glutamate has been shown to involve postsynaptic formation of NO triggered by increased levels of intracellular calcium (Knowles et al., 1989; Wang et al., 2006; Wang et al., 2007).
4.3. Distribution of VGluT3 in the NTS
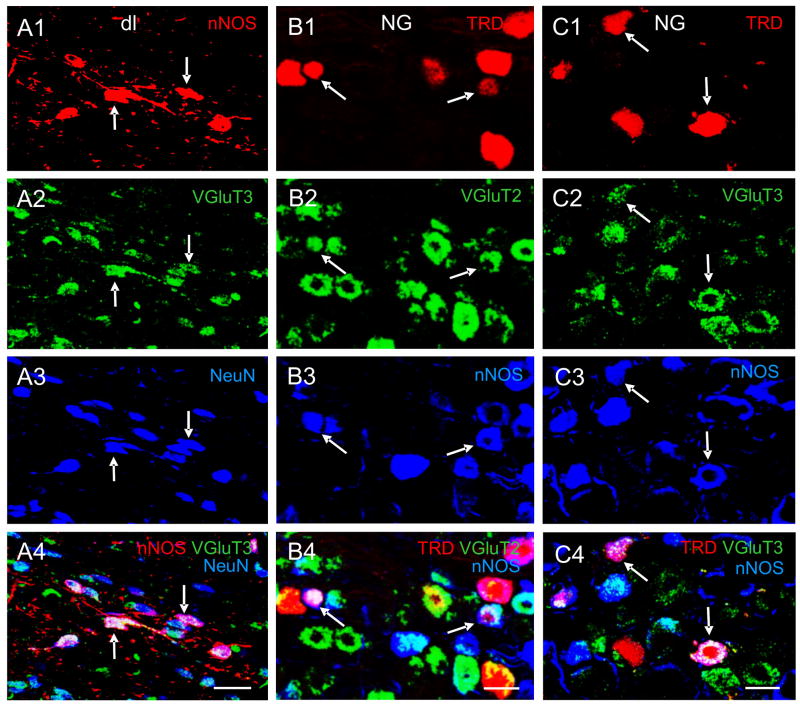
VGluT3 staining in the rat NTS was weaker than in many other brain stem structures such as the gracilis nucleus, hypoglossal nucleus, dorsal motor nucleus of vagus and nucleus ambiguus. In the rat NTS, the densities of cells and processes stained for VGluT3 were higher in the intermediate, medial and interstitial subnuclei than those in the central, dorsolateral, ventral, and commissural subnuclei (Lin and Talman, 2005a). While VGluT1 and VGluT2 staining were observed only in processes, VGluT3 staining was observed in processes as well as cell bodies in the rat NTS (Lin and Talman, 2005a). In fact, staining in cell bodies was often more prominent than in processes. Our observation that VGluT3 was present in the rat NTS is consistent with a previous publication that shows VGluT3 mRNA in the rat NTS (Schafer et al., 2002). In addition, VGlulT3 staining was noted not only in neurons, as demonstrated by its colocalization with a neuronal marker, neuronal nuclear antigen (NeuN) (Fig 2. panelsA2-A3), but also it was seen in glial structures, as demonstrated by its colocalization with a glial marker, glial fibrillary acidic protein (Lin and Talman, 2005a). This observation is in line with findings from other laboratories that show the presence of VGluT3 in axons, dendrites, neurons, astrocytes and glial end feet (Fremeau et al., 2002). In addition, VGluT3 is expressed not only in excitatory, typically glutamatergic, neurons but also at inhibitory synapses and neurons in rat (Gras et al., 2001; Fremeau et al., 2002).
Fig. 2.
Pseudo-colored confocal images showing multiple-label immunofluorescent staining of NTS cells in the dorsolateral subnucleus (dl) subnucleus of the NTS and the NG. A1–A4: A merged image (A4) of the dl demonstrates cells labeled for nNOS (red, A1), VGluT3 (green, A2), NeuN (blue, A3). Neurons that are triple-labeled appear white, as indicated by arrows. B1–B4: A merged image (B4) showing NG cells triple-labeled (indicated by arrows) for TRD (red, B2), VGluT2 (green, B2) and nNOS (blue, B3). C1–C4: A merged image (C4) demonstrating NG cells triple-labeled (indicated by arrows) for TRD (red, C1), VGluT3 (green, C2) and nNOS (blue, C3). Scale bar = 20 μm. Data were extracted from previous publications (Lin and Talman, 2005a; Lin and Talman, 2006) with permission.
Because VGluT1, VGluT2 and VGluT3 were found in the same rat NTS subnuclei, we performed additional double-labeling immunofluorescent staining to examine their relationship in the NTS. Immunoreactivity of VGluT3 never colocalized with either that of VGluT1 or VGluT2 in fibers or cells in NTS subnuclei. The segregation of VGluT3 staining and VGluT1 staining was observed even in areas where the density of VGluT3-IR processes and the density of VGluT1-IR fibers were relatively high, such as the intermediate subnucleus. This can be expected since VGluT3 is found in axons, dendrites, neurons, astrocytes and glial end feet, while VGluT1 and VGluT2 are present solely in axonal terminals in rat (Fremeau et al., 2002). Another report has also shown that VGluT3 was not found in VGluT1 or VGluT2 synapses in the rat neocortex (Schafer et al., 2002). Therefore, although VGLUT3 is structurally and functionally closely related to VGluT1 and VGluT2, the distinct distribution of VGluT3 has prompted the suggestion that the VGluT3 system is a distinct glutamatergic marker of its own and that VGluT3 is neither a part of the VGluT1 nor the VGluT2 system (Schafer et al., 2002). Investigators have suggested that VGluT3 might contribute to exocytotic release of glutamate by non-neural cells (Fremeau et al., 2002).
4.4. Colocalization of VGluT3 and nNOS in the NTS
Our immunofluorescent staining showed that cells stained for both VGluT3-IR and nNOS-IR were observed in all subnuclei of the rat NTS although some subnuclei contained more double-labeled neurons than did others (Lin and Talman, 2005a). Among rat NTS subnuclei, the central subnucleus contained the highest percentage (77 ± 9%) of VGluT3-IR cells that also was nNOS-IR. The percentages of VGluT3-IR cells that also were nNOS-IR in the dorsolateral and medial subnuclei were 42 ± 8% and 48 ± 9%, respectively. On the other hand, the majority of the nNOS-IR neurons, ranging from 80% to 90%, in NTS subnuclei contained VGlu3-IR. These double-labeled cells in the rat NTS likely were neurons because they were additionally stained for NeuN (Fig. 2, panels A1-4). We also noted that some nNOS-IR fibers also were VGluT3-IR in some rat NTS subnuclei. These double-labeled processes likely were fibers because nNOS is found in axons and dendrites, but not in glial processes, in the rat NTS (Lin et al., 1998). Therefore, although VGluT3 is not specifically associated with neurons, the VGluT3-IR fibers that also contain nNOS staining in the NTS likely were of neuronal origin. If that is the case, these data would provide useful information for the hypothesis that glutamate and NO in the NTS may interact in different subregions that are implicated in mediating physiologic functions.
5. Additional anatomical support: Colocalization of nNOS and NMDA receptors in the NTS
5.1. Distribution of NMDA receptors in the NTS
Glutamate receptors may be categorized as ionotropic and metabotropic (Hollmann and Heinemann, 1994; Dingledine et al., 1999). Based on agonist preferences, three classes of ionotropic receptors are recognized: NMDA and two non-NMDA classes, α-amino-3-hydroxy-5-methylisoxozole-proprionic acid (AMPA) and kainic acid (Dingledine et al., 1999). NMDA glutamate receptors in the NTS are capable of modulating the aortic baroreceptor heart rate reflex in rat (Andresen and Kunze, 1994; Ohta and Talman, 1994). They may also participate in the NTS in regulating ventilation (Mizusawa et al., 1994) and swallowing (Kessler and Jean, 1991).
We have studied the distribution of NMDA receptors in the rat NTS by immunofluorescent staining using antibody made against NMDA receptor subunit 1 (NMDAR1), the fundamental subunit of NMDA receptor (Lin and Talman, 2000). The NMDAR1 antibody that we used recognized all splice variants. Consistent with findings in the cat NTS (Ambalavanar et al., 1998), we observed NMDAR1-IR neurons and fibers in all NTS subnuclei, with the highest density of NMDAR1-IR cells being found in the central subnucleus. The medial, dorsolateral, ventral and interstitial subnuclei contained a moderate density of NMDAR1-IR cells. The presence of NMDAR1-IR in these subnuclei supports pharmacological findings that NMDA receptors may play a role in glutamate-mediated functions.
5.2. Colocalization of NMDA receptors and nNOS in the NTS
Our previous reports showed that NMDA-induced depressor responses are attenuated by the nNOS inhibitor 7-nitroindazole and sGC inhibitor in rat (Talman et al., 2001; Chianca, Jr. et al., 2004). Other investigators have found that prior administration of the NMDA receptor antagonist MK-801 significantly attenuated depressor and bradycardiac effects of L-arginine, a precursor of NO in rat (Lo et al., 1997). Because these pharmacological data suggest that NMDA receptors may be involved in the interaction of NO and glutamate, we hypothesized that nNOS and NMDA receptors are colocalized or are found on neuronal structures that lie in close proximity in the NTS. We tested that hypothesis by performing double-label immunofluorescent staining for nNOS and NMDAR1 receptor in the rat NTS (Lin and Talman, 2000). Analysis of confocal microscopic images of the immunofluorescent labeled NTS showed that NMDAR1-IR and nNOS-IR colocalized in neurons and fibers in almost all rat NTS subnuclei (see Fig. 1C and 1F for examples). In addition, NMDAR1-IR cells and fibers were often apposed to nNOS-IR neurons and fibers. While almost all nNOS-IR neurons were NMDAR1-IR, the percentages of NMAR1-IR cells that also were nNOS-IR varied among NTS subnuclei. The central subnucleus contained the highest percentage (94 ± 2%) of double-labeled NMDAR1-IR cells. The percentages of NMDAR1-IR cells that were double-labeled in the other NTS subnuclei ranged from 13% – 34%. Colocalization of NMDA receptors and NOS in the central subnucleus of rat NTS was reported in a previous study, although NADPH diaphorase was used as a marker for NOS (Broussard et al., 1995). Thus, results from our NMDAR1 and nNOS double-labeling study supported the hypothesis that potential interactions between NO and glutamate in the NTS may involve NMDA receptors.
The exact mechanism of NO and glutamate interaction is not known. The interaction may be intercellular and involve formation of NO triggered by increased levels of calcium (Knowles et al., 1989; Wang et al., 2006) as a result of the activation of NMDA type glutamate receptor (Garthwaite et al., 1988). NO may inhibit NMDA receptor-mediated increases in intracellular calcium and calcium current and act as a feedback regulator of the calcium-calmodulin dependent production of NO (Manzoni et al., 1992; Wang et al., 2006). On the other hand, the interaction between glutamate and NO in the NTS may be intracellular and effected through a common transduction pathway that involves sGC (Garthwaite and Garthwaite, 1987; Bredt and Snyder, 1989).
6. Additional anatomical support: Colocalization of nNOS and AMPA receptors in the NTS
6.1. Distribution of AMPA receptors in the NTS
In addition to NMDA receptors non-NMDA glutamate receptors may also play important roles in the cardiovascular regulation in the NTS (Ohta and Talman, 1994; Yen et al., 1999). For example, microinjection of a non-NMDA receptor antagonist blocks depressor responses produced by electrical simulation of the aortic depressor nerve (ADN) in rat (Gordon and Leone, 1991). Likewise the sensitivity of baroreceptor reflex is decreased by introduction of a non-NMDA receptor antagonist into the rat NTS (Ohta and Talman, 1994). Non-NMDA receptors may not only participate in cardiovascular regulation, they may also contribute to responses elicited by stimulation of gustatory afferents as was observed in goldfish and hamster (Smith et al., 1998; Smeraski et al., 1999).
There are 4 types of AMPA-selective glutamate receptor subunits (GluR): GluR1, GluR2, GluR3 and GluR4. Combination of different subunits into hetero-oligomeric complexes contributes to the functional diversity of AMPA receptors (Gasic and Hollmann, 1992). We studied the distribution of AMPA receptors in the rat NTS (Lin and Talman, 2001) using antibody against GluR1. The rat NTS exhibited moderate staining for GluR1 in perikarya, proximal dendrites and processes in all of its subnuclei. A moderate density of stained cells was present in the interstitial, intermediate, dorsolateral, ventral, medial and commissural subnuclei. Although a higher density of GluR1-IR cells was present in the central subnucleus, these cells contained a low level of GluR1-IR. These observations are consistent with studies using immunohistochemical methods (Petralia and Wenthold, 1992; Kessler and Baude, 1999) and in situ hybridization techniques (Sato et al., 1993) to examine the distribution of AMPA receptors in the rat NTS.
6.2. Colocalization of AMPA receptors and nNOS in the NTS
Prior administration of the non-NMDA receptor antagonist, 6,7-dinitroquinoxaline-2,3-dione, significantly attenuated depressor and bradycardiac effects of L-arginine (Lo et al., 1997). Therefore, it is possible that the interaction between NO and glutamate in the NTS may be mediated through AMPA-selective glutamate receptors as well as NMDA receptors. This suggestion is supported by the presence of both nNOS and AMPA-selective glutamate receptors in the NTS (Lin et al., 1998; Ambalavanar et al., 1998; Lin and Talman, 2001). To further delineate the anatomical relationship between nNOS containing neurons and those with AMPA receptors, we performed double-label immunofluorescent staining in the rat NTS (Lin and Talman, 2001). Confocal analysis showed that immunoreactivity of GluR1 and immunoreactivity of nNOS colocalized in neurons and fibers of the rat NTS (see Fig. 1D and 1G for examples). In addition, GluR1-IR cells and fibers were often apposed to nNOS-IR neurons and fibers. While almost all nNOS-IR neurons were also GluR1-IR, only a portion of GluR1-IR cells were nNOS-IR. The central subnucleus had the highest percentage (91 ± 3%) of GluR1-IR cells that also were nNOS-IR, the dorsolateral and medial subnuclei contained 44 ± 9% and 43 ± 5%, respectively.
The high percentages of double-labeled neurons found in the dorsolateral and medial subnuclei suggest that AMPA receptors may be important in putative interactions between NO and glutamate in these subnuclei. Our data are consistent with the hypothesis that the interaction between NO and glutamate may involve non-NMDA receptors (Lo et al., 1997). As discussed earlier, the interaction between NO and glutamate in the NTS may go through NMDA receptors and this interaction may involve formation of NO triggered by increased levels of calcium (Knowles et al., 1989; Wang et al., 2006) as a result of the activation of NMDA type glutamate receptors (Garthwaite et al., 1988). Interestingly, it is now known that non-NMDA receptors can also induce the generation of NO through a calcium-mediated process (Gunasekar et al., 1995; Yamada and Nabeshima, 1997; Wang et al., 2006). Our finding that calcium permeable GluR1 subunit and nNOS are colocalized or apposed to each other in the NTS is consistent with this observation. Similarly, results from this study also suggest that the putative interaction between glutamate and NO in the central subnucleus may in part involve AMPA receptors, even though the level of GluR1-IR is low in neurons of this subnucleus. However, as mentioned before, there are no pharmacological data available to support an interaction between NO and glutamate in mediating gustatory functions by the NTS. Future studies are required to support this hypothesis.
7. Relationship of eNOS and nNOS in the NTS
The role of eNOS in cardiovascular regulation in the NTS has been implicated in recent studies that utilized gene transfer methodology. Researchers have shown that spontaneous baroreceptor reflex gain was increased, while the heart rate was decreased, in rats that had received an adenoviral vector that expresses a dominant negative mutant of eNOS to down-regulate eNOS expression in the NTS (Waki et al., 2003; Waki et al., 2006). In addition, the dominant negative mutant of eNOS also prevented angiotensin II-induced baroreflex attenuation (Paton et al., 2001). While these studies have suggested that NO derived from eNOS has an inhibitory effect on baroreflex transmission in the NTS, results of other studies may suggest a different role for NO from eNOS. For example, increasing NO production in the NTS with an adenoviral vector encoding eNOS caused hypotension and bradycardia in conscious rats (Sakai et al., 2000; Hirooka et al., 2001; Hirooka et al., 2003). Although the authors of the latter studies utilized eNOS as a tool for synthesis of NO in the NTS, their results suggest that NO may be excitatory in the NTS.
While one report briefly mentioned that eNOS positive neurons were not detected by immunohistochemistry in the medulla (Hirooka et al., 2001), another report showed that eNOS staining was found in neurons of the NTS where it colocalized with angiotensin II-IR (Paton et al., 2001). Our own study demonstrated that many brain stem areas, including the NTS, area postrema, nucleus ambiguus, hypoglossal nucleus and dorsal motor nucleus of vagus, exhibited eNOS staining (Lin et al., 2007). However, none of the eNOS-IR structures were neuronal in nature, based on the observation that none of these structures was positive for neuronal marker (Lin et al., 2007). In contrast, nNOS-IR cells always stained for neuronal marker (Lin and Talman, 2005a). Double-label immunofluorescent staining of eNOS and nNOS showed that the two isoforms never colocalized in the NTS although both were abundant in the nucleus (Lin et al., 2007). Therefore, eNOS and nNOS appear to be expressed in different types of cells in the NTS. Different cellular compartmentalization of eNOS-IR and nNOS-IR in the NTS is similar to that described in other central sites. For example, nNOS has been localized mainly in neurons in the suprachiasmatic nucleus, whereas eNOS has been found in other type of cells in the nucleus (Caillol et al., 2000). The differential localization of eNOS and nNOS may suggest that eNOS and nNOS mediate different functions in the NTS. This suggestion was supported by investigators who demonstrated that the angiotensin II-induced baroreceptor reflex depression can be prevented by eNOS specific inhibitors, but not by an nNOS specific inhibitor (Paton et al., 2001). On the other hand, although eNOS and nNOS are present in different compartments in the NTS, they are often found less than 1–2 μm of each other. Considering the close proximity of these point sources of NO and the highly diffusible nature of the molecule (Garthwaite, 1995), it is difficult to reconcile how the same compound released at nearly the same site may lead to such different physiological effects. The observation again raises the possibility that NO may be a component of a larger biologically active compound, which could effect more complex, receptor mediated functions (Ohta et al., 1997; Lipton et al., 2001).
8. Glutamate and nNOS in the NG and vagal afferents
8.1 Expression of glutamate, glutamate markers and glutamate receptors in the NG and vagal afferents
The NG contains cell bodies of vagal afferents, which carry visceral information to the NTS (Kalia and Mesulam, 1980). It has been shown that a fraction of NG neurons was labeled after injection of 3H-asparate into the NTS, indicating that primary vagal afferent fibers may utilize glutamate as a neurotransmitter (Schaffar et al., 1990). Supporting that finding, injection of horseradish peroxidase into the NG anterogradely labeled numerous axon terminals that contained glutamate (Saha et al., 1995a; Sykes et al., 1997) and removal of the NG resulted in reduction of glutamate release in the NTS (Meeley et al., 1989). In addition, investigators also reported that vagal afferents contain glutamate (Lawrence, 1995; Schaffar et al., 1997) and the R1 subunit of NMDA receptors (Aicher et al., 1999). Recently, VGluT1 and VGluT2 have been identified in vagal afferents in the NTS when cholera toxin B-subunit was use as anterograde tracer (Lachamp et al., 2006). Because vagal afferent fibers from the NG also transmit visceral signals other than those from cardiovascular receptors, those data did not provide a definitive neuroanatomical link between cardiovascular afferents and glutamate as a neurotransmitter of cardiovascular transmission. Injecting cholera toxin B-subunit into the pericardial sac or the aortic nerve of the rat, some investigators showed specifically that cardiac vagal afferents expressed VGluT1 and VGluT2, and provided direct evidence that cardiac vagal axons release glutamate as neurotransmitter (Corbett et al., 2005).
8.2. Expression of nNOS in the NG and vagal afferents
It has been reported that NG neurons express nNOS (Nozaki et al., 1993; Lawrence et al., 1996). There is also indirect evidence that NOS containing neurons in the NG contribute to NOS positive terminals in the NTS in that NOS positive NG neurons can be retrogradely labeled from the NTS (Ruggiero et al., 1996). In addition, the rat vagus nerve appears to bidirectionally transports nNOS, indicated by accumulation of NADPH-diaphorase reactivity proximal to the proximal ligature and distal to the distal ligature (Fong et al., 2000). Therefore, we sought to provide direct evidence that vagal afferents to the NTS contained nNOS by combining degeneration of vagal afferents after removal of the NG with nNOS immunoelectron microscopy (Lin et al., 1998). We identified degenerating axonal terminals by the presence of highly clustered clear vesicles in the terminal axoplasm (Knyihar-Csillik et al., 1982; Lapa and Bauer, 1992). Axons in the NTS that exhibited features of degeneration were considered to have arisen from pseudounipolar NG neurons that had been removed with the nodose ganglionectomy. We noted 67% of degenerating axon terminals were nNOS-IR fibers. These nNOS-IR fibers formed synaptic contact with dendrites that were either nNOS-IR or not nNOS-IR. The observation that the majority of vagal afferents contained nNOS-IR indicates that NO may play an important role in this pathway. In contrast to our results, a lack of nNOS in vagal afferent terminals in the NTS when tracers were injected to the NG or the heart has been reported by other investigators (Atkinson et al., 2003). The reason for the discrepancy between that report and ours is not clear, although it is possible that the neuronal tracers used in the latter study may have been preferentially accumulated in a different subcellular compartment (for example, endosomes) than that in which nNOS is localized (for example, synaptic active zone) in these terminals. In that case, even if the tracer and nNOS were present in the same axon, their colocalization might not be demonstrable because sections through the axon could cut through only one compartment at a time. Such limitations on the ability to demonstrate colocalization have been inferred from another study in which differential compartmentalization has been shown for cholera toxin B-subunit and VGluT1 (Corbett et al., 2005). On the other hand, it is also possible that an up-regulation of nNOS following nerve damage may have occurred in our degeneration study. However, our suggestion that nNOS is presence in presynaptic terminals innervating the NTS from the NG cell bodies is strengthened by studies that demonstrated reduction in nNOS ipsilateral to nodose ganglionectomy (Ruggiero et al., 1996; Lawrence et al., 1998).
8.3. Colocalization of VGluT and nNOS in aortic depressor neurons
The ADN conveys information predominantly from aortic baroreceptors to the NTS (Kalia and Welles, 1980; Ciriello, 1983; Mendelowitz et al., 1992). Cell bodies of ADN afferent fibers are located in the NG (Kalia and Welles, 1980; Donoghue et al., 1982). An increase in efflux of glutamate in the NTS was observed after applying high potassium containing Krebs solution during stimulation of the ADN (Kubo and Kihara, 1988). Glutamate and glutamate agonists produce responses in baroreflex-related NTS neurons during ADN stimulation (Zhang and Mifflin, 1997). These data suggest that ADN neurons in the NG are glutamatergic and therefore should contain glutamate or markers for glutamatergic transmission. To support that hypothesis, we applied a fluorescent tracer, tetramethyl rhodamine dextran (TRD), to the rostral end of the cut ADN in rats to identify ADN neurons and then performed immunofluorescent staining for glutamatergic markers in the NG (Lin and Talman, 2006). Our results showed that many NG neurons were VGluT2-IR or VGluT3-IR. In contrast, the majority of NG neurons were not VGluT1-IR. Of the NG neurons that were TRD positive (i.e. ADN neurons), 8 ± 8% were VGluT1-IR, 40 ± 9% were VGluT2-IR, and 40 ± 9% were VGluT3-IR. In addition, we found that the percentage of ADN neurons that were VGluT2-IR was significantly higher than that of non-ADN neurons. The percentage of ADN neurons that were VGluT3-IR was significantly higher than that of non-ADN neurons. These data suggest that ADN neurons are more likely to be glutamatergic than those that transmit other visceral information.
Using similar methods to test if ADN neurons express nNOS, we found that 38% of ADN neurons were nNOS-IR (Lin and Talman, 2006). Therefore, the ADN may use NO to transmit cardiovascular signals. This suggestion is in line with our previously discussed EM data that show the presence of nNOS in degenerating vagal terminals. In contrast to what we observed for VGluT2-IR ADN neurons and VGluT3-IR ADN neurons, we found that the percentage of TRD positive neurons that were nNOS-IR was not significantly different from that of TRD negative neurons. This may indicate that ADN neurons are as likely to use NO as a neurotransmitter as are NG neurons that transmit other visceral signals. In fact, a number of studies have provided support for a role of NO in gastric regulation and respiratory control (Ogawa et al., 1995; Krowicki et al., 1997; Lipton et al., 2001).
We also performed double-label immunofluorescent staining for nNOS and glutamate markers in the NG after its ADN neurons had been labeled with TRD (Lin and Talman, 2006). Neurons triple labeled for TRD, nNOS and VGluT2 were noted in the NG (Fig. 2, B1–B4). Among all ADN neurons counted, 27 ± 9% were both nNOS-IR and VGluT2-IR. These observations provide neuroanatomical support for the hypothesis that glutamate and NO may interact in transmitting aortic afferent signals from ADN neurons to NTS neurons. They are also consistent with the finding that VGluT2 and nNOS are colocalized in neurons and fibers in the NTS (Lin et al., 2004). Furthermore, we also noted that VGluT3 and nNOS were colocalized in 21 ± 7% TRD neurons and some NG fibers (Fig. 2, C1-C4), a finding that is also consistent with our earlier observation that VGluT3-IR and nNOS-IR colocalize in neurons and fibers in the NTS (Lin and Talman, 2005a). Taken together, these neuroanatomical data support the hypothesis that NO and glutamate may interact in mediating cardiovascular function at the level of the NTS. Our observation that VGluT2 and nNOS colocalized and that VGluT3 and nNOS colocalized in ADN neurons suggest that there is a cellular framework for simultaneous release of NO and glutamate in cardiovascular afferents in the NTS. The observation would also support potential modulation of glutamate receptors or glutamate-mediated signal transduction by NO acting at the postsynaptic neuron. Of course, glutamate may be released from terminals that contain VGluT2 or VGluT3 and act upon postsynaptic glutamate receptors to activate nNOS. Such a mode of action is supported by our data that showed VGluT2-IR fibers and VGluT3-IR fibers closely apposed to nNOS-IR containing fibers in the NTS (Lin et al., 2004; Lin and Talman, 2005a). As discussed earlier, NO formed and released by terminals could also diffuse into the postsynaptic cell to modulate glutamate receptor mediated cellular activities such as intracellular calcium levels and calcium currents (Southam and Garthwaite, 1993; Wang et al., 2006).
9. Summary
Glutamate and NO may mediate similar physiological responses in the NTS and may interact to modulate cardiovascular and respiratory functions. In addition to pharmacological studies that support their roles as neurotransmitters in the NTS and their interaction with each other there, chemical neuroanatomical studies also provide an anatomical basis for a potentially relevant physiological link between glutamate and NO in the NTS. Neurons or fibers containing glutamate, glutamate neuronal markers or glutamate receptors have been shown to colocalize with, be in close apposition to, or even make synaptic contact with neurons or fibers expressing nNOS in the NTS (summarized in Table 1), NG, vagal afferents and ADN neurons. Mechanisms underlining the interaction in the NTS appear to be similar to those in other areas of the brain. In the NTS, NO produced by nNOS may affect glutamate release in the presynaptic site via sGC activation as a result of activated NMDA receptors or AMPA receptors. NO formed in the presynaptic neuron may also diffuse into the target (postsynaptic) cell and act directly on sGC to modulate NMDA receptors and AMPA receptors. Glutamate release from presynaptic sites may act on postsynaptic NMDA receptors and AMPA receptors to increase calcium levels that lead to the activation of nNOS and enhanced NO production. NO produced at the postsynaptic site may inhibit NMDA receptors or AMPA receptors and act as a feedback regulator of NO production. One of the most intriguing questions regarding the glutamate-NO interaction in the NTS is how NO modulates glutamate signal transduction. Indeed, how does NO participate both in stimulation and inhibition of glutamate receptors? It will be interesting to know if NO induces release of glutamate by stimulating VGluTs trafficking in the NTS, and if it does, how? Are metabotropic glutamate receptors or kainate receptors involved in this interaction? Future studies designed to answer these questions could clarify glutamate-NO interactions in regulation of cardiovascular as well as non-cardiovascular systems both in health and in disease.
Table 1.
Anatomical Relationships between nNOS and glutamate, VGluTs, glutamate receptors, and sGC in the rat NTS.
| nNOS colocalize |
nNOS appose* | ||
|---|---|---|---|
| cell body | fiber | ||
| Glutamate | + | + | + |
| VGluT1 | − | − | − |
| VGluT2 | − | + | + |
| VGluT3 | + | + | + |
| NMDAR1 | + | + | + |
| GluR1 | + | + | + |
| sGC | + | + | + |
Fiber to fiber or fiber to cell body apposition.
"+" indicates the presence of specified relationship.
"−" indicates the lack of specified relationship.
Acknowledgments
The author would like to thank Drs. William T. Talman and Albert J. Marshall for reading this manuscript and providing helpful comments. This work was funded in part by NIH Grant R01 HL 59593 (to W. T. Talman), NIH Grant RO1 HL 088090-01A2 (to L. H. Lin and W. T. Talman), and a VA Merit Review Tab 14 (to W. T. Talman).
Abbreviations
- ADN
aortic depressor nerve
- AMPA
alpha-amino-3-hydroxy-5-methylisoxozole-proprionic acid
- ce
central subnucleus
- eNOS
endothelial nitric oxide synthase
- GluR
glutamate receptor subunit
- GluR1
glutamate receptor subunit type 1
- iNOS
induciable nitric oxide synthase
- IR
immunoreactive
- L-NAME
L-nitro-arginine methyl ester
- me
medial subnucleus
- NADPH
nicotinamide adenine dinucleotide phosphate
- NeuN
neuronal nuclear antigen
- NMDA
N-methyl-D-aspartate
- NMDAR1
NMDA receptor subunit 1
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- NG
nodose ganglion
- NTS
nucleus tractus solitarii
- PBS
phosphate buffered saline
- sGC
soluble guanylate cyclase
- TRD
tetramethyl rhodamine dextran
- VGluT
vesicular glutamate transporter
- VGluT1
vesicular glutamate transporter 1
- VGlu2
vesicular glutamate transporter 2
- VGluT3
vesicular glutamate transporter 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher SA, Sharma S, Pickel VM. N-methyl-D-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neurosci. 1999;91:119–132. doi: 10.1016/s0306-4522(98)00530-2. [DOI] [PubMed] [Google Scholar]
- Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis, Exp. Physiol. 1994;79:265–268. doi: 10.1113/expphysiol.1994.sp003761. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol. 1998;402:75–92. [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Batten TFC, Corbett EKA, Sinfield JK, Deuchars J. Subcellular localization of neuronal nitric oxide synthase in the rat nucleus of the solitary tract in relation to vagal afferent inputs. Neurosci. 2003;118:115–122. doi: 10.1016/s0306-4522(02)00946-6. [DOI] [PubMed] [Google Scholar]
- Bradley RM, King MS, Wang L, Shu X. Neurotransmitter and neuromodulator activity in the gustatory zone of the nucleus tractus solitarius. Chemical Senses. 1996;21:377–385. doi: 10.1093/chemse/21.3.377. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard DL, Bao X, Li X, Altschuler SM. Co-localization of NOS and NMDA receptor in esophageal premotor neurons of the rat. NeuroReport. 1995;6:2073–2076. doi: 10.1097/00001756-199510010-00028. [DOI] [PubMed] [Google Scholar]
- Caillol M, Devinoy E, Lacroix MC, Schirar A. Endothelial and neuronal nitric oxide synthases are present in the suprachiasmatic nuclei of Syrian hamsters and rats. Eur J Neurosci. 2000;12:649–661. doi: 10.1046/j.1460-9568.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- Chianca DA, Jr, Lin LH, Dragon DN, Talman WT. NMDA Receptors in the nucleus tractus solitarii are linked to soluble guanylate cyclase. Am J Physiol Heart Circ Physiol. 2004;286:H1521–H1527. doi: 10.1152/ajpheart.00236.2003. [DOI] [PubMed] [Google Scholar]
- Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett. 1983;36:37–42. doi: 10.1016/0304-3940(83)90482-2. [DOI] [PubMed] [Google Scholar]
- Corbett EKA, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience 2005. 2005;135:133–145. doi: 10.1016/j.neuroscience.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Di Paola ED, Vidal MJ, Nistico G. L-Glutamate evokes the release of an endothelium-derived relaxing factor-like substance from the rat nucleus tractus solitarius. J Cardiovasc Pharmacol. 1991;17(Suppl 3):S269–S272. [Google Scholar]
- Dias AC, Colombari E, Mifflin SW. Effect of nitric oxide on excitatory amino acid-evoked discharge of neurons in NTS. Am J Physiol Heart Circ Physiol. 2003;284:H234–H240. doi: 10.1152/ajpheart.00037.2002. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Donoghue S, Garcia M, Jordan D, Spyer KM. Identification and brain-stem projections of aortic baroreceptor afferent neurones in nodose ganglia of cats and rabbits. J Physiol (Lond) 1982;322:337–352. doi: 10.1113/jphysiol.1982.sp014040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Förstermann U. Nitric oxide synthase immunoreactivity in rat pontine medullary neurons. Neurosci. 1994;59:429–445. doi: 10.1016/0306-4522(94)90607-6. [DOI] [PubMed] [Google Scholar]
- Fong AY, Talman WT, Lawrence AJ. Axonal transport of NADPH-diaphorase and [(3)H]nitro-L-arginine binding, but not [(3)H]cGMP binding, by the rat vagus nerve. Brain Res. 2000;878:240–246. doi: 10.1016/s0006-8993(00)02789-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RTJ, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RTJ, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Gai WP, Messenger JP, Yu YH, Gieroba ZJ, Blessing WW. Nitric oxide-synthesising neurons in the central subnucleus of the nucleus tractus solitarius provide a major innervation of the rostral nucleus ambiguus in the rabbit. J Comp Neurol. 1995;357:348–361. doi: 10.1002/cne.903570303. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Neural nitric oxide signalling. Trends Neurosci. 1995;18:51–52. [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G. Cellular origins of cyclic GMP responses to excitatory amino acid receptor agonists in rat cerebellum in vitro. J Neurochem. 1987;48:29–39. doi: 10.1111/j.1471-4159.1987.tb13123.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gasic GP, Hollmann M. Molecular neurobiology of glutamate receptors. Ann Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res. 1991;568:319–322. doi: 10.1016/0006-8993(91)91418-z. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Talman WT. Role of excitatory amino acids and their receptors in bulbospinal control of cardiovascular function. In: Kunos G, Ciriello J, editors. Central neural mechanisms in cardiovascular regulation. Vol. 2. Birkhauser; New York: 1992. pp. 209–225. [Google Scholar]
- Granata AR, Reis DJ. Release of [3H]L-glutamic acid (L-Glu) and [3H]D-aspartic acid (D-Asp) in the area of nucleus tractus solitarius in vivo produced by stimulation of the vagus nerve. Brain Res. 1983;259:77–95. doi: 10.1016/0006-8993(83)91068-5. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El M. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2001;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: Implication for cell death. J Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- Gwyn DG, Leslie RA, Hopkins DA. Observations on the afferent and efferent organization of the vagus nerve and the innvervation of the stomach in the squirrel monkey. J Comp Neurol. 1985;239:163–175. doi: 10.1002/cne.902390204. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerve in the rat. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res. 1993;72:511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Toomim CS, McCarthy KD, Conti F, Battaglia G, Rustioni A, Petrusz P. Characterization of antisera to glutamate and aspartate. J Histochem Cytochem. 1988;36:13–22. doi: 10.1177/36.1.2891743. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El M. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Sakai K, Kishi T, Ito K, Shimokawa H, Takeshita A. Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2003;26:325–331. doi: 10.1291/hypres.26.325. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Sakai K, Kishi T, Takeshita A. Adenovirus-mediated gene transfer into the NTS in conscious rats. A new approach to examining the central control of cardiovascular regulation. Ann N Y Acad Sci. 2001;940:197–205. doi: 10.1111/j.1749-6632.2001.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Housley GD, Martin-Body RL, Dawson NJ, Sinclair JD. Brain stem projections of the glossopharyngeal nerve and its carotid sinus nerve branch in the rat. Neurosci. 1987;22:237–250. doi: 10.1016/0306-4522(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. the cervical vagus and nodose ganglion. J Comp Neurol. 1980;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–264. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Kalia M, Welles RV. Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res. 1980;188:23–32. doi: 10.1016/0006-8993(80)90553-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Baude A. Distribution of AMPA receptor subunits GluR1-4 in the dorsal vagal complex of the rat: a light and electron microscope immunocytochemical study. Synapse. 1999;34:55–67. doi: 10.1002/(SICI)1098-2396(199910)34:1<55::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Evidence that activation of N-methyl-D-aspartate (NMDA) and non-NMDA receptors within the nucleus tractus solitarii triggers swallowing. Eur J Pharmacol. 1991;201:59–67. doi: 10.1016/0014-2999(91)90323-i. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RMJ, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyihar-Csillik E, Csillik B, Rakic P. Ultrastructure of normal and degenerating glomerular terminals of dorsal root axons in the substantia gelatinosa of the rhesus monkey. J Comp Neurol. 1982;210:357–375. doi: 10.1002/cne.902100404. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Evidence of N-methyl-D-aspartate receptor-mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neurosci Lett. 1988;87:69–74. doi: 10.1016/0304-3940(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Lachamp P, Crest M, Kessler JP. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neurosci. 2006;137:73–81. doi: 10.1016/j.neuroscience.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Lapa RCRS, Bauer JA. Synaptic contacts established by primary sensory fibers innervating the teeth of rats: an ultrastructural study of the pars interpolaris of the spinal trigeminal nucleus. Tissue and Cell. 1992;24:821–827. doi: 10.1016/0040-8166(92)90017-2. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ. Neurotransmitter mechanisms of rat vagal afferent neurons. Clin Exp Pharmacol Physiol. 1995;22:869–873. doi: 10.1111/j.1440-1681.1995.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Castillo-Melendez M, McLean KJ, Jarrott B. The distribution of nitric oxide synthase-, adenosine deaminase- and neuropeptide Y-immunoreactivity through the entire rat nucleus tractus solitarius: Effect of unilateral nodose ganglionectomy. J Chem Neuroanat. 1998;15:27–40. doi: 10.1016/s0891-0618(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Nitric oxide increases interstitial excitatory amino acid release in the rat dorsomedial medulla oblongata. Neurosci Letters. 1993;151:126–129. doi: 10.1016/0304-3940(93)90002-3. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. L-glutamate as a neurotransmitter at baroreceptor afferents: evidence from in vivo microdialysis. Neurosci. 1994;58:585–591. doi: 10.1016/0306-4522(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew E, Jarrott B. Actions of nitric oxide and expression of the mRNA encoding nitric oxide synthase in rat vagal afferent neurons. Eur J Pharmacol. 1996;315:127–133. doi: 10.1016/s0014-2999(96)00606-1. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Ohta H, Machado BH, Bates JN, Talman WT. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii decreases arterial pressure and heart rate via activation of soluble guanylate cyclase. Eur J Pharmacol. 1991;202:135–136. doi: 10.1016/0014-2999(91)90269-v. [DOI] [PubMed] [Google Scholar]
- Lin HC, Kang BH, Wan FJ, Huang ST, Tseng CJ. Reciprocal regulation of nitric oxide and glutamate in the nucleus tractus solitarii of rats. Eur J Pharmacol. 2000a;407:83–89. doi: 10.1016/s0014-2999(00)00684-1. [DOI] [PubMed] [Google Scholar]
- Lin LH, Cassell MD, Sandra A, Talman WT. Direct evidence for nitric oxide synthase in vagal afferents to the nucleus tractus solitarii. Neurosci. 1998;84:549–558. doi: 10.1016/s0306-4522(97)00501-0. [DOI] [PubMed] [Google Scholar]
- Lin LH, Edwards RH, Fremeau RT, Fujiyama F, Kaneda K, Talman WT. Localization of vesicular glutamate transporters colocalizes with and neuronal nitric oxide synthase in rat nucleus tractus solitarii. Neurosci. 2004;123:247–255. doi: 10.1016/j.neuroscience.2003.08.063. [DOI] [PubMed] [Google Scholar]
- Lin LH, Emson PC, Talman WT. Apposition of neuronal elements containing nitric oxide synthase and glutamate in the nucleus tractus solitarii of rat: a confocal microscopic analysis. Neurosci. 2000b;96:341–350. doi: 10.1016/s0306-4522(99)00560-6. [DOI] [PubMed] [Google Scholar]
- Lin LH, Sandra A, Boutelle S, Talman WT. Up-regulation of nitric oxide synthase and its mRNA in vagal motor nuclei following axotomy in rat. Neurosci Lett. 1997;221:97–100. doi: 10.1016/s0304-3940(96)13287-0. [DOI] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili O, Talman WT. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Talman WT. N-methyl-D-aspartate receptors on neurons that synthesize nitric oxide in rat nucleus tractus solitarii. Neurosci. 2000;100:581–588. doi: 10.1016/s0306-4522(00)00314-6. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Colocalization of GluR1 and neuronal nitric oxide synthase in rat nucleus tractus solitarii neurons. Neurosci. 2001;106:801–809. doi: 10.1016/s0306-4522(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Coexistence of NMDA and AMPA receptor subunits with nNOS in the nucleus tractus solitarii of rat. J Chem Neuroanat. 2002;24:287–296. doi: 10.1016/s0891-0618(02)00069-8. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Nitroxidergic neurons in rat nucleus tractus solitarii express vesicular glutamate transporter 3. J Chem Neuroanat. 2005a;29:179–191. doi: 10.1016/j.jchemneu.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Soluble guanylate cyclase and neuronal nitric oxide synthase colocalize in rat nucleus tractus solitarii. J Chem Neuroanat. 2005b;29:127–136. doi: 10.1016/j.jchemneu.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Vesicular glutamate transporters and neuronal nitric oxide synthase colocalize in aortic depressor afferent neurons. J Chem Neuroanat. 2006;32:54–64. doi: 10.1016/j.jchemneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- Lo WC, Lin HC, Ger LP, Tung CS, Tseng CJ. Cardiovascular effects of nitric oxide and N-methyl-D-aspartate receptors in the nucleus tractus solitarii of rats. Hypertension. 1997;30:1499–1503. doi: 10.1161/01.hyp.30.6.1499. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LGH. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii of conscious rats decreases arterial pressure but L-glutamate does not. Eur J Pharmacol. 1992;221:179–182. doi: 10.1016/0014-2999(92)90791-2. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992;8:653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Maqbool A, Batten TF, McWilliam PN. Co-localization of neurotransmitter immunoreactivities in putative nitric oxide synthesizing neurones of the cat brain stem. J Chem Neuroanat. 1995;8:191–206. doi: 10.1016/0891-0618(94)00045-u. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Hirooka Y, Hironaga K, Eshima K, Shigematsu H, Shihara M, Sakai K, Takeshita A. Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1285–R1291. doi: 10.1152/ajpregu.2001.280.5.R1285. [DOI] [PubMed] [Google Scholar]
- Meeley MP, Underwood MD, Talman WT, Reis DJ. Content and in vitro release of endogenous amino acids in the area of the nucleus of the solitary tract of the rat. J Neurochem. 1989;53:1807–1817. doi: 10.1111/j.1471-4159.1989.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol (Lond) 1994;478:55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–79. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Mizusawa A, Kikuchi Y, Hida W, Miki H, Shirato K. Nitric oxide as a retrograde messenger in the nucleus tractus solitarii of rats during hypoxia. J Physiol (Lond) 1995;486:495–504. doi: 10.1113/jphysiol.1995.sp020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Bates JN, Lewis SJ, Talman WT. Actions of S-nitrosocysteine in the nucleus tractus solitarii are unrelated to release of nitric oxide. Brain Res. 1997;746:98–104. doi: 10.1016/s0006-8993(96)01188-2. [DOI] [PubMed] [Google Scholar]
- Ohta H, Talman WT. Both NMDA and non-NMDA receptors in the NTS participate in the baroreceptor reflex in rats. Am J Physiol. 1994;267(Pt 2):R1065–70. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- Ohta H, Talman WT. Baroreceptors in the carotid sinus contribute to arterial baroreceptor reflexes in normotensive rats. Clin Exp Pharmacol Physiol. 1996;22:S62–S63. doi: 10.1111/j.1440-1681.1995.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol (Lond) 2001;531:2–58. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA- selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol. 2006;91:59–72. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mtui EP, Otake K, Anwar M. Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. J Comp Neurol. 1996;364:51–67. doi: 10.1002/(SICI)1096-9861(19960101)364:1<51::AID-CNE5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, McWilliam PN. Glutamate-immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and postembedding electron microscopic immunogold staining. Experimental Physiology. 1995a;80:193–202. doi: 10.1113/expphysiol.1995.sp003839. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TFC, McWilliam PN. Glutamate, gamma-aminobutyric acid and tachykinin-immunoreactive synapses in the cat nucleus tractus solitarii. J Neurocytol. 1995b;24:55–74. doi: 10.1007/BF01370160. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–1028. doi: 10.1161/01.hyp.36.6.1023. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. The differential expression patterns of messenger RNAs encoding non-N-methyl-D-aspartate glutamate receptor subunits (GluR1-4) in the rat brain. Neurosci. 1993;52:515–539. doi: 10.1016/0306-4522(93)90403-3. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Schaffar N, Pio J, Jean A. Selective retrograde labeling of primary vagal afferent cell-bodies after injection of [3H]D-aspartate into the rat nucleus tractus solitarii, Neurosci. Lett. 1990;114:253–258. doi: 10.1016/0304-3940(90)90572-q. [DOI] [PubMed] [Google Scholar]
- Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–308. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Smeraski CA, Dunwiddie TV, Diao L, Finger TE. NMDA and non-NMDA receptors mediate responses in the primary gustatory nucleus in goldfish. Chem Senses. 1999;24:37–46. doi: 10.1093/chemse/24.1.37. [DOI] [PubMed] [Google Scholar]
- Smith DV, Li CS, Davis BJ. Excitatory and inhibitory modulation of taste responses in the hamster brainstem. Ann N Y Acad Sci. 1998;855:450–456. doi: 10.1111/j.1749-6632.1998.tb10605.x. [DOI] [PubMed] [Google Scholar]
- Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacol. 1993;32:1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Sweazey RD. Distribution of aspartate and glutamate in the nucleus of the solitary tract of the lamb. Exp Brain Res. 1995;105:241–253. doi: 10.1007/BF00240960. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM, Izzo PN. Demonstration of glutamate immunoreactivity in vagal sensory afferents in the nucleus tractus solitarius of the rat. Brain Res. 1997;762:1–11. doi: 10.1016/s0006-8993(97)00368-5. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takayama K, Miura M. Difference in distribution of glutamate-immunoreactive neurons projecting into the subretrofacial nucleus in the rostral ventrolateral medulla of SHR and WKY: A double-labeling study. Brain Res. 1992;570:259–266. doi: 10.1016/0006-8993(92)90589-2. [DOI] [PubMed] [Google Scholar]
- Talman WT. Neuroactive substances in the control of cardiovascular and visceral responses: an overview. In: Barraco RA, editor. Nucleus of the solitary tract. CRC Press, Inc; Boca Raton, FL: 1994. pp. 233–244. [Google Scholar]
- Talman WT, Dragon DN, Ohta H, Lin LH. Nitroxidergic influences on cardiovascular control by NTS: a link with glutamate. Ann NY Acad Sci. 2001;940:169–178. doi: 10.1111/j.1749-6632.2001.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Talman WT, Lin LH. Glutamatergic and Nitroxidergic Neurotransmission in the Nucleus Tractus Solitarii. In: Misu Y, Goshima Y, editors. Neurobiology of DOPA as a Neurotransmitter. CRC Press; Boca Raton: 2006. pp. 203–216. [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980;209:813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Waki H, Kasparov S, Wong LF, Murphy D, Shimizu T, Paton JF. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J Physiol (Lond) 2003;546:1–42. doi: 10.1113/jphysiol.2002.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Murphy D, Yao ST, Kasparov S, Paton JF. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- Wang S, Paton JF, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii. Exp Physiol. 2007;92:371–382. doi: 10.1113/expphysiol.2006.036103. [DOI] [PubMed] [Google Scholar]
- Wang S, Teschemacher AG, Paton JF, Kasparov S. Mechanism of nitric oxide action on inhibitory GABAergic signaling within the nucleus tractus solitarii. FASEB J. 2006;20:1537–1539. doi: 10.1096/fj.05-5547fje. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Two pathways of nitric oxide production through glutamate receptors in the rat cerebellum in vivo. Neurosci Res. 1997;28:93–102. doi: 10.1016/s0168-0102(97)00032-1. [DOI] [PubMed] [Google Scholar]
- Yamanashi K, Miyamae T, Sasaki Y, Maeda M, Hirano H, Misu Y, Goshima Y. Involvement of nitric oxide production via kynurenic acid-sensitive glutamate receptors in DOPA-induced depressor responses in the nucleus tractus solitarii of anesthetized rats. Neurosci Res. 2002;43:231–238. doi: 10.1016/s0168-0102(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Yen JC, Chan JY, Chan SH. Differential roles of NMDA and non-NMDA receptors in synaptic responses of neurons in nucleus tractus solitarii of the rat. J Neurophysiol. 1999;81:3034–3043. doi: 10.1152/jn.1999.81.6.3034. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Influences of excitatory amino acid receptor agonists on nucleus of the solitary tract neurons receiving aortic depressor nerve inputs. J Pharmacol Exp Ther. 1997;282:639–647. [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2001;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]