Abstract
A system for electromyographic (EMG) triggering of robot-assisted therapy (dubbed the EMG game) for stroke patients is presented. The onset of a patient’s attempt to move is detected by monitoring EMG in selected muscles, whereupon the robot assists her or him to perform point-to-point movements in a horizontal plane. Besides delivering customized robot-assisted therapy, the system can record signals that may be useful to better understand the process of recovery from stroke. Preliminary experiments aimed at testing the proposed system and gaining insight into the potential of EMG-triggered, robot-assisted therapy are reported.
Index Terms: Myoelectric control, robot-assisted rehabilitation, stroke
I. INTRODUCTION
Stroke is a leading cause of disability worldwide. Every year, over 600 000 people suffer from stroke in the U.S. alone, and about 80% of acute stroke survivors lose motor skills of the arm and the hand [1], [2]. A recent innovation in rehabilitation of stroke patients is robot-assisted therapy. Several research studies have shown that intensive goal-directed repetition of movement promotes recovery following a stroke [3]–[5]. Robots can perform repetitive tasks consistently and controllably. They are also equipped with sensors and can record position, velocity, and force exerted by the patient, which may be used to measure patient’s motor performance quantitatively and objectively.
Several rehabilitation robots have been proposed so far. Examples include MIT-MANUS [6], ARM Guide [7], and MIME [8]. Clinical effectiveness was reported in several studies; patients who received robot-assisted therapy showed greater recovery than those who received sham exposure to robot therapy or a matched amount of traditional occupational therapy [9]–[14]. The potential advantages have stimulated an expanding array of research effort using customized robot-assisted therapy. Adaptive robot therapy was recently proposed and implemented on MIT-MANUS [15], [16]. The patient is asked to move the end-effector of the robot while playing a simple video-game (see Fig. 1). The robot provides assistance to complete the movements and the degree of assistance varies with the patient’s motor abilities. The patient’s efforts are detected by monitoring when the speed of the robot end-effector exceeds a certain threshold1.
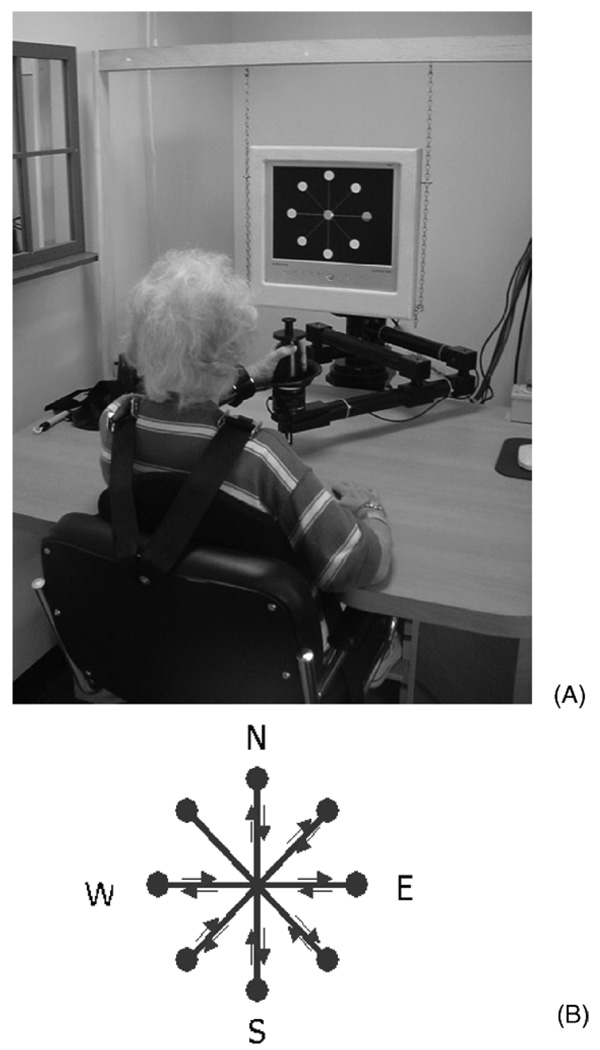
Fig. 1.
(A) Patient during robotic therapy. (B) Display of the typical game in robotic therapy. Eight targets in directions north (N), north-east (NE), east (E), south-east (SE), south (S), south-west (SW), west (W), and north-west (NW) are shown on a videoscreen. Patient is required to move the end-effector of the robot in the horizontal plane from the central target to the eight outer targets. Position of the end-effector in the plane is displayed on the screen by a small yellow cursor. Distance between the central target and each outer target position is 0.14 m.
We investigated whether it would be feasible to use electromyographic (EMG) signals, i.e., muscular electrical activity, to trigger the assistance provided by the robot. Using the EMG to trigger the robot action may offer the following advantages.
It would allow robotic therapy to be customized, e.g., through selection of specific muscle(s) to trigger the robot. This could be used to train specific muscles of patients according to their needs.
It would provide a means to verify that patients are actually attempting to generate movements during robotic therapy (e.g., rather than engaging their trunk to generate movements to exceed the speed threshold)2.
It may trigger the robot earlier than triggering based on kinematic signals3.
It may allow highly-impaired subjects to activate robot assistance; such patients might be able to generate EMG signals even though they were unable to produce sufficient movement to trigger the robot.
It may provide data critical to understanding the process of recovery from stroke, and additional measures of patient recovery, i.e., parameters derived from EMG signals may be used to quantitatively and objectively assess patient’s motor abilities.
While the use of EMG to control machines has been investigated extensively, the use of EMG in robot-assisted therapy has so far been limited to monitoring pre-treatment versus post-treatment changes in muscle activation [22]–[24] and to confirm muscle activity or inactivity in patients undergoing treatment [7], [25]. To the best of our knowledge, the use of EMG for interactive and customized robot-assisted therapy has not yet been explored.
II. BACKGROUND: MYOELECTRIC CONTROL
In myoelectric control, EMG is processed to generate a signal which is fed into a control system. Two classes of control signals are commonly derived from EMG: 1) continuous signals: typically a measure of EMG amplitude, presumably related to muscle exertion, is used to control continuous variables, such as the speed of prosthesis motion [26]–[33] and 2) discrete signals: typically the output of a pattern recognition processor that analyzes multichannel inputs is used to distinguish different classes of limb motion, such as different gestures [34]. Historically, rehabilitation engineering has been the earliest field of application for myoelectric control. The main application, explored since Wiener’s Cybernetics in the late 1940s [35], is control of arm amputation prostheses [36]–[40]. More recently, tele-operation and virtual reality have used EMG for predicting user’s intentions [29], [41]–[45].
In rehabilitation of stroke patients, a recent application is EMG-triggered neuromuscular stimulation, which seeks to promote motor relearning via retraining of voluntary control of movement [46]. EMG is recorded from a given muscle, usually the wrist or finger flexor. As soon as a patient voluntarily attempts to move and EMG exceeds a threshold, a neuromuscular stimulator assists the movement so that full extension of the limb is experienced. A theoretical neurophysiological basis for clinical effectiveness of this technique [47]–[53] is that alternative motor pathways can be recruited and activated to assist the stroke-damaged efferent pathways. This explanation is based on sensorimotor integration theory, which states that sensory input from movement of the affected limb directly influences subsequent motor output [47].
In many of the above applications, detection of the onset of muscular contractions is the first step for identifying the user’s intentions. Single-threshold detectors [54] are the main class of algorithms used for this purpose, although more sophisticated approaches have been proposed [55], [56]. In single-threshold detectors, the onset is the time when the processed EMG signal (typically the EMG envelope) exceeds a threshold, which is selected to discriminate the background noise from the component generated by active muscles. While parameters such as bandwidth of the filter for EMG processing and threshold value can influence the sensitivity of the algorithm and processing delay [54], single-threshold detectors are easy to implement; for this reason, they were chosen for the design of the EMG game.
III. EMG GAME
A. Hardware
The system was implemented using InMotion2 (Interactive Motion Technologies, Inc., Cambridge, MA), a back-drivable therapy robot and Bagnoli 4 (Delsys, Inc., Boston, MA) an EMG acquisition system with four differential electrodes.
B. The Control System
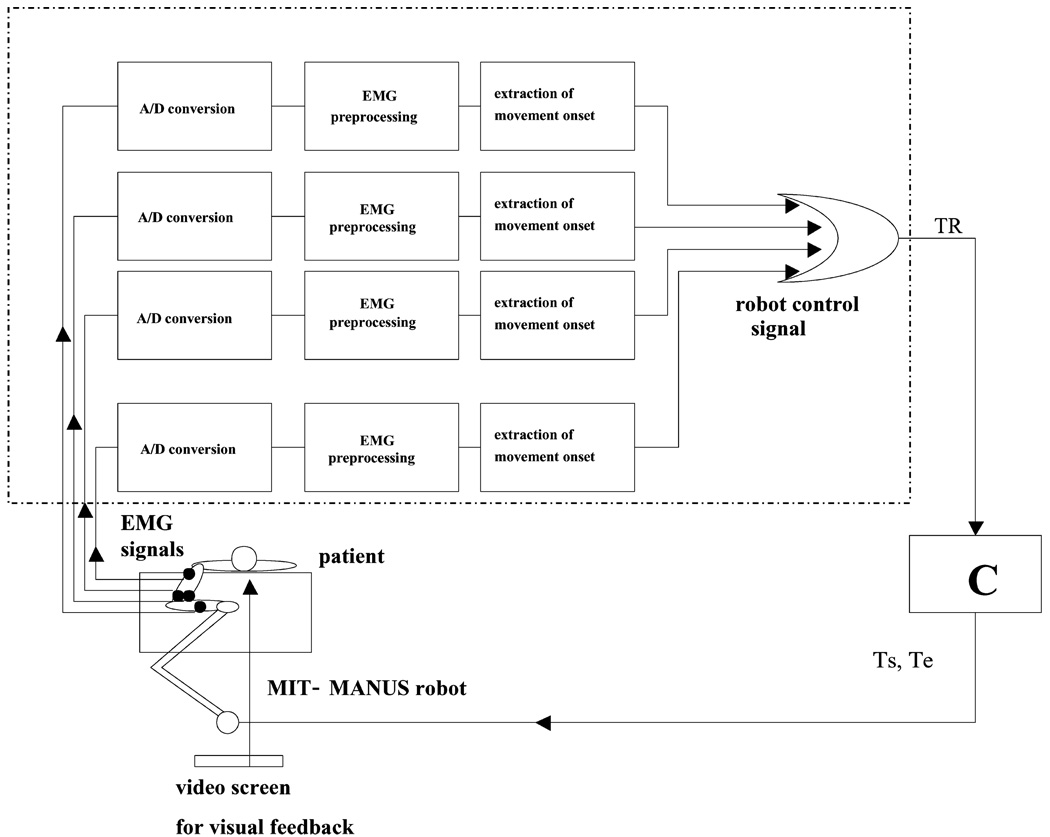
The control system is shown in Fig. 2. The control program was implemented on a personal computer in C++ using the QNX operating system4.
Fig. 2.
Triggering algorithm of the EMG game. Given four channels of EMG, each signal was processed separately to identify EMG onset, which generated a binary signal (1 if the muscle had become active, 0 otherwise). Triggering signal TR was generated as the logical OR of the binary signals generated by each channel. C is the impedance control algorithm for the robot that generated shoulder (Ts)and elbow (Te) torques.
1) EMG Preprocessing
EMG signals were recorded with a gain of 1000 V/V and a bandpass of 20 ± 5 Hz to 450 ± 50 Hz and digitized with sampling frequency fc = 1.0 kHz and 16-bit quantization (United Electronics Industries, Inc., Canton, MA). Signals were high-pass filtered (second order Butterworth, cut-off 10 Hz), full-wave rectified, and low-pass filtered to construct an estimate of the EMG envelope (moving average, length Md, where d = 1,…,4 indicates a data acquisition channel).
2) Generation of the Control Signal
In the following, the ith sample of the processed EMG signals recorded from each data acquisition channel d (d = 1,…,4) is defined as EMGd(i). The robot action was triggered when a robot boolean control signal TR was enabled (became 1).
| (1) |
where ∥ is the logical OR operator and TCd is a binary triggering signal associated with each EMG channel, defined as follows:
| (2) |
where
| (3) |
| (4) |
| (5) |
and N is the number of samples used to calculate the parameters above (see below). Equations (2)–(5) implement a single-threshold detector: for each channel d, the onset was detected when EMGd exceeded Td (calculated as the mean Ad plus αd times the standard deviation Sd of EMGd) for at least Pd consecutive samples. If onset was not detected in any of the EMG channels, the robot control signal TR was kept at 0 and no robot action was triggered. As soon as onset was detected in any of the four channels, the robot control signal TR became 1, thereby triggering robot action.
The performance of the single-threshold detector can be varied by adjusting the values of parameters Pd, Md, and αd [54]. These parameters were tuned to provide satisfactory onset detection for this application (e.g., to reject electrocardiographic artifacts that affected some of the EMG traces).
3) EMG Game Controller Timing
The controller generated the targets on a video-screen, similar to previous robot therapy (see Fig. 1) [9], [10]. Fig. 3 shows the timing of the EMG game controller.
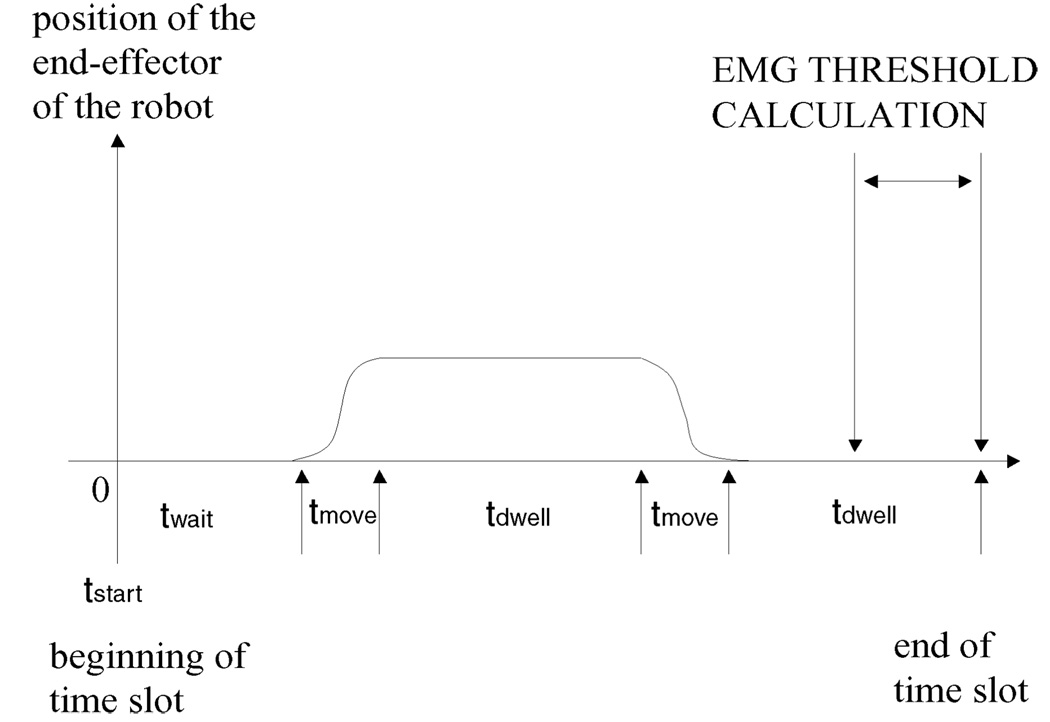
Fig. 3.
Timing of the EMG game controller for each time slot, i.e., movement from the central target to each outer target and back. Patient’s arm was positioned in the center of the robot workspace, which corresponds to the central target on the videoscreen (see Fig. 1(b), part B). At tstart, the outer target was shown to the patient; the controller waited twait seconds before triggering the robot action if the patient did not attempt to move. Game started as soon as the patient’s attempt to move was detected. Patient had to reach the target position within tmove seconds; if needed, the robot assisted the patient’s arm to move. After the outer target was reached, the controller waited tdwell seconds and then showed the patient the central target, which had to be reached within tmove seconds. After tdwell seconds, the controller showed a new target in a new direction. For each channel the threshold Td (d = 1,…, 4) was calculated in a time window of length N samples (1 s) before the end of the time slot. For target North, Td was calculated before tstart; for the seven remaining outer targets, Td was calculated at the end of the second tdwell time.
4) Impedance Controller
Once the robot action had been triggered, the robot impedance controller assisted the patient’s arm movements in the same way as in the adaptive game described elsewhere [15], [16]. The controller allowed patients capable of movement to reach the targets unassisted, but it provided assistance to patients who could not reach the targets. The amount of assistance was based on the patient’s performance.
Excessive muscular tone in pathological subjects may induce involuntary arm movements that can cause the end-effector of the robot to move far from the starting position prior to triggering robot action. To prevent abrupt motion of the patient’s arm when the robot was triggered, the robot stiffness was initialized at 150 N/m to ensure gentle arm positioning.
5) Selective Triggering
Different ways to generate the signal TR can be implemented by modifying (1). As an example, let channel 1 record the EMG signal taken from anterior deltoid (AD) and modify (1) in a target-dependent way as follows:
| (6) |
For each i, robot action would be triggered only when EMG1 exceeds T1. Such an option could be useful to train muscle AD for given directions (in this example the directions of targets N, NE, W, NW). Targets and muscles could be chosen in a different way for each patient to implement personalized therapies.
IV. EXPERIMENTS
Three different experiments were conducted. Experiment I was aimed at exploring the characteristics of EMG signals of stroke patients to better understand the potential of EMG-triggered, robotic therapy (robot assistance off). Experiments II and III were aimed at testing the EMG game under unassisted and assisted conditions (robot assistance off and on, respectively).
Similar to ongoing robot therapy [15], subjects were seated in a comfortable chair in front of the InMotion2 robot, restrained by a seat-belt with shoulder straps, and had their elbow supported with a padded wooden support attached to the elbow with Velcro straps. They were asked to move the end-effector of the robot in the horizontal plane to targets shown on a video-screen located in front of them. Targets were presented sequentially in a clockwise direction, starting from target North (see Fig. 1). The sequence was repeated five times. In the unassisted experiments, if a patient was unable to move back to the central target, the therapist gently guided the patient’s arm back to the center position.
In all the experiments, electrodes for EMG recording were placed by an occupational therapist according to established recommendations [57]. Subjects who gave informed consent to participate were tested at Burke Rehabilitation Hospital, White Plains, NY. The experiments and informed consent procedures were approved by the Massachusetts Institute of Technology (MIT) Committee on the Use of Humans as Experimental Subjects and the Institutional Review Board of Burke Rehabilitation Hospital.
A. Experiment I: EMG Exploration
Two unimpaired subjects (unimpaired subjects 1 and 2) and five stroke patients participated in this experiment (patients 1–5). Patients 1 and 2 were out-patients with moderate and mild hemiparesis; patients 3, 4, and 5 were in-patients with severe hemiparesis (upper extremity component of the Fugl-Meyer (FM) at admission 18/66, 30/66, 8/66, 0/66, and 4/66, respectively). For unimpaired subjects and out-patients, EMG was recorded from the following muscles: pectoralis major clavicularis part (PEC), latissimus dorsi (LAT), triceps long-head (TRIC), middle deltoid (MD), brachioradialis (BRA), teres major (TM), infraspinatus/teres minor (INF), anterior deltoid (AD), biceps (BIC), posterior deltoid (PD), upper trapezius (UT), lower trapezius (LT), and middle trapezius (MT). These muscles were chosen because they were expected to become active according to previous studies on unimpaired subjects [58], [59]. Since the Bagnoli 4 records from four channels the protocol had to be repeated four times in order to gather data from all the muscles selected for recording. For in-patients, due to the tight treatment schedule required by their rehabilitation programs, EMG was only recorded from a subset of the above muscles: PEC, BIC, TRIC, and UT. These muscles were chosen because they were expected to be among the muscles displaying the highest modulation.
B. Experiments II and III: EMG Triggering With Unassisted and Assisted Games
One unimpaired subject (unimpaired subject 3) and two stroke out-patients with severe hemiparesis (patient 6 and 7; upper extremity component of the FM at admission 11/66 and unavailable in medical records) participated in these experiments. EMG was recorded from PEC, AD, BIC, and PD. These muscles were chosen because during experiment I, they were among the muscles that displayed the highest modulation5 and they were easier to record than other muscles in the 13-muscle list (i.e., easier than UT used in experiment 1 with in-patients).
V. RESULTS
A. Experiment I
1) Need for Personalized Therapy
All subjects apart from patient 5 (see below) were able to move toward the outer targets and back. With each subject, given a movement toward a specific target, several muscles were found to be active (EMG displayed modulation). In unimpaired subjects, muscular activation patterns were similar across subjects and consistent with the results of previous studies [58]–[60]. In stroke patients, patterns varied highly across subjects, i.e., patients accomplished similar movements by activating different muscles with different timings6. For example, the EMG taken from TRIC in patient 2 showed almost no modulation in any direction, but showed modulation in patients 1, 3, and 4. In the movement from center to target North, with patient 1, BIC was found to be highly active, while with unimpaired subjects, BIC was found to be active mainly during movements from target North back to the central target.
These findings suggest that different patients may need to re-learn how to activate different muscles. EMG game might allow selective training of specific muscles.
2) EMG Triggering in Highly-Impaired Subjects
Fig. 4 shows data taken from patient 5. This patient was not capable of moving in any direction. However, her EMG data showed some modulation, which in several cases occurred without movement. In the remaining patients, several muscles presented weaker or slower activation profiles than the corresponding muscles in unimpaired subjects. For example, in movements toward target North with patient 1, the AD envelope profile slowly increased as movement was performed (while in unimpaired subjects it rapidly increased before or as soon as movement was initiated). However, it was still able to trigger the robot action (see Table I). This shows that highly-impaired patients, who may be able to activate muscles only weakly or even unable to produce movement, may still produce EMG signals that can trigger robot action. This suggests that EMG triggering might be particularly useful for highly-impaired subjects.
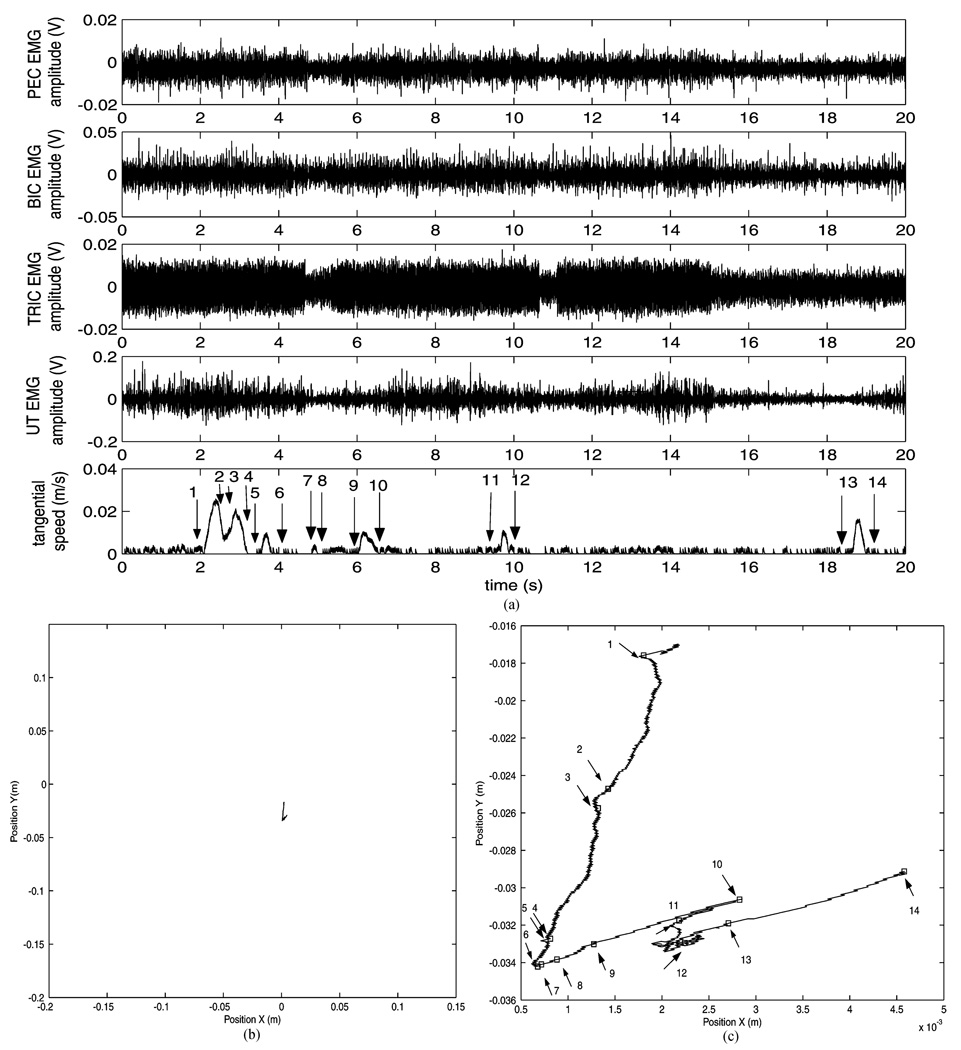
Fig. 4.
(A) Recording from patient 5 during a part of trial 4 in experiment I. EMG modulation is evident even when speed is measurably zero (see for example time windows [7–9 s] and [12–18 s]). Speed is composed of several low-amplitude peaks, i.e., the subject generates isolated small movements. Also, increases in speed do not always correspond to modulation in EMG signals (e.g., see time window [18–20 s]); movements might have been generated by muscles not monitored, or by trunk muscles engaged by the patient in the attempt to move. (B) Plan view. (C) Plan view magnified. Note that the squares labeled 1, 14, correspond to the intervals 1, 14 indicated in the tangential speed plot in (A).
TABLE I.
Mean and standard deviation of d1 and d2 values for AD across the 5 trials of experiment I. tEMG was automatically calculated with the single-threshold algorithm described in section III with parameters M = 100, and P = 120. Note the different behavior of the muscle across different subjects. Negative values indicate that the EMG onset occurs before the instant that υ exceeds the speed threshold. positive values, especially if high suggest that muscle action was not related to movement; other muscles, either external (e.g., trunk muscles) or internal to the subset recorded for the subject, could have generated the movement (see text)
| α | Subject | d1vy(s) | d2vy(s) | d1vt(s) | d2vt(s) |
|---|---|---|---|---|---|
| α = 1 | Subj. 1 | 0.079 ± 0.117 | −0.064 ± 0.129 | 0.081 ± 0.116 | −0.060 ± 0.130 |
| Subj. 2 | −0.133 ± 0.228 | −0.175 ± 0.224 | −0.066 ± 0.270 | −0.172 ± 0.222 | |
| Pat. 1 | 0.419 ± 2.999 | −1.506 ± 2.426 | 0.420 ± 2.898 | −1.499 ± 2.430 | |
| Pat. 2 | −0.270 ± 0.316 | −0.371 ± 0.327 | −0.265 ± 0.317 | −0.360 ± 0.331 | |
| α = 3 | Subj. 1 | 0.113 ± 0.105 | −0.031 ± 0.125 | 0.115 ± 0.104 | −0.026 ± 0.126 |
| Subj. 2 | −0.009 ± 0.030 | −0.052 ± 0.025 | 0.057 ± 0.105 | −0.049 ± 0.023 | |
| Pat. 1 | 3.629 ± 3.646 | 1.703 ± 1.936 | 3.630 ± 3.645 | 1.710 ± 1.942 | |
| Pat. 2 | 0.136 ± 0.199 | 0.035 ± 0.218 | 0.141 ± 0.199 | 0.046 ± 0.221 |
3) Comparison and Combination of EMG, Velocity, and Force Triggering
Besides EMG, velocity (υ) or force signals could be used to detect a patient’s attempt to move. We explored whether a control signal for triggering the robot action could be generated earlier by using EMG signals rather than velocity or force signals.
In order to compare EMG and υ triggering, we investigated movements from center to target North. For each subject’s trial and EMG trace, we calculated the difference d = tEMG − tυTH’ with tEMG the onset of muscular contraction and tVTH the instant when υ exceeded a given threshold VTH. As for VTH, we considered two values: υTH1 = 5.83. 10−3 m/s and υTH2 = 1.75 · 10−2 m/s. Accordingly, we calculated the parameters d1 = tEMG − tυTH1 and d2 = tEMG − tυTH2. Threshold values υTH1 and υTH2 were chosen because they defined the range for the speed threshold υTH used in the adaptive game [15], [16], which was calculated as follows:
| (7) |
where tmove is the time allotted by the adaptive game controller for the movement of the patient’s arm from the central target to each of the eight outer targets. This value represents 10% of the peak velocity of a reaching movement assuming a minimum jerk trajectory [62]. υTH ∈ [5.83·10−3 m/s−1.75·10−2m/s]. Also, υTH1 corresponds to the resolution for υ measurements, since its value is just above the noise level present in the filtered signal. As for υ, we considered two signals: υt and its component υy along the direction pointing to the outer target. υt was calculated as follows: , with x(i) and y(i) the sampled positions of the end-effector of the robot at time i,dx(i) = (x(i) − x(i − 1)) · fc and dy(i) = (y(i) − y(i − 1)) · fc with fc sampling frequency, dxs(i) and dys(i) obtained by filtering dx(i) and dy(i) with a low pass-filter (first order, cut-off frequency 10 Hz). The following values were then calculated:
For both υ = υt and υ = υy, d2 < d1 since tυTH2 > tυTH1. d1υy(d2υy) ≤ d1υt(d2υt) since tυy ≥ tυt. With each subject and α = 1, d1υy values were negative in several different muscles and trials. For example, they were negative in more than 1 trial for MD, INF, PD, and UT with subject 1; for AD and BIC with subject 2; for INF and AD with patient 1; for AD, BRACH, MD, and INF with patient 2; for TRIC, BIC, and UT with patient 3; for TRIC, PEC, BIC, and UT with patient 4. In stroke patients, d1 negative values of the order of several hundreds of milliseconds were not uncommon. For example, with patient 3,d1υy was negative in four cases in TRIC (average value −0.296 s) and in UT (average value −0.608 s); similar data was found with patient 4 in UT, as well as in several muscles of patient 2. Then, as for timing, triggering with EMG versus velocity based signals (and vice-versa) might make a difference. As α was increased, e.g., from 1 to 3, the number of times EMG onset was detected before speed decreased. The increase of α seemed to have a greater effect in patients than in unimpaired subjects. Table I reports d1 and d2 values for movements performed from center to target North for AD, a muscle primarily involved in such movements in unimpaired subjects. With α = land α = 3, d1υy values were negative in 10 and 5 trials out of 20 (5 trials for each subject), respectively, all differences localized in patients data.
With patient 1, we found negative values for d1 for very few trials and muscles. Although strapped to a chair, patients could have engaged their trunk to attempt to generate enough movement to trigger the robot. While trunk data were not recorded during our experiments, making it difficult to draw a definite conclusion, this scenario enlightens a potential advantage of EMG selective triggering. Triggering with EMG taken from upper limb muscles might “force” the patient to use the upper limb to generate movement to trigger the robot.
To increase the chance of generating EMG triggering signals more quickly than speed triggering signals the number of channels for (simultaneous) EMG recording could be increased; lower α values could also be used to allow weaker activations to trigger the robot. To increase the chance of triggering the robot as quickly as possible, a hybrid EMG and speed triggering strategy could be used. Thus, to allow the fastest signal (either EMG or speed) trigger the robot (1) could be modified as:
| (8) |
Force signals were recorded using a 6-DOF ATI US-30-100 force transducer (ATI Industrial Automation, Apex, NC). The transducer was placed at the end-effector. Force signals became distinguishable from the baseline before υ signals, but after EMG signals.
B. Experiments II and III
The parameters of the timing of the EMG game controller were chosen as follows: twait = tdwell = 2 s (see Fig. 3). For experiment II, tmove was set to 4 s for the patients, so that they had enough time to move from the central target to the outer targets or vice-versa, and to 3 s for the unimpaired subject. For experiment III, tmove was reduced to 2 s; the impedance controller parameters, such as stiffness and damping, were set to the typical values used in the adaptive game [15], [16].
1) Generation of the Robot Triggering Signal
All subjects were able to generate TR signals via their EMG. Results are summarized in Table II and Table III for experiment II and III, respectively.
TABLE II.
Results of experiment II. For each subject, columns report the following values. Column 2: number of TR signals generated by the EMG game ((1)); column 3–4: number of times υy exceeded υTF1-υTH2; column 5–6: number of times υt exceeded υTH1−υTH2; column 7–10: number of times d1υy, d2υy, d1υt, and d2υt was negative and average value (in seconds). This data was obtained with M = Md = 100, P = Pd = 120, α = αd = 3 (d = 1,…,4). The values were calculated on a total of 40 trials and in the intervals [tstart − twait] of each time slot (see Fig. 3). Triggering signals based on υt are generated more easily (e.g., compare column 5 with columns 2 and 3) and quickly (e.g., compare column 9 with column 7) than EMG and υy triggering signals
| Subj. | EMG | υy, υTH1 | υy, υTH2 | υt, υTH1 | υy, υTH2 | d1vy | d2vy | d1vt | d2vt |
|---|---|---|---|---|---|---|---|---|---|
| Subj. 3 | 40 | 40 | 40 | 40 | 40 | 12, −0.057 | 27, −0.059 | 11, −0.058 | 26, −0.061 |
| Pat. 6 | 26 | 40 | 37 | 40 | 40 | 6, −0.281 | 10, −0.270 | 2, −0.310 | 3, −0.395 |
| Pat. 7 | 40 | 35 | 32 | 40 | 39 | 6, −0.451 | 7, −0.436 | 2, −0.089 | 2, −0.195 |
TABLE III.
Results of experiment III. See Table II for descriptions of columns. Note that d1 and d2 values are not reported: when EMG enabled the robot action before υ signals, υTH thresholds were likely to be exceeded due to robot assistance and not to the patient movement. This data was obtained with M = Md = 100, P = Pd = 180, α = αd = 3 (d = 1,…,4)
| Subj. | EMG | υy, υTH1 | υy, υTH2 | υt, υTH1 | υy, υTH2 | d1vy | d2vy | d1vt | d2vt |
|---|---|---|---|---|---|---|---|---|---|
| Subj. 3 | 39 | 40 | 40 | 40 | 40 | 6 | 21 | 5 | 20 |
| Pat. 6 | 27 | 37 | 37 | 40 | 39 | 6 | 8 | 2 | 4 |
| Pat. 7 | 33 | 38 | 38 | 40 | 40 | 4 | 8 | 0 | 1 |
In experiment II, TR signals were generated as follows. For the southern targets, they were mainly generated by PD with unimpaired subject 3, by PEC and AD with patient 6, and by PEC with patient 7; for the northern targets by PEC, AD, and PD with unimpaired subject 3, by BIC with patient 6 and by PEC and BIC with patient 7. For the northern targets, note that with patients, AD and PEC usually became active, although weakly and later than BIC, which displayed a strong modulation. For experiment III, the number of TR signals generated and the muscles that produced them were consistent with the results of experiment II.
2) Comparison of EMG and Velocity Triggering
Table II reports the results of the comparison between υ and EMG triggering for experiment II. Note that with α = αd = 1 (d = 1,…,4) for patient 6 the number of TR signals increased to 40. Also, for unimpaired subject 3, patients 6, and 7 the number of cases where d1υy was negative increased to 24, 19, and 23 (average d1υy values −0.104 s, −0.419 s, and −0.351 s), and where d1υt was negative to 24, 7, and 12 (average d1υt values −0.102 s, −0.311 s, and −0.206 s), respectively. Average d1υy positive values were 0.022 s, 0.276 s, and 0.422 s, and average positive d1υt values were 0.023 s, 0.320 s, and 0.397 s.
Although the patients generally generated vt triggering signals more easily and quickly than υy and EMG triggering signals, analysis of position data showed that they were only able to reach some targets. Specifically they could not reach the northern targets and, in some cases, targets East and West; when shown these targets, they moved toward southern directions. However, even when patients moved toward the wrong directions, triggering signals based on vt were still generated. While triggering signals based on υt enable robot assistance as soon as any movement is generated, triggering signals based on υy ensure that the robot action is enabled only when the patient moves toward the direction of the target shown (and of robot assistance). With the EMG game, triggering signals based on EMG are generated only when the patient voluntarily activates the muscles of the upper-limb, and selective EMG triggering signals are generated only when the patient voluntarily activates preselected muscles when moving toward preselected targets. To correlate assistance to a movement with activation of muscles primarily responsible for generating the same movement might enhance motor recovery from stroke.
3) Effects of Robot Assistance
A comparison of EMG signals of stroke patients recorded during experiment II and III showed that with robot assistance on, the EMG level during the rest periods decreased (significant in 6/8 muscles, p < 0.10). The mean EMG level of stroke patients during moves tended to decrease (significant in 5/8 muscles, p < 0.10), suggesting that patients completed the task with less effort.
VI. CONCLUSION
A system for EMG-triggered, robot-assisted therapy (dubbed the EMG game) was introduced. The EMG game enables robot action only when the patient voluntarily initiates a movement; it then assists the patient in completing the movements, thereby providing sensorimotor feedback. The EMG game can implement customized treatments; its parameters can be adapted to train specific patient muscles and to deliver robotic treatment even when the patient is only able to generate weak bursts of muscular contractions. While it remains unclear whether EMG allows faster triggering than speed based triggering signals, experiment II showed that with speed triggering the direction of robot action might be uncorrelated with the direction of the movement generated by the patient. Similarly to EMG-triggered neuromuscular stimulation, the EMG game can be programmed to trigger the robot action as soon as specific muscles are activated. The EMG game also records information about a patient’s motor performance, such as which muscles triggered the robot action and at what time instants. This data, together with kinematic signals recorded during the therapy, may be useful to monitor, quantify, and better understand patient recovery from stroke. Our future research will be aimed at evaluating its clinical effectiveness.
ACKNOWLEDGMENT
Dr. H.I. Krebs and Dr. N. Hogan are co-inventors in the MITheld patent for the robotic device used in this work. They hold equity positions in Interactive Motion Technologies, Inc., the company that manufactures this type of technology under license to MIT.
This work was supported by in part by the National Institute of Child Health and Human Development under Grant R01-HD-37397 and Grant R01-HD-045343 and in part by the Burke Medical Research Institute.
Biographies

Laura Dipietro received the Laurea Degree (summa cum laude) in electrical engineering (biomedical curriculum) from the University of Florence, Florence, Italy, in 1998 and the Ph.D. degree in biomedical robotics from Scuola Superiore S. Anna, Pisa, Italy, in 2003.
She is currently a Postdoctoral Associate at the Newman Laboratory, Massachusetts Institute of Technology, Cambridge. Her current research interests include robot-assisted neuro-rehabilitation, motor control and learning, and biomedical signal processing.

Mark Ferraro received the B.S. degree from Quinnipiac University, Hamdane CT, and did advanced course work at the International Academy of Orthopedic Medicine, Tucson, AZ, which culminated in the M.S. degree from Texas Tech University, Lubbock, TX.
He was a Senior Occupational Therapist from 1998 to 2000, and ran the Robotics Stroke Recovery Unit at the Burke Medical Research Institute, White Plains, NY, until 2004. He is currently with Aventis.

Jerome Joseph Palazzolo received the B.S. and M.S. degrees in mechanical engineering from Michigan State University, East Lansing, in 1992 and 1994, respectively, and the Ph.D. degree in mechanical engineering from the Massachusetts Institute of Technology (MIT), Cambridge, in 2005.
He is currently a Postdoctoral Associate at the Newman Laboratory for Biomechanics and Human Rehabilitation, MIT. His research interests are in the areas of dynamic systems and control, adaptive robotic therapy/training, and human–machine interactions.

Hermano Igo Krebs (M’91) received the electrician degree from Escola Tecnica Federal de Sao Paulo, Sao Paulo, Brazil, in 1976, the B.S. and M.S. degrees in naval engineering (option electrical) from University of Sao Paulo, Sao Paulo, Brazil, in 1980 and 1987, respectively, the M.S. degree in ocean engineering from Yokohama National University, Yokohama, Japan, in 1989, and the Ph.D. degree in ocean engineering from the Massachusetts Institute of Technology (MIT), Cambridge, in 1997, with the dissertation entitled “Robot-Aided Neuro-Rehabilitation and Functional Imaging.”
From 1977 to 1978, he taught electrical design at Escola Tecnica Federal de Sao Paulo. From 1978 to 1979, he worked at the University of Sao Paulo in a project aiming at the identification of hydrodynamic coefficients during ship maneuvers. From 1980 to 1986, he was a surveyor of ships, offshore platforms, and containercranes at the American Bureau of Shipping, Sao Paulo office. In 1989, he was a Visiting Researcher at Sumitomo Heavy Industries, Hiratsuka Laboratories, Japan. From 1993 to 1996, he worked at Casper, Phillips & Associates in containercranes and control systems. He joined the Newman Laboratory for Biomechanics and Human Rehabilitation, Mechanical Engineering Department, MIT, in 1997, where he is a Principal Research Scientist and Lecturer. He also holds an affiliate position as an Adjunct Research Professor of Neuroscience a Weill Medical College, Cornell University, White Plains, NY. He is one of the founders of Interactive Motion Technologies, a Cambridge, MA, based startup company commercializing robot technology for rehabilitation. His research interests are in the areas of neuro-rehabilitation, functional imaging, human-machine interactions, robotics, and dynamic systems modeling and control.
Bruce T. Volpe received the B.S. and M.D. degrees from Yale University, New Haven, CT.
He is currently a Professorin the Department of Neurology and Neuroscience, Weill Medical College, Cornell University, White Plains, NY. He is interested in a model of antibody mediated disease.

Neville Hogan was born in Dublin, Ireland. He received the Dip. Eng. (with distinction) from Dublin College of Technology, Dublin, Ireland, and the M.S., M.E., and Ph.D. degrees from Massachusetts Institute of Technology (MIT), Cambridge.
Following industrial experience in engineering design, he joined the Faculty of the School of Engineering, MIT, in 1979 and has served as Head and Associate Head of the System Dynamics and Control Division, Department of Mechanical Engineering. He is Professor of Mechanical Engineering, Professor of Brain and Cognitive Sciences, and Director of the Newman Laboratory for Biomechanics and Human Rehabilitation, MIT. He is a co-founder of Interactive Motion Technologies, Inc., Cambridge, MA, and a Board Member of Advanced Mechanical Technologies, Inc., Watertown, MA. His research is broad and multidisciplinary, extending from biology to engineering; it has made significant contributions to motor neuroscience, rehabilitation engineering and robotics, but its focus converges on an emerging class of machines designed to cooperate physically with humans. Recent work pioneered the creation of robots sufficiently gentle to provide physiotherapy to frail and elderly patients recovering from neurological injury such as stroke, a novel therapy that has already proven its clinical significance.
Prof. Hogan had received several awards including an Honorary Doctorate from the Delft University of Technology, the Silver Medal of the Royal Academy of Medicine in Ireland, and an Honorary Doctorate from the Dublin Institute of Technology.
Footnotes
Reprinted from IEEE Transactions on Neural Systems and Rehabilitation Engineering.
This material is posted here with permission of the IEEE. Such permission of the IEEE does not in any way imply IEEE endorsement of any of this web site's products or services. Internal or personal use of this material is permitted. However, permission to reprint/republish this material for advertising or promotional purposes or for creating new collective works for resale or redistribution must be obtained from the IEEE by writing to pubs-permissions@ieee.org.
By choosing to view this document, you agree to all provisions of the copyright laws protecting it.
If the patient is not able to move the end-effector within a given time (typically 2 s), the robot will start providing assistance.
Voluntary physical exercise is considered to be a key factor in promoting recovery after traumatic injury of the central nervous system (CNS) on rats [17] and monkeys [4]. It has been shown that it induces neurogenesis in the adult CNS [18], increases trophic factor production in select regions of the brain [19], and can modulate crucial aspects of plasticity [20].
Generating faster triggering signals may improve the correlation between a patient’s attempts to move and the robot’s assistance. This may make robotic therapy more effective since the timing of stimulus seems to be important for induction of plasticity [21].
Although we are presently moving to C code using Real-Time Linux.
During experiment 1, for all directions, among the 13 muscles recorded for out-patients and unimpaired subjects the subset PEC, BIC, UT, MD, BRA, PD, and AD displayed the highest modulation.
Stroke affects motor control mechanisms and stroke patients might accomplish movements by using compensatory muscular synergies [61].
Contributor Information
Laura Dipietro, Newman Laboratory for Biomechanics and Human Rehabilitation, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 USA (e-mail: lauradp@mit.edu)..
Mark Ferraro, Department of Neurology and Neuroscience, Burke Medical Research Institute, Weill Medical College, Cornell University, White Plains, NY 10605 USA..
Jerome Joseph Palazzolo, Newman Laboratory for Biomechanics and Human Rehabilitation, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 USA..
Hermano Igo Krebs, Newman Laboratory for Biomechanics and Human Rehabilitation, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 USA, and also with the Department of Neurology and Neuroscience, Burke Medical Research Institute, Weill Medical College, Cornell University, White Plains, NY 10605 USA..
Bruce T. Volpe, Newman Laboratory for Biomechanics and Human Rehabilitation, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 USA, and also with the Department of Neurology and Neuroscience, Burke Medical Research Institute, Weill Medical College, Cornell University, White Plains, NY 10605 USA.
Neville Hogan, Newman Laboratory for Biomechanics and Human Rehabilitation, the Departments of Mechanical Engineering and Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139 USA..
REFERENCES
- 1.Heart and Stroke Facts and Statistics. Amer. Heart Assoc.; 2001. [Google Scholar]
- 2.Gresham GE, Duncan PW, Stason WB. Rockville, MD: U.S. Dept. Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; Post-Stroke Rehabilitation. Clinical Practice Guideline, no. 16. 1995 AHCPR Publication. no. 95–0662. [PubMed]
- 3.Whitall J, McCombe, Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;vol. 31(no 10):2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 4.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: Role of use versus learning. Neurobiol. Learning Memory. 2000;vol. 74(no 1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 5.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: Results from a single-blind randomized clinical trial. Stroke. 1999;vol. 30(no 11):2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 6.Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neuro-rehabilitation. IEEE Trans. Rehabil. Eng. 1998 Mar.vol. 6(no 1):75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinkensmeyer D, Schmit BD, Rymer Z. Assessment of active and passive restraint during guided reaching after chronic brain injury. Ann. Biomed. Eng. 1999;vol. 27(no 6):805–814. doi: 10.1114/1.233. [DOI] [PubMed] [Google Scholar]
- 8.Lum PS, Burgar CG, Kenney D, Van der Loos HFM. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans. Biomed. Eng. 1999 Jun.vol. 46(no 6):652–662. doi: 10.1109/10.764942. [DOI] [PubMed] [Google Scholar]
- 9.Volpe B, Krebs HI, Nogan N, Edelstein L, Diels C, Aisen M. A novel approach to stroke rehabilitation: Robot-aided sensorimotor stimulation. Neurology. 2000;vol. 54(no 10):1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- 10.Volpe B, Krebs HI, Nogan N, Edelstein L, Diels C, Aisen M. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;vol. 53(no 8):1874–1876. doi: 10.1212/wnl.53.8.1874. [DOI] [PubMed] [Google Scholar]
- 11.Lum PS, Burgar CG, Kenney D, Van der Loos HFM. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper limb motor function following stroke. Arch. Phys. Med. Rehab. 2002;vol. 83(no 7):952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 12.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch. Phys. Med. Rehab. 2003;vol. 84(no 4):477–482. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- 13.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hughes R, Hogan N. Robotic therapy for chronic motor impairment after stroke: Follow-up results. Arch. Phys. Med. Rehab. 2004;vol. 85(no 7):1106–1111. doi: 10.1016/j.apmr.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Ferraro M, Palazzolo JJ, Krol J, Krebs HI, Hogan N, Volpe BT. Robot aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;vol. 61(no 11):1604–1607. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- 15.Krebs HI, Volpe BT, Palazzolo JJ, Fasoli SE, Ferraro M, Edelstein L, Hogan N. Disturbances of higher level neural control—Robotic applications in stroke. Proc. IEEE Int. Annu. Conf. Eng. Med. Biol. Soc. 2001 Oct.vol. 4:4069–4074. [Google Scholar]
- 16.Krebs HI, Palazzolo JJ, Dipietro L, Ferraro M, Krol J, Rannekleiv K, Volpe BT, Hogan N. Rehabilitation robotics: Performance-based progressive robot-assisted therapy. Autonomous Robots. 2003;vol. 15(no 1):7–20. [Google Scholar]
- 17.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J. Neurosci. 1999;vol. 19(no 22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempermann G, Van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self-repair. Prog. Brain Res. 2000;vol. 127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- 19.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;vol. 373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R. Voluntary exercise induces a bdnf-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002;vol. 88(no 5):2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 21.Stefan K, Kunesch E, Choen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;vol. 123(no 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 22.Reinkensmeyer D, Kahn LE, Averbuch M, McKenna-Cole AN, Schimt BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: Progress with the ARM guide. J. Rehab. Res. Devel. 2000;vol. 37(no 6):653–662. [PubMed] [Google Scholar]
- 23.Lum PS, Van der Loos M, Shor P, Burgar CG. A robotic system for upper limb exercises to promote recovery of motor function following stroke; Proc. IEEE Int. Conf. Rehabil. Robot; 1999. pp. 235–238. [Google Scholar]
- 24.Lum PS, Burgar CG, Shor P, Van der Loos M. Robot-assisted upper limb movement promotes improved motor function in post-stroke hemiparesis; Proc. Nat. Rehab. Res. Devel. Service Meeting; 2000. [Google Scholar]
- 25.Cozens JA. Robotic assistance of an active upper limb exercise in neurologically impaired patients. IEEE Trans. Rehabil. Eng. 1999 Jun.vol. 7(no 2):254–256. doi: 10.1109/86.769416. [DOI] [PubMed] [Google Scholar]
- 26.Hogan N. A review of the methods of processing EMG for use as a proportional control signal. J. Biomed. Eng. 1976;vol. 11:81–86. [PubMed] [Google Scholar]
- 27.Kreifeldt J. Signal versus noise characteristics of filtered EMG used as a control source. IEEE Trans. Biomed. Eng. 1974;vol. BME-21:298–308. doi: 10.1109/tbme.1971.4502784. [DOI] [PubMed] [Google Scholar]
- 28.Hogan N, Mann R. Myoelectric signal processing: Optimal estimation applied to electromyography. IEEE Trans. Biomed. Eng. 1980;vol. BME-7:382–410. doi: 10.1109/tbme.1980.326652. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen SC, Knutti DF, Johnson RT, Sears HH. Development of the Utah artificial arm. IEEE Trans. Biomed. Eng. 1982;vol. BME-29:249–269. doi: 10.1109/TBME.1982.325033. [DOI] [PubMed] [Google Scholar]
- 30.Clancy EA, Bouchard S, Rancourt D. Estimation and application of EMG amplitude during dynamic contractions. IEEE Eng. Med. Biol. Mag. 2001 Nov./Dec.vol. 20(no 6):47–54. doi: 10.1109/51.982275. [DOI] [PubMed] [Google Scholar]
- 31.Clancy EA, Farry EA. Adaptive whitening of the electromyogram to improve amplitude estimation. IEEE Trans. Biomed. Eng. 2000 Jun.vol. 47(no 6):709–719. doi: 10.1109/10.844217. [DOI] [PubMed] [Google Scholar]
- 32.Clancy EA, Hogan N. Multiple site electromyograph amplitude estimation. IEEE Trans. Biomed. Eng. 1995 Feb.vol. 42(no 2):203–211. doi: 10.1109/10.341833. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H-S, Ju M-S, Lin C-CK. Improving elbow torque output of stroke patients with assistive torque controlled by EMG signals. J. Biomech. Eng. 2003;vol. 125(no 6):881–886. doi: 10.1115/1.1634284. [DOI] [PubMed] [Google Scholar]
- 34.Saridis GN, Gootee TP. EMG pattern analysis and classification for a prosthetic arm. IEEE Trans. Biomed. Eng. 1982;vol. 29:403–412. doi: 10.1109/TBME.1982.324954. [DOI] [PubMed] [Google Scholar]
- 35.Wiener N. Cybernetics: Control and Communication in the Animal and the Machine. Cambridge, MA: MIT Press; 1948. [Google Scholar]
- 36.Rotchild RD, Mann RW. An EMG controlled, force sensing, proportional rate, elbow prosthesis; Marquette Univ. Proc. Symp. Biomed. Eng; 1966. pp. 106–109. [Google Scholar]
- 37.Mann RW. Efferent and afferent control of an electromyographic proportional-rate, force sensing artificial elbow with cutaneous display of joing angle. Symp. Basic Problems Prehension Movement Control Artificial Limbs, Proc. Inst. Mech. Engrs.; London, U.K.; 1968. pp. 86–92. [Google Scholar]
- 38.Mann RW. Technical and Biological Problems of Control—A Cybernetic View. Pittsburgh, PA: Instrum. Soc. Amer.; 1970. Design criteria, development and pre- and post-fitting amputee evaluation of an EMG controlled, force sensing, proportional-rate, elbow prosthesis with cutaneous kinesthetic feedback; pp. 579–586. [Google Scholar]
- 39.Zardoshti-Kermani M, Wheeler B, Badie K, Hashemi R. EMG feature evaluation for movement control of upper extremity prostheses. IEEE Trans. Rehab. Eng. 1995 Dec.vol. 3(no 4):324–333. [Google Scholar]
- 40.Park S-H, Lee S-P. EMG pattern recognition based on artificial intelligence techniques. IEEE Trans. Rehab. Eng. 1998;vol. 6:400–405. doi: 10.1109/86.736154. [DOI] [PubMed] [Google Scholar]
- 41.Farry KA, Walker ID, Baraniuk RG. Myolectric tele-operation of a complex robotic hand. IEEE Trans. Robot. Autom. 1996 Oct.vol. 12(no 5):775–788. [Google Scholar]
- 42.Fukuda O, Tsuji T, Kaneko M, Otsuka A. A human-assisting manipulator teleoperated by EMG signals and arm motions. IEEE Trans. Robot. Autom. 2003;vol. 19:210–222. [Google Scholar]
- 43.Alsayegh OA, Brzakovic DP. Guidance of video data acquisition by myoelectric signals for smart human-robot interfaces; Proc. IEEE Int. Conf. Robot. Autom; 1998. pp. 3179–3185. [Google Scholar]
- 44.Barreto AB, Scargle SD, Adjouadi M. A practical EMG-based human-computer interface for users with motor disabilities. J. Rehab. Res. Devel. 2000;vol. 37(no 1):53–64. [PubMed] [Google Scholar]
- 45.Nelson WT, Hettinger LJ, Cunningham JA, Roe MM, Haas MW, Dennis LB. Navigating through virtual flight environments using brain-body-actuated control; Proc. IEEE Annu. Int. Symp. Virtual Reality; 1997. pp. 30–37. [Google Scholar]
- 46.Chae J. Neuromuscular electrical stimulation for motor relearning in hemiparesis. Phys. Med. Rehabil. Clin. N. Amer. 2003;vol. 14(no 1 Suppl):93–109. doi: 10.1016/s1047-9651(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 47.Cauraugh JH, Light K, Sangbum K, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: Recovering wrist and finger extension by EMG-triggered neuromuscular stimulation. Stroke. 2000;vol. 31(no 6):1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 48.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one. Stroke. 2002;vol. 33(no 6) doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 49.Cauraugh JH, Kim SB. Chronic stroke motor recovery: Duration of active neuromuscular stimulation. J. Neurol. Sci. 2003;vol. 215(no 1–2):13–19. doi: 10.1016/s0022-510x(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 50.Chae J, Fang ZP, Walker M, Pourmehdi S. Intramuscular EMG controlled neuromuscular electrical stimulation for upper limb recovery in chronic hemiplegia. Amer. J. Phys. Med. Rehab. 2001;vol. 80(no 12):935–941. doi: 10.1097/00002060-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Chae J, Fang ZP, Walker M, Pourmehdi S, Knutson J. Intramuscular EMG controlled neuromuscular electrical stimulation for ankle dorsiflexion recovery in chronic hemiplegia. Amer. J. Phys. Med. Rehab. 2001;vol. 80(no 11):842–847. doi: 10.1097/00002060-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, Pang S. EMG-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: A randomized pilot study. Arch. Phys. Med. Rehab. 1998;vol. 79(no 5):570–575. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 53.Fields RW. Electromyographically triggered electric muscle stimulation for chronic hemiplegia. Arch. Phys. Med. Rehab. 1987;vol. 68(no 7):407–414. [PubMed] [Google Scholar]
- 54.Hodges P, Bui B. A comparison of computer based methods for the termination of onset. Electroencephalogr. Clin. Neurophysiol. 1996;vol. 101(no 6):511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 55.Bonato P, Alessio TD’, Knaflitz M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans. Biomed. Eng. 1998 Mar.vol. 45(no 3):287–299. doi: 10.1109/10.661154. [DOI] [PubMed] [Google Scholar]
- 56.Staude GH. Precise onset detection of human motor responses using a whitening filter and the log-likelihood-ratio test. IEEE Trans. Biomed. Eng. 2001 Nov.vol. 48(no 11):1292–1305. doi: 10.1109/10.959325. [DOI] [PubMed] [Google Scholar]
- 57.Basmajian JV, De Luca CJ. Muscles Alive. Baltimore, MD: Williams & Wilkins; 1985. [Google Scholar]
- 58.Yoshida N, Domen K, Koike Y, Kawato M. A method for estimating torque-vector directions of shoulder muscles using surface EMGs. Biol. Cybern. 2002;vol. 86(no 3):167–177. [PubMed] [Google Scholar]
- 59.Happee R. Goal directed arm movement. I: Analysis of EMG records of shoulder and elbow muscles. J. Electromyogr. Kinesiol. 1992;vol. 2(no 3):165–178. doi: 10.1016/1050-6411(92)90014-A. [DOI] [PubMed] [Google Scholar]
- 60.Happee R. Goal directed arm movement. II: A kinematic model and its relation to EMG records. J. Electromyogr. Kinesiol. 1993;vol. 3(no 1):13–23. doi: 10.1016/1050-6411(93)90019-S. [DOI] [PubMed] [Google Scholar]
- 61.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;vol. 123(no 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 62.Flash T, Hogan N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985;vol. 5:1688–1703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]