Abstract
Skin makes an excellent site for drug and vaccine delivery due to easy accessibility, immuno-surveillance functions, avoidance of macromolecular degradation in the gastrointestinal tract and possibility of self-administration. However, macromolecular drug delivery across the skin is primarily accomplished using hypodermic needles, which have several disadvantages including accidental needle-sticks, pain and needle phobia. These limitations have led to extensive research and development of alternative methods for drug and vaccine delivery across the skin. This review focuses on the recent trends and developments in this field of micro-scale devices for transdermal macromolecular delivery. These include liquid jet injectors, powder injectors, microneedles and thermal microablation. The historical perspective, mechanisms of action, important design parameters, applications and challenges are discussed for each method.
Keywords: Transdermal, drug delivery, vaccination, jet injection, microneedle, thermal ablation
Introduction
Transdermal drug delivery systems encompass a wide array of non-invasive or minimally invasive technologies for delivering drugs and vaccines across the skin without needles (Barry 2001; Prausnitz, Mitragotri et al. 2004; Schuetz, Naik et al. 2005). Key advantages of transdermal delivery include easy accessibility of skin, which aids in high patient compliance, avoidance of the gastrointestinal tract and the ability to achieve sustained release. The transdermal route has distinct advantages over traditional routes of drug administration, namely the oral route which has poor macromolecular bioavailability, or injections which are limited by pain, accidental needle-sticks, and possible side effects due to transiently high plasma drug concentration (Kermode 2004; Mitragotri 2005). These advantages of transdermal delivery coupled with a boom in the rate of macromolecular drug discovery have led to significant advances in transdermal device development over the last decade.
Skin has evolved to be a highly effective barrier around the human body (Scheuple and Blank 1971). This has made it very challenging to deliver large molecular weight hydrophilic drugs such as proteins and peptides. The outermost layer of skin, stratum corneum, is primarily composed of dead corneocytes embedded in lipid layers (Elias 1983). This brick and mortar like arrangement offers a substantial barrier to small hydrophilic compounds as well as to essentially all high molecular weight drugs. Molecules which are successful in crossing stratum corneum may enter the blood circulation via diffusion (Flynn, Yalkowsk. Sh et al. 1974). The rate of diffusion depends on molecular weight as well as concentration gradient, making it even more difficult to deliver large molecules in a time controlled manner, as macromolecules diffuse slowly and may have limited solubility in aqueous medium. This has limited the number of drugs delivered with passive methods to highly lipophilic molecules under 500 Da (Prausnitz, Mitragotri et al. 2004). Therefore, there is a need for methods and devices to deliver hydrophilic and high molecular weight drugs in a controlled and reproducible fashion.
Technologies used by transdermal devices can be divided into passive or active methods based on whether an external source of energy is used for skin permeation enhancement. Passive methods include use of chemical enhancers, emulsions and lipid assemblies as well as biological methods such as peptides (Schreier and Bouwstra 1994; Karande, Jain et al. 2004; Prausnitz, Mitragotri et al. 2004; Schuetz, Naik et al. 2005; Chen, Shen et al. 2006; El Maghraby, Williams et al. 2006). Chemical methods are relatively easy to incorporate into transdermal patches and can be used to deliver varying dosage amounts by changing the application area. However, these methods may have a lag time up to hours and thus cannot be easily adapted for rapid onset or time varying delivery which may be needed for drugs such as insulin.
Increasing numbers of academic and industrial researchers are focusing on transdermal devices with active mechanisms for skin permeation (Brown, Martin et al. 2006). A similar trend is seen in the type of systems that have entered the transdermal market in the last decade, and those under clinical development (Gordon and Peterson 2003; Brown, Martin et al. 2006). These active methods of skin permeation enhancement include jet injectors, iontophoresis, electroporation, ultrasound, microneedles, powder injection, ablation and tape stripping (Prausnitz, Bose et al. 1993; Mitragotri, Blankschtein et al. 1995; Zhang, Shung et al. 1996; Bashir, Chew et al. 2001; Doukas and Kollias 2004; Kalia, Naik et al. 2004; Karande, Jain et al. 2004; Habash, Bansal et al. 2006; Arora, Hakim et al. 2007). Active methods increase transport across the skin typically by using an added driving force for drug transport or by physically disrupting the barrier. This enables delivery of many hydrophilic drugs and macromolecules. In addition, active methods also offer more control over delivery profile, thus resulting in shorter delays between application and drug reaching systemic circulation compared to passive methods. Also, the device and application parameters can be adjusted to better match individual’s skin properties (Tezel, Sens et al. 2001; Davis, Landis et al. 2004; Baxter and Mitragotri 2005).
For the same reasons, devices using active methods can have additional requirements including power supply, possible feedback/sensor mechanism to adjust the rate of delivery and user interface for parameter control (LaVan, McGuire et al. 2003). This stretches the challenges of active device development beyond simply breaching the permeability barrier of skin and into varying engineering fields of microelectomechanical systems (MEMS), micro fluidics and embedded software (Grayson, Shawgo et al. 2004; Ekinci and Roukes 2005). It is this complexity of implementation of active permeation methods into devices that makes this task challenging.
In addition to the complexity of device fabrication and integration, issues related to maximizing delivery efficiency while minimizing undesirable reactions require significant research and development efforts. Over the last decade, great progress on this front has been made with the advent of devices which have at least one working parameter in micrometer range and are collectively referred to as micro-scale devices in this review. Operation at micron scale is important because micron-sized breaches in the stratum corneum barrier are large enough to let most drugs through, since most drugs are of nanometer dimensions. At the same time, they are small enough that they appear to be safe, well tolerated by patients and allow rapid skin recovery post-administration. Such micro-scale devices include liquid jet injectors, solid powder injectors, microneedles and thermal microporation devices. We discuss their mechanisms of permeation enhancement, the current devices using each method, health effects and future directions for device development.
Liquid Jet Injectors
Liquid jet injections employ a high-speed jet to puncture the skin and deliver drugs without the use of a needle. Research on jet injectors began in the early 1930s with Arnold Sutermesiter, an engineer who noticed accidental injections of diesel oil into the hands of workers when small leaks occurred in high-pressure lines (Bremseth and Pass 2001). Since then, two main classes of liquid jet injectors have been developed. These are single-dose jet injectors, known as DCJIs (Disposable Cartridge Jet Injectors) and MUNJIs (Multi-Use-Nozzle Jet Injectors) (Mitragotri 2006). Some DCJIs are only partly disposable while others are fully disposable. MUNJIs did not have any disposable parts and were introduced for rapid mass immunization. Their use, however, was discontinued in the wake of reports of spread of hepatitis B in the 1980s due to their use. The cause of outbreak was thought to be cross contamination due to splash back of interstitial liquid from the skin onto the nozzle (Canter, Mackey et al. 1990). The focus of most studies on jet injectors since then and those discussed in this review therefore is on liquid DCJIs.
Mechanism
The basic design of commercial liquid jet injectors consists of a power source (compressed gas or spring), piston, drug-loaded compartment and a nozzle with orifice size typically ranging between 150–300 μm (Mitragotri 2006). Upon triggering the actuation mechanism, the power source pushes the piston which impacts the drug-loaded compartment, thereby leading to a quick increase in pressure (Schramm and Mitragotri 2002). This forces the drug solution through the nozzle orifice as a liquid jet with velocity ranging between 100–200 m/s. A schematic of injection process is shown in Figure 1. The jet is turbulent in nature and the diameter of the jet is comparable to that of the orifice but increases with distance traveled. Upon impinging on skin, the jet punctures through the skin and initiates hole formation. The formation of a hole is believed to be due to a combination of skin erosion and fracture and is completed during the first few hundred microseconds (Baxter and Mitragotri 2005). As the jet progresses deeper in the skin, velocity decreases until it does not have sufficient energy to continue hole formation. This completes the first phase of injection i.e. unidirectional skin puncture and is followed by the second phase, multidirectional jet dispersion from the end point of penetration. Further, the dispersion of liquid from this point appears to be approximately hemispherical, whose shape is governed by jet power (Schramm-Baxter and Mitragotri 2004).
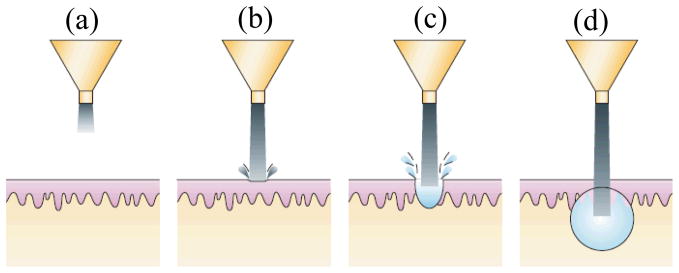
Figure 1. Schematic of drug delivery using liquid jet injector.
(to be printed after permission from (Mitragotri 2006)) (a) Formation of liquid jet (b) Initiation of hole formation due to impact of jet on skin surface (c) Development of hole inside skin with progress of injection (d) Deposition of drug at the end of hole in a near spherical or hemispherical pattern (spherical pattern shown).
Design Parameters
The depth of penetration and shape of liquid dispersion is governed by the orifice diameter and jet exit velocity. Nozzle diameters between 31–559 μm and exit velocities between 115–200 m/s have been used in experimental studies (Baxter and Mitragotri 2005; Baxter and Mitragotri 2006). An increase in penetration depth is reported both with increasing nozzle diameter at constant exit velocity and increasing jet exit velocity at constant diameter, when injection volumes were kept constant. Increasing diameter also increased size of dispersion. More recently, jet power (Po) has been suggested as a combined parameter for describing dependence of jet penetration depth and dispersion on velocity and nozzle diameter. Jet power is calculated as:
where Do is nozzle diameter, uo is exit velocity and ρ is liquid density. Penetration depth increased from 0.2 mm at a power of 1 W to 2.8 mm at a power of 62.4 W. With increasing power, the shape of liquid dispersion at the end of hole also changed from resembling a lower hemisphere with end of eroded hole as center to an upper hemisphere with end of hole lying at the top of hemisphere (Schramm-Baxter, Katrencik et al. 2004). With variation in jet parameters, it is possible to span the full thickness of skin and control the depth where the bulk of drug solution is being delivered. The percent completeness of injection, defined as the percent of drug solution delivered across the skin, also increased linearly from near zero at a power of 1 W to >90% at a power of ~30 W, beyond which the delivery remained constant at or above 90%. Other factors which may affect penetration depth but need further investigation include mechanical properties of skin, injection volume and stand-off distance. The stand-off distance is defined as the distance which the liquid jet travels after leaving the injector’s orifice until it makes contact with the skin.
Applications
MUNJIs have been used for mass immunization programs for diseases including measles, smallpox, cholera, hepatitis B, influenza and polio (Weniger 2003). DCJIs have been used for delivery of several proteins. Most work has been done on delivery of insulin (Weller and Linder 1966; Lindmayer, Menassa et al. 1986) and growth hormones (Verhagen, Ebels et al. 1995; Bareille, MacSwiney et al. 1997; Agerso, Moller-Pedersen et al. 2002; Dorr, Zabransky et al. 2003), while erythropoietin (Suzuki, Takahashi et al. 1995) and interferon (Brodell and Bredle 1995) have also been delivered. Insulin administration by jet injectors led to a faster delivery into systemic circulation, possibly due to better dispersion at the injection site. However, the acceptance of jet injectors has been low due to variable reactions at the site of administration (see ‘Safety’ below).
To counter the challenges faced by traditional jet injectors, a novel pulsed microjet has been developed (Arora, Hakim et al. 2007). This new approach focuses on minimizing pain and bruising by minimizing injection volumes and depth of penetration. The actuation mechanism is based on a piezoelectric transducer and offers strict control over delivery volumes and injection velocity. The high velocity (> 100 m/s) of microjets allowed their entry into skin, whereas the small jet diameters (50–100 μm) and extremely small volumes (2–15 nl) limited the penetration depth (~ 200 μm). The efficacy of this design was confirmed by delivering therapeutic doses of insulin in a rat model.
Safety
The acceptance of conventional jet injectors has been mixed due to variable reactions at the administration site. Some reports state no difference in level of pain compared to that experienced by hypodermic needles (Sarno, Blase et al. 2000), but others have reported higher levels of pain (Jackson, Austin et al. 2001). Variable reports in local reactions further augmented this fact, with some researchers reporting absence of local reactions (Resman, Metelko et al. 1985) while others have reported significantly more reactions including pain, bleeding and haematomas (Houtzagers, Visser et al. 1988). It has been shown that the depth of penetration and percent delivery decrease with increasing Young’s modulus (i.e. mechanical strength) of skin (Baxter and Mitragotri 2005). Commercial injectors come with very limited choice of settings and owing to the person-to-person variability in skin’s mechanical properties, variability in patient response may be due to the failure of this “one size fits all” approach of current devices. Future devices such as pulsed microjets are being designed to address these problems by offering superior control over injection profile.
Powder Injectors
Powder jet injectors deliver vaccines or drugs in dry powdered form into superficial layers of skin. The terms biolistic injectors and gene guns have also been commonly used for these injectors, with the latter term used exclusively for DNA delivery (Peachman, Rao et al. 2003; Kendall 2006). The early work on injecting solid micro-particles in biological samples was reported by Klein and coworkers in 1987 (Klein, Wolf et al. 1987), who demonstrated transfection of plant cells with DNA and RNA using nucleic acid-coated tungsten particles. Since then, researchers have explored the potential of this technique for applications in protein delivery, gene therapy as well as traditional and DNA vaccination (Sarphie, Johnson et al. 1997; Burkoth, Bellhouse et al. 1999; Chen, Endres et al. 2000; Chen, Weis et al. 2001; Chen, Endres et al. 2002).
Mechanism
Basic design of solid jet injectors include compressed gas as the power source, a drug compartment containing particulate drug formulation, and a nozzle to direct the flow of particles (Kendall, Mitchell et al. 2004; Mulholland, Kendall et al. 2004). The drug compartment is closed with diaphragms on either side, which are typically few microns thick. Upon triggering the actuation mechanism, compressed gas from a storage canister expands and pushes against the diaphragms, sequentially rupturing them. The flow of gas carries the drug particles with it. The particles then exit through a nozzle and impinge on skin (Figure 2). Upon impacting on the skin, particles puncture micron-sized holes into stratum corneum by virtue of their momentum. Some particles are contained in stratum corneum while a significant percent reach the viable epidermis for the desired therapeutic effect.
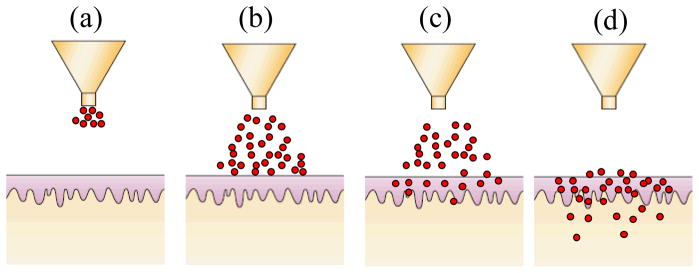
Figure 2. Schematic of drug delivery using powder injector.
(Modified from (Mitragotri 2006)) (a) Ejection of particles from nozzle (b) Impact of particles on skin surface (c) Penetration of particles across stratum corneum (d) Completion of delivery. Particles which penetrate into the skin are mostly distributed in stratum corneum and viable epidermis.
Another design used for studying powder injection mechanisms is light gas gun, which uses an accelerating piston for imparting desired particle velocity (Crozier and Hume 1957). Upon triggering the actuation mechanism, the piston accelerates and carries the particles with it. A deceleration mechanism forces the piston to slow down and makes the particles leave the surface of piston. The particles are ejected and impact on target tissue surface.
Design Parameters
Key parameters in determining particle delivery across the stratum corneum are impact velocity, particle radius and particle density. The particles constitute powdered preparation of drugs or vaccines and range between 10–20 μm. For DNA vaccination, coated metal particles between 0.5–3 μm have been used. A much broader range of particle sizes (0.5–52.6 μm) and densities (1.08–18.2 g/cm3) have been studied for injector development (Kendall, Mitchell et al. 2004). For studying correlations between particle properties and skin penetration, a combined parameter, namely particle impact parameter, has been defined as ρvr, where ρ, v and r are particle density, impact velocity and radius, respectively. Particle impact parameter represents momentum per unit cross-sectional area of the particle. Depth of penetration and fraction of particles penetrating stratum corneum were found to be directly proportional to this parameter. At a fixed value of particle impact parameter, an increase in particle radius corresponds to a decrease in particle velocity at constant density and resulted in a decrease in penetration depth. For a given set of particle properties, velocity of particles can be controlled by varying gas pressure (200–900 psi). Since keeping particle impact parameter uniform is necessary for targeting specific skin layers, various internal contour designs have been studied for achieving narrow velocity profiles. This has led to optimization of internal sections of the injector, namely driver tube and shock tube through which the carrier gas flows before reaching the nozzle (Kendall 2002; Kendall, Quinlan et al. 2004). A recent study has revealed a correlation between epidermal cell death and particles delivered per unit area of target tissue, making particle payload another important parameter (Raju, McSloy et al. 2006).
Applications
Solid jet injectors have been studied for delivery of DNA encoding for viral and bacterial antigens using coated gold micro-particles (Morel, Falkner et al. 2004; Matthews, Rhind et al. 2007; Matthews, Rhind et al. 2007). Induction of humoral and cell mediated immune response against influenza, hepatitis B and rabies has been shown in mice (Chen, Endres et al. 2000; Lodmell, Ray et al. 2000; Chen, Periwal et al. 2001; Chen, Weis et al. 2001; Chen, Endres et al. 2002). Protection against tumors has also been demonstrated by injecting DNA coated gold micro-particles and DNA encapsulated in polymeric particles (Han, Cladel et al. 1999; Han, Cladel et al. 2000; Han, Cladel et al. 2000; Han, Peng et al. 2002; McKeever, Barman et al. 2002; Frelin, Alheim et al. 2003). An extensive review of preclinical DNA vaccination studies using solid jet injector systems in large animal models (swine and non human primates) has been published by Fuller and co-workers (Fuller, Loudon et al. 2006). Clinical efficacy in humans has been demonstrated by induction of cell mediated and humoral immune response against hepatitis B using DNA coated gold micro-particles (Chen, Endres et al. 2000; Roy, Wu et al. 2000). Phase I clinical studies for delivery of DNA vaccine against influenza showed humoral response (Drape, Macklin et al. 2006). Another human clinical study used cross-immunization regime with primary immunization using powder injector followed by intradermal injection as booster, and showed cell mediated response against malaria (McConkey, Reece et al. 2003).
Safety
Human clinical trials have reported painless delivery at the time of injection with DNA vaccines being well tolerated (Tacket, Roy et al. 1999; Roy, Wu et al. 2000; McConkey, Reece et al. 2003; Rottinghaus, Poland et al. 2003; Roberts, Barr et al. 2005; Drape, Macklin et al. 2006). Post injection symptoms have been reported to develop quickly after the injection and include mild erythema, hyper-pigmentation, flaking and discoloration at the injection site. In some cases, transient sensations of mild tingling, tightening or burning have also been reported. Most symptoms disappeared within the first month except mild discoloration, which has been reported to persist for up to 6 months.
Microneedles
Microneedles, as the name suggests, are micron-scale needles that are employed for transdermal vaccination and drug delivery (Reed and Lye 2004). The recognition that very small needles may be sufficient for transport across the 10–20 μm-thick stratum corneum was first proposed in the 1970s (Gerstel and Place 1976) but progress was delayed largely due to lack of techniques to fabricate such small structures. The first work on use of microneedles for transdermal drug delivery was reported in the late 1990’s (Henry, McAllister et al. 1998). Established techniques of the microelectronics industry are now being adapted and expanded upon for microneedle fabrication. Earlier designs of microneedles had silicon as the fabrication material due to easy adaptability to microelectronic fabrication processes. Current designs emphasize metal and polymeric microneedles.
Four different types of microneedle designs have been developed, which include solid microneedles that pierce the skin to make it more permeable, solid microneedles coated with dry powder drugs or vaccines for dissolution in the skin, microneedles prepared from polymer with encapsulated vaccine for rapid or controlled release in the skin, and hollow microneedles for injections (Matriano, Cormier et al. 2000; Cormier and Daddona 2003; Prausnitz, Ackley et al. 2003; Prausnitz 2004; Reed and Lye 2004; Prausnitz 2005; Prausnitz, Mikszta et al. 2005; Birchall 2006; Coulman, Barrow et al. 2006; Sivamani, Liepmann et al. 2007). Metals used in solid microneedles include stainless steel, titanium and nickel-iron. Polymeric needles use engineering plastics, biodegradable polymers and water soluble polymers such as polycarbonate, polylactic-coglycolic acid, and carboxymethyl-cellulose respectively.
Mechanism
The mechanism of action depends on the microneedle design and is summarized in Figure 3. All types of microneedles are typically fabricated as an array of up to hundreds of microneedles over a base substrate. Solid microneedles can either be pressed onto the skin or scraped on the skin for creating microscopic holes, thereby increasing skin permeability by up to four orders of magnitude (Mikszta, Alarcon et al. 2002; McAllister, Wang et al. 2003). This is followed by application of drugs or vaccines from a patch or topical formulation. Residual holes after microneedle removal measure microns in size and have a lifetime more than a day when kept under occlusion, but less than 2 h when left uncovered (unpublished data).
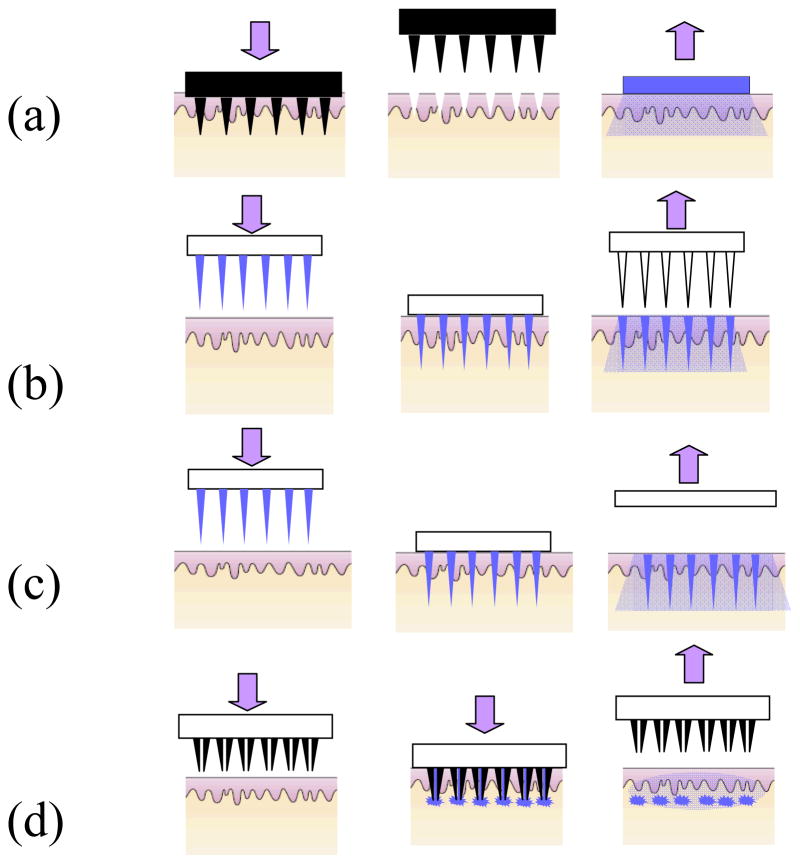
Figure 3. Schematic of drug delivery using different designs of microneedles.
(a) Solid microneedles for permeabilizing skin via formation of micron-sized holes across stratum corneum. The needle patch is withdrawn followed by application of drug-containing patch (b) Solid microneedles coated with dry drugs or vaccine for rapid dissolution in the skin (c) Polymeric microneedles with encapsulated drug or vaccine for rapid or controlled release in the skin (d) Hollow microneedles for injection of drug solution.
The second strategy is to have vaccines or drugs encapsulated in a dry coating onto solid microneedles (Matriano, Cormier et al. 2002; Gill and Prausnitz 2007). This coating can dissolve within 1 minute after insertion into skin, after which the microneedles can be withdrawn and discarded. As an alternative to using insoluble metal or polymer microneedles, complete microneedles have been fabricated out of biodegradable or water-soluble polymers. Model drugs have been encapsulated within PLGA microneedles for controlled release over hours to months (Park, Allen et al. 2006) and, more recently, within water-soluble carboxymethyl-cellulose, polyvinyl-pyrrolidone and maltose for rapid release within minutes (Ito, Yoshimitsu et al. 2006; Kolli and Banga 2008; Lee, Park et al. 2008; Sullivan, Murthy et al. 2008). The final approach consists of using hollow microneedles to puncture the skin followed by infusion of liquid formulation through the needle bores in a manner similar to hypodermic injection (Gardeniers, Luttge et al. 2003; Wang, Cornwell et al. 2006).
Design Parameters
Microneedle design is constrained by a number of parameters. First, microneedles must be capable of inserting into skin without breaking. While metals are typically strong enough, polymers must be selected to have sufficient mechanical strength. Microneedle geometry is also important, where sharpness of tip strongly affects the force required for microneedle insertion into skin. Other parameters, including microneedle length, width and shape all influence force required for microneedle fracture (Davis, Landis et al. 2004; Park, Allen et al. 2005). Typical microneedle geometries vary from 150–1500 μm in length, 50–250 μm in base width and 1–25 μm in tip diameter.
Microneedles can also be designed to minimize pain. Initial studies showed that specific microneedles of a couple hundred microns length were reported painless (Kaushik, Hord et al. 2001; Mikszta, Alarcon et al. 2002). More recently, a detailed study has shown that microneedle length strongly affects pain, where a three-fold increase in needle length (i.e. 500–1500 μm) increased pain seven fold (i.e. from 5% to 35% of the pain caused by a hypodermic needle) (Gill and Prausnitz in press). Increasing the number of microneedles (620 μm long) ten-fold from 5 to 50 increased pain by a factor of three. Other geometrical parameters did not influence pain significantly.
Fabrication methods for microneedles need to be designed appropriately. As single-use, disposable devices, manufacturing costs should be kept low. Lithographic etching and micro-molding methods are typically used and are expected to have mass production costs well under US $1.00 and possibly as low as US $0.10 per device. Fabrication methods also need to avoid denaturing of vaccines and drugs and have therefore emphasized room temperature processing with aqueous solvents and GRAS excipients (Prausnitz, Ackley et al. 2003; Prausnitz 2005; Prausnitz, Mikszta et al. 2005).
Applications
Microneedles have been studied in vitro, in animals and in humans for a variety of applications. Microneedle piercing has been shown to increase skin permeability by orders of magnitude to a variety of compounds ranging from low molecular weight tracers to proteins, DNA and even nanoparticles (Mikszta, Alarcon et al. 2002; McAllister, Wang et al. 2003). A recent study reported on delivery of naltrexone, which is used to treat alcohol and opioid addiction, at therapeutic levels in normal human subjects using this approach (Wermeling, Banks et al. 2008). Solid microneedles have also been coated with a number of different compounds, including low molecular weight drugs, proteins, DNA, virus particles and microparticles (Gill and Prausnitz 2007). Human clinical trials by Zosano Pharmaceuticals (Freemont, CA, USA) were in Phase II clinical trials for delivery of parathyroid hormone from coated microneedles at the time of writing this review. Dissolving polymer microneedles have similarly encapsulated various compounds, including erythropoietin and enzymes that were shown to retain activity after encapsulation and even after at least two months of storage at room temperature (Ito, Hagiwara et al. 2006; Lee, Park et al. 2008; Sullivan, Murthy et al. 2008). Hollow microneedles have been shown to deliver insulin to rodent models and modulate blood glucose levels (Gardeniers, Luttge et al. 2003; McAllister, Wang et al. 2003). Recent work in human subjects has demonstrated insulin delivery to control blood glucose levels in diabetic human subjects and lidocaine delivery to induce local anesthesia in normal human subjects (unpublished data).
Vaccine delivery via microneedles has attracted considerable attention. For example, administration of influenza vaccine via microneedles elicited immune responses comparable to or better than intramuscular injections in mouse model (Alarcon, Hartley et al. 2007). Human clinical trials on influenza vaccination using hollow microneedles have completed Phase III and have been submitted as the basis for registration in Europe through collaboration between Becton Dickenson (Franklin Lakes, NJ, USA) and Sanofi Pasteur (Lyon, France) (Dean, Alarcon et al. 2005). Other vaccine studies include administration of ChimeriVax™-JE for yellow fever, plasmid DNA encoding hepatitis B surface antigen, and recombinant protective antigen of Bacillus anthracis (Mikszta, Sullivan et al. 2005; Mikszta, Dekker et al. 2006). In all these studies, microneedles generated immune responses at least as strong as those generated by subcutaneous or intramuscular injections. Studies also demonstrated dose sparing ability of microneedles, where lower antigen dosage via microneedles elicited immune response comparable to higher antigen doses via alternate routes, i.e. subcutaneous and intramuscular injections (Matriano, Cormier et al. 2002; Widera, Johnson et al. 2006). Recently, a device which uses an electrically active microneedle array to cause electroporation in the skin has effectively enhanced DNA vaccination (Hooper, Golden et al. 2007).
Safety
Although data has not yet been published from ongoing human clinical trials, their progression through phase II and III suggests an acceptable safety profile. Other data from animal and human studies have been published and generally report no significant adverse reactions to microneedles. More specifically, no infections caused by microneedles have been reported (Matriano, Cormier et al. 2002; Cormier, Johnson et al. 2004; Widera, Johnson et al. 2006). In addition, skin irritation has been reported to be mild and transient when it exists at all (Lin, Cormier et al. 2001; Matriano, Cormier et al. 2002; Mikszta, Alarcon et al. 2002; Gardeniers, Luttge et al. 2003; McAllister, Wang et al. 2003; Martanto, Davis et al. 2004; Wang, Cornwell et al. 2006), and bleeding is generally not associated with use of microneedles (Mikszta, Alarcon et al. 2002; McAllister, Wang et al. 2003; Martanto, Davis et al. 2004; Davis, Martanto et al. 2005; Dean, Alarcon et al. 2005; Mikszta, Sullivan et al. 2005; Alarcon, Hartley et al. 2007). As discussed above, a variety of microneedle designs have been reported to be painless in human subjects. Additional studies are needed to fully assess safety.
Thermal Ablation
Use of thermal energy for surgical removal of selected tissue has been reported by medical practitioners as early as Hippocrates (460–370 BC), who used hot iron rods for cauterization of wounds (Karpozilos and Pavlidis 2004). In modern medicine, thermal ablation generally refers to tissue removal due to high temperature induced by various energy sources. Percutaneous thermal ablation for tumor targeting is well established but does not use devices with micron-sized operating dimensions and is discussed elsewhere (De Sanctis, Goldberg et al. 1998; Van Rhoon and Wust 2005). More recently, devices with micro-scale ablation elements have been developed for controlled removal of stratum corneum and thus thermally microporate the skin for enhanced transdermal drug delivery.
Mechanism
Thermal ablation of skin that selectively removes stratum corneum without damaging deeper tissues is achieved through careful control of skin surface temperature over short duration of time. A schematic of this process is shown in Figure 4. By heating the skin surface briefly (eg. ≪ 1 s), heat penetration is largely limited to stratum corneum, with local temperatures up to hundreds of degrees Celsius, while deeper viable tissue remains much cooler and structurally intact (Bramson, Dayball et al. 2003). Formation of micropores of 30 μm diameter and 70 μm depth and absence of necrosis in surrounding tissue has been reported using selective ablation techniques (Bramson, Dayball et al. 2003). In another study, micropores exhibiting an elliptical geometry of 80 μm width, 300 μm length ad 40–50 μm in depth were formed corresponding to the geometry of ablation elements (Sintov, Krymberk et al. 2003).
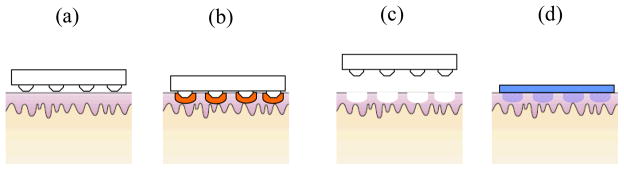
Figure 4. Schematic of drug delivery using thermal ablation.
(a) Microelectrodes are pressed against the skin. (b) Skin is ablated via heating due to RF energy or resistive heating in the electrodes. (c) After removing the ablation device, micropores formed are covered with drug patch for delivery.
One mechanistic hypothesis is that bound water in the stratum corneum must be heated beyond its boiling point, upon which the water vaporizes (Apitz and Vogel 2005). This sudden increase in volume of water blasts micro-craters locally in stratum corneum. In this way, thermal treatment of the stratum corneum triggers a mechanical event that actually causes tissue ablation. Other experiments suggest that temperatures much higher than boiling point of water are needed for extensive tissue ablation and that stratum corneum combustion is mechanistically responsible (Prausnitz et al. unpublished data).
Design Parameters
The temperature, duration, and localization of thermal energy applied to the skin are all critical design parameters. Skin should be heated well above 100°C and possibly up to many hundreds of degrees Celsius. Because skin heating is done for a very short time and extreme temperature gradients exist within skin (e.g. > 10,000 °C/mm), it has been difficult to make precise measurements of skin temperature. To localize heating within the stratum corneum, thermal pulses are applied typically on the millisecond time scale or shorter. Longer pulses lead to heating of deeper skin tissue, which can cause undesirable damage to living tissues. Heating should also be localized to specific areas on the skin surface. Since it would generally be undesirable to ablate large areas on the skin surface for safety reasons, heating elements measuring just microns in size have been used. By employing an array of these micro-heaters, large area of skin can be treated for drug delivery, but only small spots of stratum corneum area are ablated within the treated area.
One approach to achieving controlled heating in this way involves a two dimensional grid of wires having micron-scale resistors between each of the nodes. Using such a device, a brief surge of electric current through the network causes the resistors to suddenly heat up due to ohmic resistance. The electrodes cool down as soon as the current is turned off. This transiently heats the skin surface and ablates stratum corneum. PassPort™ system fabricated by Altea Therapeutics Corp (Atlanta, GA, USA) (Banga 2006) is based on this concept. A prototype of this device used an array of 80 μm diameter tungsten wires (72–75 wires/cm2) as resistive elements for producing focused short bursts of thermal energy for ablation of stratum corneum (Bramson, Dayball et al. 2003).
Another approach involves an array of electrodes that are activated one by one or through a feedback mechanism to briefly pass radiofrequency (RF) current into the skin. The resulting heat generated within the stratum corneum selectively heats this tissue for localized ablation. One such handheld device based on RF energy is ViaDerm™ which has been developed by Transpharma Ltd (Israel) (Levin, Gershonowitz et al. 2005). The device employs a disposable array of stainless steel micro-electrodes (100 μm length and 40 μm diameter; 200 electrodes/cm2) mounted on a polycarbonate body. The activation of device is governed by pressure as the device is pressed on skin at the site of application. Repeated applications of up to 250 V and 380 V for in vivo and in vitro respectively, were used at a frequency of 100 kHz for duration of 1 ms each.
Applications
ViaDerm™ has been extensively tested in vitro for delivery across porcine skin and in vivo on pigs and Sprague-Dawley rats for delivery of testosterone, granisetron hydrochloride, diclofenac sodium and plasmid DNA (Sintov, Krymberk et al. 2003; Levin, Gershonowitz et al. 2005; Birchall, Coulman et al. 2006). The studies consisted of either topical application of model drug or application of transdermal drug patch post ablation. Following in vivo testing, a number of human clinical studies have been reported for ViaDerm™ (Sarphie, Johnson et al. 1997). Delivery of grainsetron was tested over a period of 24 hours in human clinical trials. A steady increase in plasma grainsetron levels for up to 12 hours after patch administration followed by maintenance of a constant level till patch removal at 24 hours was reported. Phase I human clinical trials were conducted for delivery of hPTH [1–34], a peptide fragment of human parathyroid hormone, as an anabolic treatment for osteoporosis. The study was carried out over a period of 7 days with daily administration of hPTH [1–34]. Absence of drug accumulation or degradation of hPTH [1–34] and drug bioavailability of 40% has been reported. In addition, ViaDerm™ system is currently in Phase I/II clinical trials for hGH delivery. A human clinical study has also been performed for delivery of insulin (Sarphie, Johnson et al. 1997).
Thermal ablation by Passport system™ has been tested for administration of adenovirus vaccine with approximately 120 fold increase in reporter gene expression in various mice strains (Bramson, Dayball et al. 2003). More recently, delivery of interferon α2β has been shown with passive and iontophoretic patch in rat model (Badkar, Smith et al. 2007). The device has also been tested for delivery of influenza antigens, tetanus antigen, erythropoietin and fentanyl citrate in pre-clinical studies. Human clinical trials are currently underway for transdermal delivery of basal levels of insulin, hydromorphone HCl, fentanyl citrate, and apomorphine HCl (Sarphie, Johnson et al. 1997).
Safety
Thermal ablation devices have shown acceptable safety profiles. In a recent human clinical trial for evaluating safety, administration sites were examined and results quantified using Draize irritation index for irritation on a scale of 0–8 and Visual Analogie Scale (VAS) for pain on a scale of 0–100. Draize index was 0.75 while VAS score was 5, confirming low degree of erythema and pain. Similar results have been reported in clinical trial for grainsetron delivery, where no irritation was detected after 24 h patch application. Slight erythema has been reported for use of prototype for PassPort™ system (Sarphie, Johnson et al. 1997).
Conclusions
The concepts which form the basis of transdermal micro-devices discussed here were discovered and first described several decades ago. The literature reviewed here strongly indicates that our fundamental understanding of device design parameters and how they affect device interaction with skin has significantly advanced over the last decade. These advances have resulted in novel device designs with increased therapeutic potential and minimal patient discomfort. Ongoing challenges include increasing therapeutic potential still further for some of these devices. Overall, promising trends for the next generation of transdermal vaccination and drug delivery micro-scale devices have emerged.
Micro-scale disruption of skin using the devices discussed here offers several advantages. Micron-sized pores can deliver several therapeutic molecules over a broad molecular weight range in shorter duration of time. These microscopic holes are still small enough to limit undesired effects including pain, irritation and infection. Other advantages include better delivery control over physical and physiological impact on skin. Current disadvantages are big size of some devices and high cost for single use devices or difficulties in component re-use. Future challenges lie principally in device engineering for making devices more portable, affordable and give reproducible results across a wide range of subjects.
Acknowledgments
This work was supported in part by the National Institutes of Health. Mark Prausnitz is the Emerson-Lewis Faculty Fellow and is a member of the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at Georgia Institute of Technology, Atlanta, GA.
References
- Agerso H, Moller-Pedersen J, et al. Pharmacokinetics and pharmacodynamics of a new formulation of recombinant human growth hormone administered by ZomaJet 2 Vision, a new needle-free device, compared to subcutaneous administration using a conventional syringe. Journal of Clinical Pharmacology. 2002;42(11):1262–1268. doi: 10.1177/009127002762491361. [DOI] [PubMed] [Google Scholar]
- Alarcon JB, Hartley AW, et al. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clinical and Vaccine Immunology. 2007;14(4):375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apitz I, Vogel A. Material ejection in nanosecond Er : YAG laser ablation of water, liver, and skin. Applied Physics a-Materials Science & Processing. 2005;81(2):329–338. [Google Scholar]
- Arora A, Hakim I, et al. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4255–4260. doi: 10.1073/pnas.0700182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badkar AV, Smith AM, et al. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharmaceutical Research. 2007;24(7):1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- Banga AK. New technologies to allow transdermal delivery of therapeutic proteins and small water-soluble drugs. American Journal of Drug Delivery. 2006;4(4):221–230. [Google Scholar]
- Bareille P, MacSwiney M, et al. Growth hormone treatment without a needle using the Preci-Jet 50 transjector. Archives of Disease in Childhood. 1997;76(1):65–67. doi: 10.1136/adc.76.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–14. doi: 10.1016/s0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Bashir SJ, Chew AL, et al. Physical and physiological effects of stratum corneum tape stripping. Skin Research and Technology. 2001;7(1):40–48. doi: 10.1034/j.1600-0846.2001.007001040.x. [DOI] [PubMed] [Google Scholar]
- Baxter J, Mitragotri S. Jet-induced skin puncture and its impact on needle-free jet injections: Experimental studies and a predictive model. Journal of Controlled Release. 2005;106(3):361–373. doi: 10.1016/j.jconrel.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Baxter J, Mitragotri S. Needle-free liquid jet injections: mechanisms and applications. Expert Review of Medical Devices. 2006;3(5):565–574. doi: 10.1586/17434440.3.5.565. [DOI] [PubMed] [Google Scholar]
- Birchall J, Coulman S, et al. Cutaneous gene expression of plasmid DNA in excised human skin following delivery via microchannels created by radio frequency ablation. International Journal of Pharmaceutics. 2006;312(1–2):15–23. doi: 10.1016/j.ijpharm.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Birchall JC. Microneedle array technology: the time is right but is the science ready? Expert Review of Medical Devices. 2006;3(1):1–4. doi: 10.1586/17434440.3.1.1. [DOI] [PubMed] [Google Scholar]
- Bramson J, Dayball K, et al. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Therapy. 2003;10(3):251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- Bremseth DL, Pass F. Delivery of insulin by jet injection: recent observations. Diabetes Technology & Therapeutics. 2001;3(2):225–232. doi: 10.1089/152091501300209598. [DOI] [PubMed] [Google Scholar]
- Brodell RT, Bredle DL. The treatment of palmar and plantar warts using natural alpha-interferon and a needleless injector. Dermatologic Surgery. 1995;21(3):213–218. doi: 10.1111/j.1524-4725.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Brown MB, Martin GP, et al. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Delivery. 2006;13(3):175–187. doi: 10.1080/10717540500455975. [DOI] [PubMed] [Google Scholar]
- Burkoth TL, Bellhouse BJ, et al. Transdermal and transmucosal powdered drug delivery. Critical Reviews in Therapeutic Drug Carrier Systems. 1999;16(4):331–384. doi: 10.1615/critrevtherdrugcarriersyst.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- Canter J, Mackey K, et al. An Outbreak of Hepatitis-B Associated with Jet Injections in a Weight-Reduction Clinic. Archives of Internal Medicine. 1990;150(9):1923–1927. [PubMed] [Google Scholar]
- Chen DX, Endres RL, et al. Epidermal powder immunization using non-toxic bacterial enterotoxin adjuvants with influenza vaccine augments protective immunity. Vaccine. 2002;20(21–22):2671–2679. doi: 10.1016/s0264-410x(02)00215-3. [DOI] [PubMed] [Google Scholar]
- Chen DX, Endres RL, et al. Epidermal immunization by a needle-free powder delivery technology: Immunogenicity of influenza vaccine and protection in mice. Nature Medicine. 2000;6(10):1187–1190. doi: 10.1038/80538. [DOI] [PubMed] [Google Scholar]
- Chen DX, Periwal SB, et al. Serum and mucosal immune responses to an inactivated influenza virus vaccine induced by epidermal powder immunization. Journal of Virology. 2001;75(17):7956–7965. doi: 10.1128/JVI.75.17.7956-7965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DX, Weis KF, et al. Epidermal powder immunization induces both cytotoxic T-lymphocyte and antibody responses to protein antigens of influenza and hepatitis B viruses. Journal of Virology. 2001;75(23):11630–11640. doi: 10.1128/JVI.75.23.11630-11640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Shen YY, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nature Biotechnology. 2006;24(4):455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- Cormier M, Daddona PE. Macroflux technology for transdermal delivery of therapeutic proteins and vaccines. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified-Release Drug Delivery Technology. New York: Marcel Dekker; 2003. pp. 589–598. [Google Scholar]
- Cormier M, Johnson B, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. Journal of Controlled Release. 2004;97(3):503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Coulman SA, Barrow D, et al. Minimally invasive delivery of macromolecules and plasmid DNA via microneedles. Current Drug Delivery. 2006;3:65–75. doi: 10.2174/156720106775197510. [DOI] [PubMed] [Google Scholar]
- Crozier WD, Hume W. High-velocity, light-gas gun. Journal of Applied Physics. 1957;28(8):892–894. [Google Scholar]
- Davis SP, Landis BJ, et al. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. Journal of Biomechanics. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Davis SP, Martanto W, et al. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Transactions on Biomedical Engineering. 2005;52(5):909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- De Sanctis JT, Goldberg SN, et al. Percutaneous treatment of hepatic neoplasms: A review of current techniques. Cardiovascular and Interventional Radiology. 1998;21(4):273–296. doi: 10.1007/s002709900263. [DOI] [PubMed] [Google Scholar]
- Dean CH, Alarcon JB, et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005;1(3):106–11. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- Dorr HG, Zabransky S, et al. Are needle-free injections a useful alternative for growth hormone therapy in children? Safety and pharmacokinetics of growth hormone delivered by a new needle-free injection device compared to a fine gauge needle. Journal of Pediatric Endocrinology & Metabolism. 2003;16(3):383–392. doi: 10.1515/jpem.2003.16.3.383. [DOI] [PubMed] [Google Scholar]
- Doukas AG, Kollias N. Transdermal drug delivery with a pressure wave. Advanced Drug Delivery Reviews. 2004;56(5):559–579. doi: 10.1016/j.addr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Drape RJ, Macklin MD, et al. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24(21):4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ekinci KL, Roukes ML. Nanoelectromechanical systems. Review of Scientific Instruments. 2005;76(6):061101 1–12. [Google Scholar]
- El Maghraby GMM, Williams AC, et al. Can drug-bearing liposomes penetrate intact skin? Journal of Pharmacy and Pharmacology. 2006;58(4):415–429. doi: 10.1211/jpp.58.4.0001. [DOI] [PubMed] [Google Scholar]
- Elias PM. Epidermal Lipids, Barrier Function, and Desquamation. Journal of Investigative Dermatology. 1983;80:S44–S49. doi: 10.1038/jid.1983.12. [DOI] [PubMed] [Google Scholar]
- Flynn GL, Yalkowsk Sh, et al. Mass-Transport Phenomena and Models - Theoretical Concepts. Journal of Pharmaceutical Sciences. 1974;63(4):479–510. doi: 10.1002/jps.2600630403. [DOI] [PubMed] [Google Scholar]
- Frelin L, Alheim M, et al. Low dose and gene gun immunization with a hepatitis C virus nonstructural (NS) 3 DNA-based vaccine containing NS4A inhibit NS3/4A-expressing tumors in vivo. Gene Therapy. 2003;10(8):686–699. doi: 10.1038/sj.gt.3301933. [DOI] [PubMed] [Google Scholar]
- Fuller DH, Loudon P, et al. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40(1):86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Gardeniers HJGE, Luttge R, et al. Silicon micromachined hollow microneedles for transdermal liquid transport. Journal of Microelectromechanical Systems. 2003;12(6):855–862. [Google Scholar]
- Gerstel MS, Place VA. Drug delivery device. USA: 1976. [Google Scholar]
- Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. Journal of Controlled Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clinical Journal of Pain. doi: 10.1097/AJP.0b013e31816778f9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RD, Peterson T. 4 myths about transdermal drug delivery. Drug Delivery Technology. 2003;3(4):44–50. [Google Scholar]
- Grayson ACR, Shawgo RS, et al. A BioMEMS review: MEMS technology for physiologically integrated devices. Proceedings of the Ieee. 2004;92(1):6–21. [Google Scholar]
- Habash RWY, Bansal R, et al. Thermal therapy part 1: An introduction to thermal therapy. Critical Reviews in Biomedical Engineering. 2006;34(6):459–489. doi: 10.1615/critrevbiomedeng.v34.i6.20. [DOI] [PubMed] [Google Scholar]
- Han R, Cladel NM, et al. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. Journal of Virology. 2000;74(20):9712–9716. doi: 10.1128/jvi.74.20.9712-9716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Peng XW, et al. Gene gun-mediated intracutaneous vaccination with papillomavirus E7 gene delays cancer development of papillomavirus-induced skin papillomas on rabbits. Cancer Detection and Prevention. 2002;26(6):458–467. doi: 10.1016/s0361-090x(02)00125-3. [DOI] [PubMed] [Google Scholar]
- Han RC, Cladel NM, et al. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. Cancer Gene Therapy. 2000;7(12):S25–S25. doi: 10.1128/jvi.74.20.9712-9716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RC, Cladel NM, et al. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. Journal of Virology. 1999;73(8):7039–7043. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S, McAllister DV, et al. Microfabricated microneedles: A novel approach to transdermal drug delivery. Journal of Pharmaceutical Sciences. 1998;87(8):922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Golden JW, et al. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtzagers CMGJ, Visser AP, et al. The Medi-Jector-II - efficacy and acceptability in insulin-dependent diabetic-patients with and without needle phobia. Diabetic Medicine. 1988;5(2):135–138. doi: 10.1111/j.1464-5491.1988.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hagiwara E, et al. Feasibility of microneedles for percutaneous absorption of insulin. European Journal of Pharmaceutical Sciences. 2006;29(1):82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yoshimitsu JI, et al. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. Journal of Drug Targeting. 2006;14(5):255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- Jackson LA, Austin G, et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine. 2001;19(32):4703–4709. doi: 10.1016/s0264-410x(01)00225-0. [DOI] [PubMed] [Google Scholar]
- Kalia YN, Naik A, et al. Iontophoretic drug delivery. Advanced Drug Delivery Reviews. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Karande P, Jain A, et al. Discovery of transdermal penetration enhancers by high-throughput screening. Nature Biotechnology. 2004;22(2):192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- Karpozilos A, Pavlidis N. The treatment of cancer in Greek antiquity. European Journal of Cancer. 2004;40(14):2033–2040. doi: 10.1016/j.ejca.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Hord AH, et al. Lack of pain associated with microfabricated microneedles. Anesthesia and Analgesia. 2001;92(2):502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- Kendall M. Engineering of needle-free physical methods to target epidermal cells for DNA vaccination. Vaccine. 2006;24(21):4651–4656. doi: 10.1016/j.vaccine.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kendall M, Mitchell T, et al. Intradermal ballistic delivery of micro-particles into excised human skin for pharmaceutical applications. Journal of Biomechanics. 2004;37(11):1733–1741. doi: 10.1016/j.jbiomech.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Kendall MAF. The delivery of particulate vaccines and drugs to human skin with a practical, hand-held shock tube-based system. Shock Waves. 2002;12(1):23–30. [Google Scholar]
- Kendall MAF, Quinlan NJ, et al. Measurements of the gas and particle flow within a converging-diverging nozzle for high speed powdered vaccine and drug delivery. Experiments in Fluids. 2004;37(1):128–136. [Google Scholar]
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promotion International. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- Klein TM, Wolf ED, et al. High-Velocity Microprojectiles for Delivering Nucleic-Acids into Living Cells. Nature. 1987;327(6117):70–73. [PubMed] [Google Scholar]
- Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharmaceutical Research. 2008;25(1):104–113. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- LaVan DA, McGuire T, et al. Small-scale systems for in vivo drug delivery. Nature Biotechnology. 2003;21(10):1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, et al. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin G, Gershonowitz A, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharmaceutical Research. 2005;22(4):550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- Lin WQ, Cormier M, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux (R)) technology. Pharmaceutical Research. 2001;18(12):1789–1793. doi: 10.1023/a:1013395102049. [DOI] [PubMed] [Google Scholar]
- Lindmayer I, Menassa K, et al. Development of New Jet Injector for Insulin Therapy. Diabetes Care. 1986;9(3):294–297. doi: 10.2337/diacare.9.3.294. [DOI] [PubMed] [Google Scholar]
- Lodmell DL, Ray NB, et al. DNA vaccination of mice against rabies virus: effects of the route of vaccination and the adjuvant monophosphoryl lipid A (MPL (R)) Vaccine. 2000;18(11–12):1059–1066. doi: 10.1016/s0264-410x(99)00352-7. [DOI] [PubMed] [Google Scholar]
- Martanto W, Davis SP, et al. Transdermal delivery of insulin using microneedles in vivo. Pharmaceutical Research. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- Matriano JA, Cormier M, et al. Microfabricated microneedles for gene and drug delivery. Annual Reviews in Biomedical Engineering. 2000;2:289–313. doi: 10.1146/annurev.bioeng.2.1.289. [DOI] [PubMed] [Google Scholar]
- Matriano JA, Cormier M, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- Matthews K, Rhind SM, et al. The effects of gene gun delivered pIL-3 adjuvant on skin pathology and cytokine expression. Veterinary Immunology and Immunopathology. 2007;119(3–4):233–242. doi: 10.1016/j.vetimm.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Matthews K, Rhind SM, et al. The effect of gene gun-delivered pGM-CSF on the immunopathology of the vaccinated skin. Scandinavian Journal of Immunology. 2007;65(3):298–307. doi: 10.1111/j.1365-3083.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- McAllister DV, Wang PM, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey SJ, Reece WHH, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nature Medicine. 2003;9(6):729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- McKeever U, Barman S, et al. Protective immune responses elicited in mice by immunization with formulations of poly (lactide-co-glycolide) microparticles. Vaccine. 2002;20(11–12):1524–1531. doi: 10.1016/s0264-410x(01)00509-6. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Alarcon JB, et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nature Medicine. 2002;8(4):415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Dekker JP, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infection and Immunity. 2006;74(12):6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikszta JA, V, Sullivan J, et al. Protective immunization against inhalational anthrax: A comparison of minimally invasive delivery platforms. Journal of Infectious Diseases. 2005;191(2):278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Immunization without needles. Nature Reviews Immunology. 2005;5(12):905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Innovation - Current status and future prospects of needle-free liquid jet injectors. Nature Reviews Drug Discovery. 2006;5(7):543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- Mitragotri S, Blankschtein D, et al. “Ultrasound-Mediated Transdermal Prot”in Delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- Morel PA, Falkner D, et al. DNA immunisation: altering the cellular localisation of expressed protein and the immunisation route allows manipulation of the immune response. Vaccine. 2004;22(3–4):447–456. doi: 10.1016/j.vaccine.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Mulholland WJ, Kendall MAF, et al. Characterization of powdered epidermal vaccine delivery with multiphoton microscopy. Physics in Medicine and Biology. 2004;49(22):5043–5058. doi: 10.1088/0031-9155/49/22/002. [DOI] [PubMed] [Google Scholar]
- Park JH, Allen MG, et al. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. Journal of Controlled Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Park JH, Allen MG, et al. Polymer microneedles for controlled-release drug delivery. Pharmaceutical Research. 2006;23(5):1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- Peachman KK, Rao M, et al. Immunization with DNA through the skin. Methods. 2003;31(3):232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. Microneedles for transdermal drug delivery. Advanced Drug Delivery Reviews. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. Assessment of microneedles for transdermal drug delivery. In: Bronaugh R, Maibach HI, editors. Percutaneous Absorption. New York: Marcel Dekker; 2005. pp. 497–507. [Google Scholar]
- Prausnitz MR, Ackley D, et al. Microneedles for transdermal drug delivery. In: Rathbone HJ, Roberts MM, editors. Modified Release Drug Delivery Systems. New York: Marcel Dekker; 2003. pp. 513–22. [Google Scholar]
- Prausnitz MR, V, Bose G, et al. Electroporation of Mammalian Skin - a Mechanism to Enhance Transdermal Drug-Delivery. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(22):10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz MR, Mikszta J, et al. Percutaneous Penetration Enhancers. Boca Raton, FL: CRC Press; 2005. Microneedles; pp. 239–55. [Google Scholar]
- Prausnitz MR, Mitragotri S, et al. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–24. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- Raju PA, McSloy N, et al. Assessment of epidermal cell viability by near infrared multi-photon microscopy following ballistic delivery of gold micro-particles. Vaccine. 2006;24(21):4644–4647. doi: 10.1016/j.vaccine.2005.08.067. [DOI] [PubMed] [Google Scholar]
- Reed ML, Lye WK. Microsystems for drug and gene delivery. Proceedings of the IEEE. 2004;92(1):56–75. [Google Scholar]
- Resman Z, Metelko Z, et al. The Application of Insulin Using the Jet Injector Dg-77. Acta Diabetologica Latina. 1985;22(2):119–125. doi: 10.1007/BF02590785. [DOI] [PubMed] [Google Scholar]
- Roberts LK, Barr LJ, et al. Clinical safety and efficacy of a powdered Hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine. 2005;23(40):4867–4878. doi: 10.1016/j.vaccine.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Rottinghaus ST, Poland GA, et al. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine. 2003;21(31):4604–4608. doi: 10.1016/s0264-410x(03)00447-x. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Wu MS, et al. Induction of antigen-specific CD8+T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19(7–8):764–778. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- Sarno MJ, Blase E, et al. Clinical immunogenicity of measles, mumps and rubella vaccine delivered by the Injex jet injector: comparison with standard syringe injection. Pediatric Infectious Disease Journal. 2000;19(9):839–842. doi: 10.1097/00006454-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Sarphie DF, Johnson B, et al. Bioavailability following transdermal powdered delivery (TPD) of radiolabeled inulin to hairless guinea pigs. Journal of Controlled Release. 1997;47(1):61–69. [Google Scholar]
- Scheuple RJ, I, Blank H. Permeability of Skin. Physiological Reviews. 1971;51(4):702–746. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- Schramm-Baxter J, Katrencik J, et al. Jet injection into polyacrylamide gels: investigation of jet injection mechanics. Journal of Biomechanics. 2004;37(8):1181–1188. doi: 10.1016/j.jbiomech.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Schramm-Baxter J, Mitragotri S. Needle-free jet injections: dependence of jet penetration and dispersion in the skin on jet power. Journal of Controlled Release. 2004;97(3):527–535. doi: 10.1016/j.jconrel.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Schramm J, Mitragotri S. Transdermal drug delivery by jet injectors: Energetics of jet formation and penetration. Pharmaceutical Research. 2002;19(11):1673–1679. doi: 10.1023/a:1020753329492. [DOI] [PubMed] [Google Scholar]
- Schreier H, Bouwstra J. Liposomes and Niosomes as Topical Drug Carriers -Dermal and Transdermal Drug-Delivery. Journal of Controlled Release. 1994;30(1):1–15. [Google Scholar]
- Schuetz YB, Naik A, et al. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2005;2(3):533–48. doi: 10.1517/17425247.2.3.533. [DOI] [PubMed] [Google Scholar]
- Sintov AC, Krymberk I, et al. Radiofrequency-driven skin microchanneling as a new way for electrically assisted transdermal delivery of hydrophilic drugs. Journal of Controlled Release. 2003;89(2):311–320. doi: 10.1016/s0168-3659(03)00123-8. [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Liepmann D, et al. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4(1):19–25. doi: 10.1517/17425247.4.1.19. [DOI] [PubMed] [Google Scholar]
- Sullivan SP, Murthy N, et al. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Advanced Materials. 2008;20(5):933-+. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Takahashi I, et al. Daily Subcutaneous Erythropoietin by Jet Injection in Pediatric Dialysis Patients. Nephron. 1995;69(3):347–347. doi: 10.1159/000188489. [DOI] [PubMed] [Google Scholar]
- Tacket CO, Roy MJ, et al. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17(22):2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- Tezel A, Sens A, et al. Frequency dependence of sonophoresis. Pharmaceutical Research. 2001;18(12):1694–1700. doi: 10.1023/a:1013366328457. [DOI] [PubMed] [Google Scholar]
- Van Rhoon GC, Wust P. Introduction: Non-invasive thermometry forthermotherapy. International Journal of Hyperthermia. 2005;21(6):489–495. doi: 10.1080/02656730500272963. [DOI] [PubMed] [Google Scholar]
- Verhagen A, Ebels JT, et al. Pharmacokinetics and Pharmacodynamics of a Single-Dose of Recombinant Human Growth-Hormone after Subcutaneous Administration by Jet-Injection - Comparison with Conventional Needle-Injection. European Journal of Clinical Pharmacology. 1995;49(1–2):69–72. doi: 10.1007/BF00192361. [DOI] [PubMed] [Google Scholar]
- Wang PM, Cornwell M, et al. Precise microinjection into skin using hollow microneedles. Journal of Investigative Dermatology. 2006;126(5):1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- Weller C, Linder M. Jet Injection of Insulin Vs Syringe-and-Needle Method. Journal of the American Medical Association. 1966;195(10):844. doi: 10.1001/jama.1966.03100100096027. [DOI] [PubMed] [Google Scholar]
- Weniger BG. Innovative Administration Systems for Vaccines. Rockville; Maryland: 2003. Jet Injection of Vaccines: Overview and Challenges for Mass Vaccination with Jet Injections (JIs) [Google Scholar]
- Wermeling DP, Banks SL, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058–63. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G, Johnson J, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Zhang I, Shung KK, et al. Hydrogels with enhanced mass transfer for transdermal drug delivery. Journal of Pharmaceutical Sciences. 1996;85(12):1312–1316. doi: 10.1021/js9601142. [DOI] [PubMed] [Google Scholar]