Abstract
The ability of isolated brain mitochondria to accumulate, store and release calcium has been extensively characterized. Extrapolation to the intact neuron led to predictions that the in situ mitochondria would reversibly accumulate Ca2+ when the concentration of the cation in the vicinity of the mitochondria rose above the ‘set-point’ at which uptake and efflux were in balance, storing Ca2+ as a complex with phosphate, and slowly releasing the cation when plasma membrane ion pumps lowered the cytoplasmic free Ca2+. Excessive accumulation of the cation was predicted to lead to activation of the permeability transition, with catastrophic consequences for the neuron. Each of these predictions has been confirmed with intact neurons, and there is convincing evidence for the permeability transition in cellular Ca2+ overload associated with glutamate excitotoxicity and stroke, while the neurodegenerative disease in which possible defects in mitochondrial Ca2+ handling have been most intensively investigated is Huntington's Disease. In this brief review evidence that mitochondrial Ca2+ transport is relevant to neuronal survival in these conditions will be discussed.
Keywords: mitochondria, brain, calcium, excitotoxicity, permeability transition, Huntington's Disease
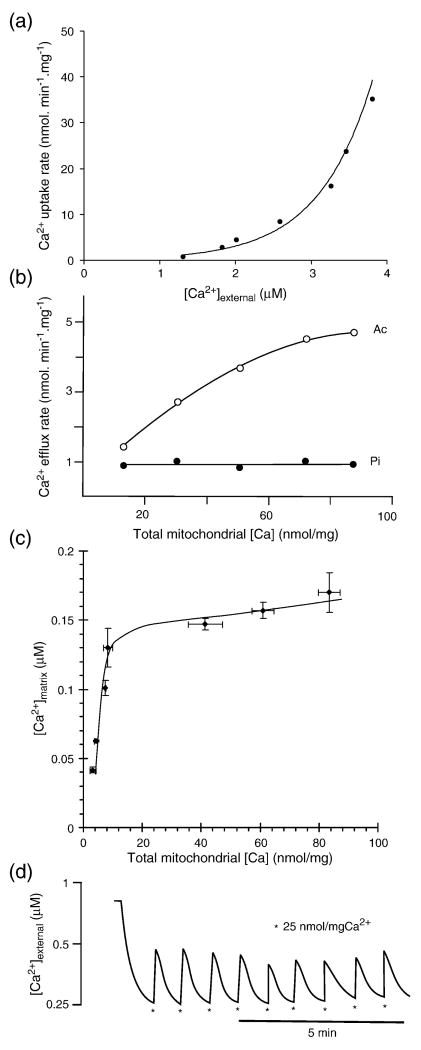
Isolated, respiring mitochondria accumulate Ca2+ when the concentration of the cation in their immediate environment rises above the ‘set-point’ at which the rates of uptake via the Ca2+ uniporter and efflux via the mitochondrial Na+/Ca2+ exchanger are in balance [1] (Fig. 1a). With isolated brain mitochondria under plausibly physiological conditions in the presence of external adenine nucleotides and phosphate the set-point is close to 0.5μM [2], Fig. 1a, and this closely corresponds to values estimated for net Ca2+ accumulation by mitochondria in situ within cultured neurons [3-6]. When more than 10nmol of Ca2+ /mg protein has been taken up, the parallel accumulation of phosphate leads to the formation of a calcium phosphate complex in the matrix which effectively buffers the free matrix Ca2+ ([Ca2+]m) at about 0.2 to 2μM[7,8], Fig. 1c, similar values being reported for in situ neuronal mitochondria [9]. The calcium phosphate complex is maintained by the high matrix pH [8] and its composition has been investigated by Kristian et al. [10] who performed X-ray spectral analysis of calcium phosphate precipitates in rapidly frozen Ca2+-loaded brain mitochondria and reported a variable calcium to phosphate ratio depending on loading conditions, suggesting that the composition of the complex is somewhat flexible. This matrix Ca2+ complexation in turn clamps the activity of the Na+/Ca2+ exchanger at a value that is independent of any further increase in total matrix Ca2+ [11], Fig. 1b, and under these conditions the set-point is independent of the total matrix Ca2+. The result is that the isolated mitochondria can act as perfect ‘buffers’ of free Ca2+ in their vicinity, responding to multiple additions of Ca2+ to the medium by lowering the extra-mitochondrial free Ca2+ concentration ([Ca2+]e) back to the set-point after each addition (Fig. 1d). This can continue until in excess of 500nmol Ca2+ /mg protein has been accumulated and the onset of bioenergetic collapse associated with the permeability transition [8].
Fig. 1.
Ca2+ transport by isolated mitochondrial. (a) Kinetics of Ca2+ uptake via the Ca2+ uniporter in rat liver mitochondria as a function of external free Ca2+ concentration. (b) Kinetics of Ca2+ release via the Ca2+/2H+ exchanger in rat liver mitochondria as a function of matrix Ca2+ load; Ac, phosphate depleted mitochondria in the presence of 5mM acetate, Pi, mitochondria in the presence of 3.3mM phosphate. The efflux rate in Pi is independent of total matrix Ca2+ because free matrix Ca2+ is buffered by the Ca2+-phosphate complex. (c) Rat liver mitochondrial matrix free Ca2+ concentration as a function of matrix Ca2+ load. Note the transition from varying to buffered free Ca2+ at 10nmol Ca2+/mg when the Ca2+ phosphate complex starts to form. (d) Rat brain mitochondria buffer the external free Ca2+ concentration close to 0.25μM in Mg2+-free media. For experimental details see [2,8,11]
The inference from these in vitro studies is that mitochondria within intact neurons will act as temporary reversible stores of Ca2+, accumulating the cation when the cytoplasmic free Ca2+ ([Ca2+]c) is above the set-point, for example following a train of action potentials, and releasing the cation back to the cytoplasm when the plasma membrane Ca2+-ATPases succeed in pumping down [Ca2+]c to below the set-point in the subsequent resting phase. As will be discussed below, there is convincing evidence that this occurs in intact neurons, lowering peak values of [Ca2+]c but extending the duration of elevated cytoplasmic Ca2+, and modulating cell signaling and transmitter release.
For this cytoplasmic buffering to occur with no deleterious consequences for the mitochondria and hence the cell, it is clearly essential that the period during which [Ca2+]c is elevated above the set-point is brief to avoid mitochondrial Ca2+ overload. If however a tonic activation of Ca2+ entry pathways across the plasma membrane is sufficient to retain [Ca2+]c above the set-point, then an inexorable accumulation of Ca2+ within the matrix will occur until maximal capacity is exceeded and the permeability transition ensues, initiating rapid necrotic cell death. Conversely, in a quiescent neuron where [Ca2+]c is close to 0.1μM the mitochondrial matrix will be largely depleted of Ca2+, the calcium-phosphate complex will not form and [Ca2+]m will vary as a function of [Ca2+]c. Isolated mitochondria smoothly transition into the regulatory mode when the total matrix Ca2+ is decreased below 10nmol/mg [8]. This ‘regulatory’ rather than ‘storage’ range can control the activity of the TCA cycle in many cells (reviewed in [12]).
Under conditions of respectively, low, intermediate and excess matrix Ca2+ accumulation, mitochondria in neurons may therefore be involved in intra-mitochondrial metabolic regulation, in controlling the extent and duration of cytoplasmic Ca2+ elevations and in the extreme case in the very life or death of the neuron. Studies on the roles of mitochondrial Ca2+ transport in the brain may thus be divided into the ‘physiological’ where the uptake of Ca2+ into the matrix is reversible and serves a regulatory role and the ‘pathological’ that generally involves activation of the mitochondrial permeability transition. These will now be considered separately.
1. ‘Physiological’ transport of Ca2+ by brain mitochondria
Mitochondrial Ca2+ uptake in neurons may play multiple roles, including facilitating the uptake of Ca2+ through voltage and receptor activated Ca2+ channels and decreasing feedback inhibition by a sub-membrane ‘calcium cloud’ [13]. Mitochondria will also compete for cytoplasmic Ca2+ with the plasma membrane and endoplasmic reticulum Ca2+-ATPases for cytoplasmic Ca2+. Mitochondria could also prolong periods of elevated [Ca2+]c in stimulated neurons by reversibly sequestering and releasing the cation. David and Barrett [14,15] have investigated how inhibition of mitochondrial Ca2+ transport affects quantal release of transmitter during repetitive stimulation of mouse motor nerve terminals. Comparing responses in the presence of oligomycin with those after oligomycin plus antimycin A (to control for bioenergetic effects) the authors concluded that mitochondrial Ca2+ uptake prevented desynchronization of quantal release and minimized depletion of releasable vesicles during tetanic stimulation. In contrast, inhibition of e.r. Ca2+ uptake with cyclopiazonic acid had minimal effects on [Ca2+]c or quantal release.
In investigating the physiology and pathology of neuronal mitochondrial Ca2+ transport, however, it is important to take a holistic view of the mitochondrion and its in situ bioenergetics, rather than focusing purely on the transport of the cation. Thus an elevation in [Ca2+]c sufficient to allow net mitochondrial Ca2+ accumulation imposes a dual energetic demand on the mitochondrial proton circuit. Firstly the elevation in [Ca2+]c will activate plasma membrane and endoplasmic reticulum Ca2+-ATPases with a resultant increase in ATP demand. Secondly, the direct uptake of the cation into the matrix utilizes the proton circuit and directly competes with mitochondrial ATP synthesis. Indeed it was shown many years ago that a high rate of Ca2+ uptake could even lower the protonmotive force sufficiently to cause a transient reversal of the ATP synthase [16].
1.1 Isolated mitochondria
Brain mitochondrial preparations are inherently heterogeneous [7], as well as differing in Ca2+-sensitivity between strains [17] and brain regions [18,19]. ‘Non-synaptic’ mitochondria obtained by density gradient centrifugation include mitochondria from neuronal cell bodies and glia. Treatment of the crude mitochondrial pellet with low concentrations of digitonin liberates pre-synaptic mitochondria from the associated synaptosomes [20]. Alternatively, mitochondria can be prepared from cultured neurons and glia by nitrogen cavitation [21].
When determining Ca2+ uptake by mitochondria, a clear distinction must be made between the free and total cation. Determination of total matrix Ca2+ with isolated mitochondria is straightforward, defined amounts of Ca2+ can be added to the incubation while monitoring [Ca2+]e to ensure uptake into the matrix [22]. Alternatively Ca2+ can be released into the incubation by addition of a protonophore, although experiments with isolated mitochondria suggest that not all the Ca2+ accumulated in the presence of phosphate is readily released by protonophore [10].
1.2 Mitochondrial Ca2+ transport in intact neurons
Early functional imaging studies of neurons with cytoplasmic free Ca2+ ([Ca2+]c) indicators [23] demonstrated that the neuronal cytoplasm can experience large elevations in [Ca2+]c under a variety of stimulation conditions including elevated KCl [24], high frequency field stimulation [4] and NMDA receptor activation [25]. It was therefore predicted that the in situ neuronal mitochondria would reversibly accumulate Ca2+ in response to elevated [Ca2+]c [26]. Werth and Thayer [4] obtained evidence that mitochondria in cultured rat dorsal root ganglion neurons reversibly accumulated calcium in response to high frequency field stimulation, and this was subsequently extended by different groups to KCl and glutamate induced calcium loading [13,27,28]. It is relevant to note that the ‘set-points’ determined for isolated mitochondria [2] correspond closely to those reported for intact neurons [4], and that the values for [Ca2+]m reported for Ca2+ -loaded in situ neuronal mitochondria [29] also closely correspond to values obtained with isolated mitochondria [8]. This suggests that neuronal mitochondria do not utilize adjacent endoplasmic reticulum to facilitate their Ca2+ accumulation, and may thus differ from mitochondria in cell lines with extensive e.r. [30].
As in the case of isolated mitochondria, it is important to maintain the distinction between total and free matrix Ca2+ in intact neurons. David et al. [9], see also [31,32], monitored [Ca2+]m in lizard motor terminals during trains of action potentials, using the low-affinity matrix-located indicator rhod-5N (Kd 320μM). [Ca2+]m increased to a plateau close to 1μM. Importantly, increasing the frequency of stimulation increased the rate at which [Ca2+]m rose to this plateau, but did not affect the plateau itself, suggesting that the matrix has a large capacity to buffer Ca2+ at this value. This closely corresponds to the data obtained with isolated mitochondria [8], where the calcium phosphate complex maintained [Ca2+]m at about 2μM over a 50-fold range of total Ca2+ [8]. In contrast values for [Ca2+]m reported using targeted aequorins tend to be considerably higher, e.g. [33]. The reasons for these discrepencies remain to be elucidated.
The specific, focused manipulation of mitochondrial Ca2+ in intact neurons is not trivial. Early studies where protonophores were employed to depolarize the mitochondria [6,34] of course dramatically affected the cell's bioenergetics. In cells with a high glycolytic capacity, the role of mitochondrial Ca2+ transport can be investigated by comparing cell function in the presence of oligomycin with that in the presence of oligomycin plus an electron transport chain inhibitor [13]. Since ATP generation is already glycolytic following oligomycin, the further addition of an electron transport inhibitor will allow Δψm (and hence mitochondrial Ca2+ transport) to decay with no further effect on the cell's bioenergetics. Even the widely employed Ca2+ ionophore ionomycin must be used with great care, since it induces a dissipative Ca2+ cycling across the inner mitochondrial membrane [35]. Since [Ca2+]c (and hence Ca2+ uniport activity) is elevated by the ionophore's action at the plasma membrane, this induces a potent mitochondrial uncoupling manifested by increased respiration [36]. Indeed low micromolar concentrations of the ionophore can collapse the mitochondrial membrane potential (Δψm) in cultured neurons leading to rapid necrotic cell death [35]. It would be instructive to determine the proportion of the 20,000 references in the literature utilizing such Ca2+ ionophores where this underlying bioenergetic effect is an unrecognized complication. It is generally accepted that the hexavalent cation ruthenium red is impermeant across the plasma membrane of most cells and that it acts by inhibiting Ca2+ uptake into the cell (reviewed in [37]). Ru360 has been proposed to be a more selective, cell permeant uniport inhibitor [38], but again there is considerable uncertainty as to its ability to enter cells and act specifically at the mitochondrial Ca2+ uniporter [15,37].
2. Pathological' transport of Ca2+ by brain mitochondria
2.1 Isolated mitochondria
As will become apparent in this review, much of the investigation into the role of mitochondrial Ca2+ transport in CNS diseases has focused around the induction of the permeability transition. There has been some controversy over the nature or even existence of the permeability transition in isolated brain mitochondrial preparations. In part this is because the light-scattering changes of isolated brain mitochondria during Ca2+ loading differ from e.g. liver mitochondria. The initial stages of Ca2+ accumulation by non-synaptic brain mitochondria produces an increase in light-scattering, probably due to the increased refractive index of the Ca2+loaded matrix [8]. An early study [39] showed that cyclosporine A (CsA) failed to protect cultured hippocampal neurons against mitochondrial swelling and depolarization associated with Ca2+-ionophore-induced Ca2+ loading, although as discussed above this could have been a consequence of the unrecognized bioenergetic consequences of the ionophore addition. Subsequently, the ability of CsA to decrease the Ca2+-sensitivity of isolated brain mitochondria has been shown by several groups [18,40,41]. In the presence of adenine nucleotides, it was found that the capacity of non-synaptic brain mitochondria to accumulate Ca2+ during the slow continuous infusion of the cation could be greatly increased (from 600 to 1500nmol/mg protein) in the presence of CsA [8].
The nature and regulation of the mitochondrial permeability transition have been extensively reviewed recently [42] and discussion here will be limited to the brain. The calcium loading capacity of isolated brain mitochondria has traditionally been determined by sequential bolus additions of the cation while monitoring the ability of the mitochondrial incubation to restore a low free Ca2+ concentration in the incubation. However, since each bolus addition creates a transient energy demand on the mitochondria while affecting mitochondrial membrane potential and matrix pH these may distort an accurate estimation of loading capacity which can be avoided by slowly perfusing calcium into the medium while monitoring the external free calcium concentration [8]. Under these conditions loading capacity can be separated from the bioenergetic demands that accompany bolus additions.
There is disagreement in the literature as to the ability of cyclosporine to delay the permeability transition during massive calcium loading. In the author's laboratory 1 μM cyclosporin A increased the calcium loading capacity of rat non-synaptic mitochondria from 800 to 1600 nmol/mg protein [8]. Species differences are frequently ignored, but it is significant that non-synaptic brain mitochondria from C57Bl/6J mice are able to accumulate approximately twice as much Ca2+ as the corresponding mitochondria from Sprague-Dawley rats before induction of the permeability transition [17]. Synaptic mitochondria prepared from synaptosomes are more sensitive to Ca2+, have a higher level of cyclophilin D and are more vulnerable to the permeability transition unless cyclosporine A is present [43-45]. Knock-down of cyclophilin D in PC12 cells decreases their sensitivity to the permeability transition [43] and mitochondria from CypD-knockout mice are resistant to the permeability transition [46]. Some of the variation in reported CsA sensitivity was explained by the observation of Bambrick et al. [47] that only astrocytic (but not neuronal) mitochondria displayed a CsA-sensitive Ca2+ accumulation capacity. In addition permeabilized astrocytes could accumulate more Ca2+ than permeabilized granule neurons on a per-cell or mitochondrial protein basis. Thus a failure to observe cyclosporin protection in neurons cannot be taken as evidence against a permeability transition. A number of cyclosporine A analogues have been developed that are more potent against the permeability transition while being less active as immuno-suppressants and calcineurin inhibitors [48-51] and can therefore provide less ambiguous information on the permeability transition in intact cells than CsA.
Even though the permeability transition is likely to be a stochastic process at the level of the individual mitochondrion, the release of Ca2+ from an individual mitochondrion will increase the Ca2+ concentration in its neighborhood. In a very dilute incubation (e.g. 0.05mg protein/ml in a perfusion study [8]) the likelihood is that Ca2+ released by the neuronal sub-fraction will be accumulated by the astrocytic mitochondria and that the overall incubation will show the CsA sensitivity characteristic of the latter. However, in a relatively concentrated isolated mitochondrial incubation, and even more so in the intact cell, the probability of a chain reaction of Ca2+ overload following an initial permeability transition in a sub-population will increase, in which case the mixed population might show the CsA-independent characteristics of the neuronal sub-population.
2.2 Monitoring the permeability transition in intact neurons and in vivo
The permeability transition in isolated mitochondrial preparations can be monitored by decreased light scattering, release of cytochrome c, Ca2+ and low molecular weight matrix components and collapse of Δψ. In the intact cultured neuron mitochondrial swelling can readily be detected by confocal imaging as a thread-like to globular shape transition reflecting matrix Ca2+ loading [52,53] but this cannot automatically be equated with the permeability transition since it can in many cases be reversed with no apparent loss of function [52,54]. Mitochondria that have undergone the permeability transition are depolarized and do not accumulate membrane potential indicators, and are thus not visible in fluorescent microscopy. Elmore et al. [55] have employed the loss of fluorescence resonance energy transfer (FRET) between the membrane potential-independent MitoTracker Green and TMRM+ and the resulting increase in direct MitoTracker fluorescence emission to provide a positive signal reflecting membrane potential collapse. An alternative method has involved the cytoplasmic loading of calcein, which leaves dark ‘holes’ corresponding to the mitochondria. Activation of the permeability transition leads to entry of calcein into the matrix and the disappearance of the holes [56].
3. Mitochondrial Calcium transport in CNS Disease
Neuronal dysfunction can be modeled by reproducing the actual insult in the intact animal (e.g. vessel occlusion in stroke studies) followed by isolation of mitochondria or synaptosomes. Alternatively, cell cultures can be exposed to stresses related to those experienced in vivo (e.g. glutamate exposure of neuronal cultures to simulate the glutamate excitotoxicity associated with ischemia). The limitation with this approach is that the cell cultures are neonatal and may thus lack the critical age-related changes that play such a major role in the incidence of most neurodegenerative diseases. In addition the normal physiological interconnections and glial interactions are usually missing, while in the specific case of calcium transport, the extra-cellular volume in cell culture is vast compared with in vivo and the essentially infinite extra-cellular Ca2+ compartment may exaggerate the magnitude of Ca2+ accumulation occurring in vivo.
The role of mitochondrial Ca2+ in this context can be approached at several levels, starting with the isolated mitochondria, and ranging through cell lines (including cybrids), primary neuronal cultures, isolated nerve terminals (synaptosomes), acute and organotypic tissue slices to in vivo rodent models. Except in the few cases where human material is available the neuronal dysfunction has to be created, for example by mouse genetic manipulation. If there is sufficient evidence to implicate an alteration of mitochondrial function, for example impaired Complex I function in Parkinson's Disease (PD), then the consequence of this can be investigated by in vitro or in vivo addition of Complex I inhibitors, although of course the relevance of the model is entirely dependent upon the strength of the in vivo evidence, and this approach gives little indication of the upstream or parallel events that might have caused the dysfunction in the disease itself. However, a similar pathology between the human disease and a chemical intervention is encouraging - for example the demonstration that systemic rotenone in rodents can cause a similar striatal pathology to the disease [57].
While the focus of this review is on Ca2+ and its involvement in CNS dysfunction, it is essential to bear in mind that mitochondrial Ca2+ transport, oxidative stress and bioenergetics form a closely inter-related network. Deficient ATP generation in a cell can lead to a failure of plasma membrane Ca2+ pump activity and Ca2+ overload; oxidative stress can restrict ATP generating capacity. Ca2+ uptake into the cell and its further transport into the mitochondrion can put a heavy load on the mitochondrial proton circuit, in some cases leading to neuronal cell death induced by an energy crisis [58]. While it is frequently stated that Ca2+ accumulation by in situ mitochondria results directly in oxidative stress, there is no known mechanism to cause this, and indeed a decrease rather than an increase in H2O2 is seen in respiring isolated rat brain mitochondria as they accumulate Ca2+, consistent with a lowered Δψ [59].
As previously mentioned, much of the literature on mitochondrial Ca2+ and neurological disease has focused on the permeability transition,. These studies have recently been extensively reviewed in relation to excitotoxicity [60], ischemia [61], traumatic brain injury [62], and Parkinson's and Alzheimer's Diseases [63]. A central question must be posed about any in vitro experiment in this context, and that is whether the proposed involvement of the permeability transition holds for the in vivo condition, rather than being an in vitro phenomenon, and if it does, whether it is directly mediated by some dysfunction unique to the disorder, of whether it is a more general manifestation of excitotoxicity. Finally, even if excitotoxicity is implicated as the underlying mechanism in the neuronal cell death, is it in turn caused by altered Ca2+ handling by the mitochondrion, by oxidative stress (e.g. [64-66]), by impaired mitochondrial metabolism or energetics (e.g. [58,67,68]) or mitochondrial trafficking in the neuron (e.g. [69]), by altered ion transport at the plasma membrane or by some to other subtle prime cause? These questions of course determine whether it is worthwhile to look for changes in Ca2+ handling of isolated brain mitochondria from disease models or whether cell culture or ex vivo preparations such as synaptosomes are more appropriate.
In this author's opinion, there is convincing evidence for the involvement of the permeability transition in excitotoxicity from the level of the cultured neuron to in vivo in association with stroke, ischemia, traumatic brain injury and other conditions associated with receptor-mediated cell Ca2+ overload. With the possible exception of Huntington's Disease (HD) evidence for a defect at the level of isolated mitochondrial Ca2+ transport is sparse. The remainder of this short review will therefore focus on in vitro and in vivo excitotoxicity and models of HD.
3.1 Glutamate Excitotoxicity and Stroke
The strongest evidence for a patho-physiological role the permeability transition occurs in the context of neuronal cell death in the aftermath of stroke as a result of glutamate excitotoxicity. Chronic NMDA receptor activation by glutamate released from a focal infarct underlies the progressive expansion of the volume of necrotic tissue in the aftermath of stroke [70]. The characteristics of the NMDA receptor that are important for the primary roles of the receptor in learning and memory, namely Ca2+ as well as Na+ transport and only partial desensitization in the continued presence of the ligand, are potentially lethal for the neurons in the penumbra surrounding the infarct. The mechanism of the resulting cell death as a consequence of ‘glutamate excitotoxicity’ has been extensively studied at the level of the cultured neuron and some of the earliest studies from the group of Choi [71-74] established the essential role of Ca2+ in cell death resulting from chronic NMDA receptor activation. Cortical neurons exposed to glutamate in the absence of calcium underwent extensive osmotic swelling, and in the presence of the cation this was followed by a delayed neuronal disintegration [72]. Choi proposed that calcium entry through the NMDA receptor played a dominant role in the subsequent cell death. Randall and Thayer [25] monitored real-time change is in cytoplasmic free calcium in hippocampal neurons exposed to glutamate and found that cell necrosis was preceded by a delayed massive rise in cytoplasmic free calcium concentration. This was further investigated by Tymianski and colleagues [75] who extensively examined the conditions for this stochastic ‘delayed calcium deregulation’ and concluded that the power play of calcium entry was equally or more important than the total calcium uptake, although subsequent studies with low affinity calcium probes [76] have revised this view.
The studies with isolated mitochondria discussed above led to predictions that the intra-neuronal mitochondria would be major sinks of calcium entering the neurons under conditions of extensive calcium loading and elevated cytoplasmic free calcium. In particular, if the non-desensitizing conductance of the NMDAR in the presence of exogenous glutamate is sufficient to maintain [Ca2+]c above the Ca2+ set-point for the in situ mitochondria, then the organelles will inexorably load with Ca2+ until their capacity is exceeded [13]. It is notable that an inverse relationship is seen at a single cell level in a population of cultured neurons exposed to glutamate between the extent of the immediate slightΔψm depolarization reflecting the rate of Ca2+ accumulation into the mitochondria, and the survival time before the neurons undergoes delayed Ca2+ deregulation [77]. The extent of delayed calcium deregulation was found to be proportional to the time for which mitochondria remained polarized, suggesting that a critical factor was the amount of calcium accumulated by the mitochondria [78]. Furthermore, cells with active glycolysis could be protected against delayed calcium deregulation by inhibiting mitochondrial calcium accumulation with the combination of rotenone and oligomycin [13]. Interestingly, bulk cytoplasmic free calcium elevations in response to glutamate were depressed by this combination of inhibitors in these cells [78]. Since no decrease in calcium entry was detected, it was concluded that inhibition of calcium uptake by mitochondria in close apposition to the plasma membrane calcium ATPase could remove a competition between the mitochondria and the ion pump for calcium and enhance net calcium extrusion from the cell.
Cell death in response to mitochondrial calcium loading immediately suggests the involvement of the permeability transition. However, no consistent protection was been reported for cultured cerebellar granule neurons exposed to glutamate in the presence of inhibitors of the permeability transition pore such as cyclosporine A [78], while mitochondria isolated from these cells showed no protection by cyclosporine A [79]. This is in contrast to the extensive protection afforded by the inhibitor during calcium loading of isolated brain mitochondria [8]. However, the report of cyclosporine A-sensitive Ca2+ accumulation by mitochondria prepared from the synaptosomal fraction [80] indicates that there remain questions concerning the cellular and sub-cellular localization of permeability transition-prone mitochondria.
Mitochondria in glutamate exposed cultured neurons undergo an extensive swelling that is associated with a cessation of trafficking within the neurite and dendritic beading [53,81-82]. Synchronous with the elevations in cytoplasmic free calcium mitochondria undergo a massive depolarization [77]. The matrix pH changes associated with glutamate exposure are particularly revealing; from a starting pH close to eight a transient alkalinization is seen, followed by an acidification of the matrix to pH of about 7.1. At the same time a cytoplasmic acidification occurs and the Delta pH across the membrane is largely retained [83]. Delayed calcium deregulation is associated with a further matrix acidification and the abolition of the proton gradient. It is notable that the matrix acidification will lead to a destabilization of the extensive matrix calcium phosphate precipitate, and would be predicted to greatly increase the matrix free Ca2+ [8]. Since the membrane potential and pH gradient collapse during the cytoplasmic calcium deregulation, and respiration is inhibited [84] this is consistent with a permeability transition.
While much of the focus in excitotoxicity is on Ca2+ transport, it must be born in mind that the NMDAR allows the entry of both Ca2+ and Na+. Indeed, even though a typical NMDAR may have a Ca2+/Na+ selectivity of about 10, the concentration of Na+ in the external medium is 100-fold higher than Ca2+ with the result that Na+ entry dominates. Recent studies on the bioenergetic demand of the resultant activation of the Na+/K+-ATPase to extrude the cation [85] have shown that under some circumstances the entire ATP generating capacity of the in situ mitochondria can be diverted to provide ATP for the Na+/K+-ATPase and accordingly that a variety of stresses that are in themselves non-lethal, such as partial Complex I inhibition [85], ‘mild uncoupling’ [86] and oxidative stress following glutathione depletion [84] can result in a bioenergetic deficiency that leads to rapid necrotic cell death. It is therefore important to bear in mind that Ca2+-sensitivity in ischemic models can be greatly amplified by factors that limit ATP generation, as first proposed by Henneberry [87], see also [67].
Cyclosporine A has been shown to be neuroprotective in vivo in both focal and global ischemia [88,89]. The ultimate test for the involvement of the permeability transition in glutamate excitotoxicity accompanying cerebral ischemia is to investigate the in vivo consequences of Cyp D knockout. Liver, heart and brain mitochondria from Cyp-D deficient mice are resistant to Ca2+ -induced swelling and show a capacity to accumulate Ca2+ equal to those from control mice in the presence of cyclosporine A [46,90,91]. Importantly, the infarct volume 24hr after middle cerebral artery occlusion was reduced to less than 50% of control values [90].
3.2 Mitochondrial Ca2+ transport in models of Huntington's Disease
Huntington's disease is the most studied of the group of autosomal-dominant trinucleotide repeat expansion diseases that include at least 10 poly-Q disorders (reviewed in [68,92,93]). The affected protein is huntingtin (htt) and the threshold for pathology occurs at >36Q repeats, corresponding to the appearance of aggregated htt. The severity and age of onset of symptoms are related to the repeat length which can be up to 120Q. Htt is an essential protein that is widely expressed in cells; thus the homozygous knockout mouse dies in embryo and heterozygous deletion of the htt gene results in neurodegeneration [93]. Despite this, its normal function is still debated. There is evidence that it functions as a nucleo-cytoplasmic shuttling protein involved in the regulation of transcription [92], and that it affects trafficking in neurons [94], while mitochondrial interactions have been shown [95]. HD is an aging-related disease and it has been proposed that a change in function of the expanded htt accelerates normal aging-related changes in the brain [92]. A variety of transgenic mouse models of HD have been developed. Those expressing truncated human htt (e.g. R6/2 and R6/1) have a severe and widespread pathology and are severely diabetic, while those expressing full-length expanded human htt on a yeast artificial chromosome, such as the YAC128 mouse, show more selective pathology. Knock-in models producing CAG-expanded endogenous mouse htt generally display a mild phenotype [96].
Post-mortem analysis of mitochondrial respiratory chain complex activities from HD patients revealed a greater than 50% reduction in Complex II and Complex III activity, relative to protein or citrate synthase activity in the caudate, with a lesser decrease in Complex IV and no change in Complex I[97]. The putamen showed a more than 60% reduction in complex activities (again with the exception of Complex I) [98]. Cybrid technology, where platelets from HD patients were fused with SH-SY5Y neuroblastoma cells, failed to reveal any bioenergetic differences from control samples suggesting that nuclear DNA can correct any deficiencies in the platelets' mitochondria[99]. As in the case of Parkinson's Disease there is therefore persuasive evidence for respiratory chain dysfunction in the mitochondria of the surviving cells. It is therefore important in the present context to establish whether any apparent change in mitochondrial Ca2+ handling reflects a primary action on mitochondrial transport or storage, or is a downstream consequence of the inhibited bioenergetic function.
The close association between restricted bioenergetic function and enhanced susceptibility to excitotoxicity has been discussed above. Indeed there is extensive evidence (reviewed in [100,101]) for the involvement of NMDAR function and excitotoxicity in cell death associated with HD. Significantly, a YAC transgenic mouse expressing 72 CAG repeats in htt showed enhanced glutamate excitotoxicity in vivo and in cultures of striatal medium spiny neurons [102], while the NMDAR antagonist MK801 reduced malonate-induced striatal cell death in vivo although it was without effect on lesion size in transgenic R6/1 mice [103]. The excitotoxic cascade discussed above may thus contribute to the neuronal susceptibility in HD, although the high susceptibility of the background strain (FVB/N) to excitotoxicity [104] means that the findings cannot automatically be extrapolated to the human disease. Fernandez et al. [101] monitored [Ca2+]c and Δψm in parallel in single medium spiny neurons prepared from wild-type and YAC128 mice and observed that maximal NMDAR activation induced DCD (collapse of Δψm and failure to restore basal [Ca2+]c after removal of NMDA) in the YAC128 neurons but not wild-type. No significant difference was observed in NMDAR currents and it was concluded that the mitochondria in the YAC128 cells were more sensitive to the permeability transition. A similar conclusion was reached in a study with immortalized striatal cells derived from Q111 mice [105], however again caution must be exerted in implying an excitotoxic mode of neuronal death in the disease.
While experimental Complex II deficiency with malonate or 3-nitropropionic acid (3NPA) can reproduce some of the pathology of the disease [106-108], using these inhibitors to ‘model’ the disease in vitro will not take account of any parallel or upstream consequences of the in vivo pathology and should be interpreted with caution.
The synergism between impaired mitochondrial ATP generation and susceptibility to glutamate excitotoxicity discussed above in the context of stroke is also apparent in striatal cultures exposed to 3NPA [109]. However somewhat surprisingly in view of the impaired mitochondrial bioenergetics implicated in the in vivo disease, the R6/1 and R6/2 mice show an increased resistance to glutamate excitotoxicity induced by quinolinic acid in vivo [110,111] which could not be ascribed to decreased NMDA receptor expression but which correlated with the appearance of nuclear inclusions. It was proposed that mutant htt causes a sub-lethal metabolic stress that causes cell defense mechanisms to be up-regulated against subsequent excitotoxic insult [103]. Thus medium spiny neurons from R6/2 mice showed an increased basal [Ca2+]c while at the same time being more resistant to quinolinate-induced delayed Ca2+ deregulation [111].
Many studies have been performed with isolated brain mitochondria, with the constraints due to heterogeneity discussed above. In particular, since there is evidence that a normal physiological role of htt is in the control of anterograde/retrograde transport in neurons [94] isolated mitochondrial preparations will not be able to detect bioenergetic defects that are a consequence of an altered distribution of mitochondria throughout the neuron. A study by Panov et al. [112] investigated Ca2+ transport in mitochondria prepared from transformed lymphocytes of control and HD patients and observed that the latter were depolarized by 12mV relative to the controls and showed a significant reduction in their Ca2+ accumulation capacity in the absence of exogenous adenine nucleotides. Similar reductions in Δψ and Ca2+ accumulation capacity in the presence of ADP and cyclosporine A were seen in brain mitochondria from transgenic mice expressing a yeast artificial chromosome with 72, but not 18, CAG repeats [112]. No difference in basal [Ca2+]e was shown, but the YAC72 mitochondria took up Ca2+ more slowly than those from YAC18 mice. In view of the reported respiratory chain defects it is possible that the slowed Ca2+ uptake, and the partial initial depolarization reflects an inhibited respiratory chain. Respiratory parameters were not quantified in this study [112] but a subsequent study revealed no differences in respiratory rates between lymphoblast mitochondria derived from control and HD subjects [113].
Interestingly some effect was shown from the direct addition of 5μM htt fusion proteins with long but not short Q-repeats [112]. It was however subsequently reported that the fusion proteins exerted a direct inhibitory effect on the respiration of liver and lymphoblast mitochondria [95].
A decreased Ca2+-accumulation capacity was reported for mitochondria prepared from clonal striatal cell lines containing expanded htt [114], but a detailed study by Brustovetsky et al. [18] presented different conclusions - no increase in Ca2+-sensitivity was observed between striatal mitochondria from control and HD mice (knock-in and R6/2). Indeed in young mice expanded htt produced resistance to Ca2+ [18]. Similarly, Oliveira et al. [115], using the slow Ca2+ infusion technique [8] with isolated mouse forebrain mitochondria in the presence of adenine nucleotides, albumin and oligomycin to optimize mitochondrial stability observed either no reduction in Ca2+ accumulation capacity (Hdh150 knock-in mice) or a statistically significant increase (R6/2 and YAC128 mice).
The variable and somewhat confusing data on mitochondrial Ca2+ handling obtained with the isolated organelles has favoured investigations with intact cultured neurons. The advantages are that pure neuronal mitochondria are present in their physiological milieu, that artifacts associated with the mitochondrial preparation are absent and that the mitochondria can be exposed to patho-physiologically relevant stresses. The limitation of course is that studies are restricted to neonatal preparations and that the age-related component of the disease cannot be modeled. This review has emphasized the importance of considering mitochondrial bioenergetics and Ca2+ transport in parallel. A major experimental deficiency until recently has been the lack of sensitive techniques to monitor the respiration of cultured neurons. This was resolved with the development of the cell respirometer [116] which allows the respiration of coverslip-attached cells to be monitored in parallel with single cell fluorescent functional analysis. In our experience the small amount of material means that it is difficult to prepare ‘well coupled’ isolated mitochondria from specific brain regions of single transgenic mice. Indeed, in the HD literature is difficult to find details of the basic respiratory control parameters for the various preparations used for Ca2+ transport studies, raising the suspicion that, as in our hands, the preparations may be functionally compromised. By avoiding isolation artifacts, the cell respirometer allows the respiratory parameters to be determined in situ.
A decrease in ‘spare respiratory capacity’, the ability of in situ mitochondria to increase their respiration in response to an increased ATP demand has been proposed to be an important factor defining the long-term survivability of neurons in the face of a variable energy demand [58]. In view of the evidence that Complex II may be deficient in HD it was somewhat surprising that analysis of the respiration of striatal neurons from wild-type (Hdh+/+) and heterozygous knock-in (Hdh150/+) litter mates revealed no significant difference in maximal respiratory capacity in the presence of the protonophore FCCP [115] and that in the basal state the mitochondria were only respiring at about 20% of their respiratory capacity, half of which drove the endogenous mitochondrial proton leak. In the basal state only 10% of the total ATP generating capacity was used by the wild-type mitochondria and 14% by the heterozygous knock-in. Thus, it is likely that in situ neuronal mitochondria present more robust respiratory properties than commonly isolated brain mitochondria. Despite this, parallel determination at single cell resolution of [Ca2+]c using the low-affinity Fura-5F and mitochondrial membrane potential with TMRM+ showed a statistically significant increase in the proportion of Hdh150/+ neurons undergoing DCD during a 10min exposure to NMDA relative to control Hdh+/+ neurons [115].
4. Conclusions
The four basic principles of isolated brain mitochondrial Ca2+ transport, net accumulation when [Ca2+]c rises above the ‘set-point’, storage in the alkaline matrix as a calcium-phosphate complex, controlled release when a low [Ca2+]c is restored by the plasma membrane and finally catastrophic dysfunction associated with Ca2+ overload and oxidative stress, can each be observed in intact neurons. However while all mitochondria can be induced to undergo a permeability transition, pathological conditions where such Ca2+ overload might occur are more restricted, with the strongest evidence being the association with chronic NMDA receptor activation in glutamate excitotoxicity. Since mitochondrial Ca2+ transport is driven by the proton circuit and thus competes with ATP synthesis it is essential to consider the interactions between these two processes, particularly since NMDA receptor activation induces a heavy cellular ATP demand. Finally, the severe limitation in interpreting studies with isolated brain mitochondria in models of neurodegenerative diseases such as Huntington's means that studies with intact neurons are more likely to provide some insight into the contribution of mitochondrial Ca2+ transport to the pathology of the disease.
References
- 1.Nicholls DG. Mitochondria and calcium signalling. Cell Calc. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls DG, Scott ID. The regulation of brain mitochondrial calcium-ion transport: the role of ATP in the discrimination between kinetic and membrane-potential-dependent ca efflux mechanisms. Biochem J. 1980;186:833–839. doi: 10.1042/bj1860833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol (Lond) 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:346–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GJ, Thayer SA. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. J Neurophysiol. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- 6.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol (Lond) 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristián T, Weatherby TM, Bates TE, Fiskum G. Heterogeneity of the calcium-induced permeability transition in isolated non-synaptic brain mitochondria. J Neurochem. 2002;83:1297–1308. doi: 10.1046/j.1471-4159.2002.01238.x. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;279:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 9.David G, Talbot J, Barrett EF. Quantitative estimate of mitochondrial [Ca2+] in stimulated motor nerve terminals. Cell Calc. 2003;33:197–206. doi: 10.1016/s0143-4160(02)00229-4. [DOI] [PubMed] [Google Scholar]
- 10.Kristian T, Pivovarova NB, Fiskum G, Andrews SB. Calcium-induced precipitate formation in brain mitochondria: composition, calcium capacity, and retention. J Neurochem. 2007;102:1346–1356. doi: 10.1111/j.1471-4159.2007.04626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoccarato F, Nicholls DG. The role of phosphate in the regulation of the Ca2+ efflux pathway of liver mitochondria. Eur J Biochem. 1982;127:333–338. doi: 10.1111/j.1432-1033.1982.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 12.McCormack JG, Denton RM. The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. Biochim Biophys Acta 1018. 1990:287–291. doi: 10.1016/0005-2728(90)90269-a. [DOI] [PubMed] [Google Scholar]
- 13.Budd SL, Nicholls DG. Mitochondrial calcium regulation and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 14.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca2+] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol (Lond) 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand MD, Lehninger AL. Superstoichiometric Ca2+ uptake supported by hydrolysis of endogenous ATPp in rat liver mitochondria. J Biol Chem. 1975;250:7958–7960. [PubMed] [Google Scholar]
- 17.Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, Rosenfeld J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ros in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol. 2007;292:C708–C718. doi: 10.1152/ajpcell.00202.2006. [DOI] [PubMed] [Google Scholar]
- 18.Brustovetsky N, LaFrance R, Purl KJ, Brustovetsky T, Keene CD, Low WC, Dubinsky JM. Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of huntington's disease. J Neurochem. 2005;93:1361–1370. doi: 10.1111/j.1471-4159.2005.03036.x. [DOI] [PubMed] [Google Scholar]
- 19.Friberg H, Connern C, Halestrap AP, Wieloch T. Differences in the activation of the mitochondrial permeability transition among brain regions in the rat correlate with selective vulnerability. J Neurochem. 1999;72:2488–2497. doi: 10.1046/j.1471-4159.1999.0722488.x. [DOI] [PubMed] [Google Scholar]
- 20.Nicholls DG. Calcium transport and proton electrochemical potential in mitochondria from cerebral cortex and rat heart. Biochem J. 1978;170:511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristian T, Hopkins IB, McKenna MC, Fiskum G. Isolation of mitochondria with high respiratory control from primary cultures of neurons and astrocytes using nitrogen cavitation. J Neurosci Methods. 2006;152:136–143. doi: 10.1016/j.jneumeth.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls DG. The regulation of extra-mitochondrial free calcium by rat liver mitochondria. Biochem J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor JA. Digital imaging of free Ca2+ changes and of spatial gradients in growing processes in single mammalian cns cells. Proc Natl Acad Sci USA. 1986;83:6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor JA, Tseng HY, Hockberger PE. Depolarization- and transmitter-induced changes in intracellular ca2+ of rat cerebellar granule cells in explant culture. J Neurosci. 1987;7:1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992;12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls DG, Åkerman KEO. Mitochondrial calcium transport. Biochim Biophys Acta. 1982;683:57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- 27.Thayer SA, Wang GJ. glutamate-induced calcium loads: effects on energy metabolism and neuronal viability. Clin Exp Pharmacol Physiol. 1995;22:303–304. doi: 10.1111/j.1440-1681.1995.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 28.White RJ, Reynolds IJ. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci. 1995;15:1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.reference deleted
- 30.Szabadkai G, Simoni AM, Bianchi K, De SD, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Talbot JD, David G, Barrett EF. inhibition of mitochondrial Ca2+ uptake affects phasic release from motor terminals differently depending on external [Ca2+] J Neurophysiol. 2003;90:491–502. doi: 10.1152/jn.00012.2003. [DOI] [PubMed] [Google Scholar]
- 32.David G. Mitochondrial clearance of cytosolic Ca2+ in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca2+] J Neurosci. 1999;19:7495–7506. doi: 10.1523/JNEUROSCI.19-17-07495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabadkai G, Simoni AM, Rizzuto R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem. 2003;278:15153–15161. doi: 10.1074/jbc.M300180200. [DOI] [PubMed] [Google Scholar]
- 34.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls DG. Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem. 2006;281:14864–14874. doi: 10.1074/jbc.M510916200. [DOI] [PubMed] [Google Scholar]
- 36.Åkerman KEO, Nicholls DG. Ca2+ transport by intact synaptosomes: influence of ionophore a23187 on plasma membrane potential, plasma membrane Ca2+ transport, mitochondrial membrane potential, cytosolic free Ca2+ concentration and noradrenaline release. Eur J Biochem. 1981;115:67–73. [PubMed] [Google Scholar]
- 37.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha RS, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calc. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matlib MA, Zhou Z, Knight S, Ahmen S, Choi KM, Krause-Bauer J, Phillips R, Altschuld RA, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 39.Dubinsky JM, Levi Y. Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J Neurosci Res. 1998;53:728–741. doi: 10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Chinopoulos C, Starkov AA, Fiskum G. Cyclosporin A-insensitive permeability transition in brain mitochondria - inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem. 2003;278:27382–27389. doi: 10.1074/jbc.M303808200. [DOI] [PubMed] [Google Scholar]
- 41.Brustovetsky N, Dubinsky JM. Limitations of cyclosporin a inhibition of the permeability transition in cns mitochondria. J Neurosci. 2000;20:8229–8237. doi: 10.1523/JNEUROSCI.20-22-08229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Eliseev RA, Filippov G, Velos J, Vanwinkle B, Goldman A, Rosier RN, Gunter TE. Role of cyclophilin d in the resistance of brain mitochondria to the permeability transition. Neurobiol Aging. 2006;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than non-synaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 45.Naga KK, Sullivan PG, Geddes JW. High cyclophilin d content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 47.Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G. Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr. 2006;38:43–47. doi: 10.1007/s10863-006-9004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muramatsu Y, Furuichi Y, Tojo N, Moriguchi A, Maemoto T, Nakada H, Hino M, Matsuoka N. Neuroprotective efficacy of FR901459, a novel derivative of cyclosporin a in in vitro mitochondrial damage and in vivo transient cerebral ischemia models. Brain Res. 2007;1149:181–190. doi: 10.1016/j.brainres.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 49.Alano CC, Beutner G, Dirksen RT, Gross RA, Sheu SS. Mitochondrial permeability transition and calcium dynamics in striatal neurons upon intense NMDA receptor activation. J Neurochem. 2002;80:531–538. doi: 10.1046/j.0022-3042.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- 50.Shalbuyeva N, Brustovetsky T, Brustovetsky N. lithium desensitizes brain mitochondria to calcium, antagonizes permeability transition and diminishes cytochrome c release. J Biol Chem. 2007;282:18057–18068. doi: 10.1074/jbc.M702134200. [DOI] [PubMed] [Google Scholar]
- 51.Hansson MJ, Mattiasson G, Månsson R, Karlsson J, Keep MF, Waldmeier P, Ruegg UT, Dumont JM, Besseghir K, Elmér E. The nonimmunosuppressive cyclosporin analogs NIM811 and UNILl025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 52.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerencser AA, Doczi J, Torocsik B, Bossy-Wetzel E, Adam-Vizi V. Mitochondrial swelling measurement in situ by optimized spatial filtering: astrocyte-neuron differences. Biophys J. 2008;95:2583–2598. doi: 10.1529/biophysj.107.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shalbuyeva N, Brustovetsky T, Bolshakov A, Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J Biol Chem. 2006;281:37547–37558. doi: 10.1074/jbc.M607263200. [DOI] [PubMed] [Google Scholar]
- 55.Elmore SP, Nishimura Y, Qian T, Herman B, Lemasters JJ. Discrimination of depolarized from polarized mitochondria by confocal fluorescence resonance energy transfer. Arch Biochem Biophys. 2004;422:145–152. doi: 10.1016/j.abb.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 56.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t- butylhydroperoxide. Biochem J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of parkinson's disease. Nature Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 58.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann NY Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 59.Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 60.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Current Molecular Medicine. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 61.Kristian T, Stages Metabolic. Mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calc. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 62.Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 63.Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Browne SE, Beal MF. Oxidative damage in huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 65.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 66.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006 doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Soane L, Kahraman S, Kristian T, Fiskum G. Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J Neurosci Res. 2007;85:3407–3415. doi: 10.1002/jnr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao J, Diamond MI. Polyglutamine diseases: emerging concepts in pathogenesis and therapy. Hum Mol Genet. 2007;16(Spec No 2):R115–R123. doi: 10.1093/hmg/ddm213. [DOI] [PubMed] [Google Scholar]
- 69.Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-Terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kristian T, Siesjo BK. Calcium-related damage in ischemia. Life Sci. 1996;59:357–367. doi: 10.1016/0024-3205(96)00314-1. [DOI] [PubMed] [Google Scholar]
- 71.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 72.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 75.Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stout AK, Reynolds IJ. High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neurosci. 1999;89:91–100. doi: 10.1016/s0306-4522(98)00441-2. [DOI] [PubMed] [Google Scholar]
- 77.Ward MW, Rego AC, Frenguelli BG, Nicholls DG. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci. 2000;20:7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castilho RF, Hansson O, Ward MW, Budd SL, Nicholls DG. mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci. 1998;18:10277–10286. doi: 10.1523/JNEUROSCI.18-24-10277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G. Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr. 2006;38:43–47. doi: 10.1007/s10863-006-9004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naga KK, Sullivan PG, Geddes JW. High Cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rintoul GL, Raymond LA, Baimbridge KG. Calcium buffering and protection from excitotoxic cell death by exogenous calbindin-D28k in HEK 293 Cells. Cell Calc. 2001;29:277–287. doi: 10.1054/ceca.2000.0190. [DOI] [PubMed] [Google Scholar]
- 82.Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 2007;282:26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- 83.Bolshakov AP, Mikhailova MM, Szabadkai G, Pinelis VG, Brustovetsky N, Rizzuto R, Khodorov BI. Measurements of mitochondrial ph in cultured cortical neurons clarify contribution of mitochondrial pore to the mechanism of glutamate-induced delayed Ca2+ deregulation. Cell Calc. 2007 doi: 10.1016/j.ceca.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Vesce S, Jekabsons MB, Johnson-Cadwell LI, Nicholls DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J Biol Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]
- 85.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity following partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 87.Henneberry RC. The role of neuronal energy in the neurotoxicity of excitatory amino acids. Neurobiol Aging. 1989;10:611–613. doi: 10.1016/0197-4580(89)90149-8. [DOI] [PubMed] [Google Scholar]
- 88.Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- 89.Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999;19:736–741. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Gakh O, Park S, Liu G, Macomber L, Imlay JA, Ferreira GC, Isaya G. Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum Mol Genet. 2005;467:467–479. doi: 10.1093/hmg/ddi461. [DOI] [PubMed] [Google Scholar]
- 91.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 92.Hands S, Sinadinos C, Wyttenbach A. Polyglutamine gene function and dysfunction in the ageing brain. Biochim Biophys Acta. 2008;1779:507–521. doi: 10.1016/j.bbagrm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- 94.Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+ -dependent depolarization of rat and human mitochondria: relevance to huntington's disease. Arch Biochem Biophys. 2003;410:1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- 96.Fan MMY, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in huntington's disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 97.Gu M, Gash MT, Mann VM, Javoy Agid F, Cooper JM, Schapira AHV. Mitochondrial defect in huntington's disease caudate nucleus. Ann Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 98.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in huntington's disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 99.Swerdlow RH, Parks JK, Cassarino DS, Shilling AT, Bennett JP, Jr, Harrison MB, Parker WD., Jr Characterization of cybrid cell lines containing mtDNA from huntington's disease patients. Biochem Biophys Res Commun. 1999;261:701–704. doi: 10.1006/bbrc.1999.1095. [DOI] [PubMed] [Google Scholar]
- 100.reference deleted
- 101.Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington's disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeron MM, Hansson O, Chen NS, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 103.Hansson O, Castilho RF, Korhonen L, Lindholm D, Bates GP, Brundin P. Partial resistance to malonate-induced striatal cell death in transgenic mouse models of huntington's disease is dependent on age and CAG repeat length. J Neurochem. 2001;78:694–703. doi: 10.1046/j.1471-4159.2001.00482.x. [DOI] [PubMed] [Google Scholar]
- 104.Schauwecker PE. Differences in ionotropic glutamate receptor subunit expression are not responsible for strain-dependent susceptibility to excitotoxin-induced injury. Brain Res Mol Brain Res. 2003;112:70–81. doi: 10.1016/s0169-328x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 105.Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of huntington's disease. J Biol Chem. 2007;283:5780–5790. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 106.Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993;61:1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 107.Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy Willig A, Kowall NW, Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci USA. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alexi T, Hughes PE, Faull RLM, Williams CE. 3-Nitropropionic acid's lethal triplet: cooperative pathways of neurodegeneration. Neuroreport. 1998;9:R57–R64. doi: 10.1097/00001756-199808030-00001. [DOI] [PubMed] [Google Scholar]
- 109.Greene JG, Sheu SS, Gross RA, Greenamyre JT. 3-Nitropropionic acid exacerbates N-methyl-D-aspartate toxicity in striatal culture by multiple mechanisms. Neurosci. 1998;84:503–510. doi: 10.1016/s0306-4522(97)00389-8. [DOI] [PubMed] [Google Scholar]
- 110.Hansson O, Petersn A, Leist M, Nicotera P, Castilho RF, Brundin P. Transgenic mice expressing a huntington's disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity. Proc Natl Acad Sci U S A. 1999;96:8727–8732. doi: 10.1073/pnas.96.15.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li XJ, Castilho RF, Brundin P. Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur J Neurosci. 2001;14:1492–1504. doi: 10.1046/j.0953-816x.2001.01767.x. [DOI] [PubMed] [Google Scholar]
- 112.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in huntington's disease are a direct effect of polyglutamines. Nature Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 113.Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and huntington's disease individuals. Mol Cell Biochem. 2005;269:143–152. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- 114.Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 115.Oliveira J, Ellerby LM, Rego AC, Nicholls DG. Mitochondrial dysfunction in huntington's disease: the bioenergetics of isolated and in-situ mitochondria from transgenic mice. J Neurochem. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 116.Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J Biol Chem. 2004;279:32989–33000. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]