Abstract
Background
Controversy exists over the nature and origin of reduced regional brain volumes in post-traumatic stress disorder (PTSD). At issue is whether these reductions represent pre-existing vulnerability factors for developing PTSD upon traumatic exposure or acquired PTSD signs due to the traumatic stress that caused the PTSD and/or the chronic stress of having PTSD. We employed a case-control design in monozygotic twin pairs discordant for combat exposure to address the pre-existing vs. acquired origin of brain morphometric abnormalities in this disorder.
Method
We used voxel-based morphometry to search for gray matter density reductions in magnetic resonance imaging (MRI) data obtained in a previous study of combat-exposed Vietnam veteran twins with (n=18) vs. without (n=23) PTSD and their “high-risk” vs. “low-risk” (respectively), identical, combat-unexposed co-twins.
Results
Compared to the combat-exposed twins without PTSD, the combat-exposed twins with PTSD showed significant gray matter density reductions in four predicted brain regions: right hippocampus, pregenual anterior cingulate cortex (ACC), and left and right insulae. There was a significant PTSD Diagnosis × combat Exposure interaction in pregenual ACC, in which combat-exposed PTSD twins had lower gray matter density than their own combat-unexposed co-twins as well as than the combat-exposed twins without PTSD and their co-twins.
Conclusion
The results point to gray matter volume diminutions in limbic and paralimbic structures in PTSD. The pattern of results obtained for pregenual ACC suggests that gray matter reduction in this region represents an acquired sign of PTSD that is consistent with stress-induced loss.
INTRODUCTION
Several structural magnetic resonance imaging (MRI) studies employing anatomic segmentation have found lower gray matter volumes in the hippocampus in post-traumatic stress disorder (PTSD) stemming from various traumatic events (1). One segmentation study found diminished gray matter volumes in pregenual anterior cingulate cortex (ACC) and subcallosal cortex but not dorsal ACC (s), whereas another did find dorsal ACC reduction (3). Decreased pregenual ACC activation in response to trauma-related stimuli is a prominent functional neuroimaging finding in PTSD (4–5).
The technique of voxel-based morphometry (VBM) allows an automated examination of structural brain differences using statistical parametric mapping techniques. The validity of the VBM technique for assessing regional gray matter density has been confirmed in several previous studies in comparison with conventional region-of-interest measurements (6–9). Employing VBM, the first authors found reduced dorsal ACC gray matter density in victims of an urban terrorist attack with PTSD (10). Another recent study that employed VBM found gray matter density reduction in pregenual ACC but not dorsal ACC, although manual segmentation did not confirm volumetric reduction in the former structure (11). That study also found gray matter density reduction in left insula. Yet another recent PTSD VBM study found gray matter density reductions in hippocampus, pregenual ACC, and insula (12).
Controversy exists over the nature and origin of reduced regional brain volume in PTSD. Thus far the debate has focused on the hippocampus (13). At issue is whether reduced volume represents an acquired PTSD sign, e.g., is due to the traumatic stress that caused the PTSD and/or the chronic stress of having PTSD, or a pre-existing vulnerability factor for developing PTSD upon traumatic exposure. We have been employing a case-control design in monozygotic twin pairs discordant for combat exposure in Vietnam to address the pre-existing vs. acquired origin of biological abnormalities found in PTSD (14). In a structural MRI study that manually traced the outlines of the right and left hippocampus, we found that lower total hippocampal volume constituted a “familial” vulnerability factor for PTSD, because it was found in both the combat-exposed twins with PTSD and their “high-risk,” combat-unexposed co-twins, whose hippocampal volumes were lower than those of the combat-exposed twins without PTSD and their “low-risk,” combat-unexposed co-twins (15). (Note that the term “familial” includes both heredity and shared environment, i.e., environmental experiences that both members of a twin pair have had in common.)
In the present study, we applied a VBM analysis to MRI data from the same twin sample to conduct a search throughout the entire brain for regional gray matter structural differences. Based upon the published structural imaging studies reviewed above, we predicted lower gray matter density in combat-exposed twins with PTSD compared to combat-exposed twins without PTSD in the following regions: hippocampus, dorsal ACC, pregenual ACC, subcallosal cortex, and insula. In an attempt to clarify the origin of any such differences, we used the data from the combat-unexposed co-twins. Gray matter diminution that confers familial vulnerability to PTSD would be expected in the high-risk, compared to the low-risk, combat-unexposed twins. In contrast, diminution that reflects acquired damage in PTSD would be expected to be manifest in a Diagnosis × combat Exposure interaction, in which the combat-exposed twins with PTSD had lower gray matter density than their own high-risk, combat-unexposed co-twins as well as the combat-exposed twins without PTSD and their low-risk co-twins.
METHODS AND MATERIALS
Subjects
The strategy for subject ascertainment and recruitment has been presented elsewhere (16). The present sample was described in detail in the report of our previous hippocampus manual tracing study (15). The present study reanalyzed the same MRI scans from the same subjects. In the previous study, one combat-exposed twin with PTSD and his combat-unexposed co-twin were removed from the analysis because the former was an extreme, asymmetrical outlier for manually traced hippocampal volume. This subject and his co-twin were included in the current study. (Exclusion of this pair did not alter the conclusions.) The protocol was approved by the institutional review board of the Manchester, NH VA Medical Center. All subjects had previously given written informed consent prior to participation after a complete description of the procedures.
MRI data pre-analysis
The MRI acquisition techniques were described in the previous report (15). The methods used to analyze these data in the present study were similar to those reported elsewhere (10). Image analysis was performed using ANALYZE PC 3.0 (Mayo Foundation, Rochester, MN, USA) and SPM 99 software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) running in MATLAB 6.1 (Mathworks, Sherborn, MA). In ANALYZE, image data were resampled using an algorithm to make them isotropic, with the sides measuring 0.9375 mm, and then stored. Resampled images were first spatially normalized into the standard MNI152 template (17–18). Normalized images were then segmented into gray matter, white matter, cerebrospinal fluid, and skull/scalp compartments using an automated, operator-independent process (19). The segmentation step also incorporated an image density non-uniformity correction to address image density variations caused by different positions of cranial structures within the MRI head coil (20). The spatially normalized segments of the gray matter were smoothed with a 12 mm full-width at half-maximum isotropic Gaussian kernel to accommodate individual variability in sulcal and gyral anatomy. For medial temporal regions, e.g., hippocampus, a 4 mm smoothing kernel was used instead, as has been recommended (21). By smoothing the data, the partial volume effect was used to create a spectrum of gray matter densities. Gray matter density is equivalent to the weighted average of the gray matter voxels located in the volume defined by the smoothing kernel. Because previous studies have shown a fair correlation between regional gray matter density determined by VBM and structural volumes measured by conventional, manual tracing (7,9,22), the regional gray matter density can be considered to represent the local volume of gray matter.
Statistical analyses
Demographic and psychometric data were analyzed by means of a mixed model that treated Diagnosis (in the combat-exposed twin) as a between-pairs fixed effect, combat Exposure as a within-pairs fixed effect (repeated measure), and pairs as a random effect (16). This model analysis yields a t statistic. Gray matter density was estimated on a voxel-by-voxel basis using SPM 99 (23). Contrasts were made between the 18 combat-exposed twins with PTSD and the 23 combat-exposed twins without PTSD, and separately between their high- and low-risk co-twins, using independent t-tests, adjusted for individual intracranial volume. Diagnosis × Exposure interactions were evaluated by means of a mixed, multi-group (Diagnosis), conditions (combat Exposure), and covariates (intracranial volume) model in which one twin pair was treated as though one subject with two conditions. In this analysis, 82 covariates were entered corresponding to 82 images ([18 + 23] × 2). For each of the foregoing analyses, a statistical parametric map (SPM) of the t-statistic (SPM{t}) was created, and the SPM{t} values were transformed to the normal distribution (SPM{z}). The statistical significance threshold was set at p< 0.05 corrected for multiple comparisons using the False Discovery Rate (FDR) (24).
The anatomical locations of peak coordinates were initially defined using the latest version of Talairach Daemon Client (25). These localizations were then confirmed by visually inspecting the coordinates overlaid on the mean structural image of the study sample. For peaks located within predicted brain regions, small volume correction was applied using the following a priori volume approximations from the literature: hippocampus 3.5 ml each side; insula 8 ml each side; dorsal ACC 10 ml bilaterally; pregenual ACC 5 ml bilaterally; and subcallosal cortex 5 ml bilaterally (total volume of predicted brain regions = 43 ml). For peaks located outside predicted regions, correction for whole brain was employed. Because the predictions were directional, viz. lower gray matter density in combat-exposed subjects and PTSD pairs, the tests were one-tailed, and only results in the predicted direction(s) are reported.
RESULTS
Demographics and psychometrics
Group mean age, Combat Severity score (26), total Clinician-Administered PTSD Scale (CAPS) score (27), number of potentially traumatic lifetime non-combat events (16), total Michigan Alcoholism Screening Test (MAST) score (28), and Symptom Check List-90-Revised (SCL-90-R) Depression scale score (29), along with statistical analyses, are presented in Table 1. It may be seen that age was similar among subject groups. Combat-exposed twins with PTSD had more severe combat exposure than combat-exposed twins without PTSD. As expected by virtue of selection, the former had greater combat-related symptom severity on the CAPS. PTSD twin pairs (i.e., twin pairs in which the combat-exposed twin was diagnosed with current, combat-related PTSD) reported more potentially traumatic lifetime non-combat events than non-PTSD pairs (i.e., twin pairs in which the combat-exposed twin was diagnosed with neither current nor past, combat-related PTSD). Combat-exposed twins also reported more potentially traumatic lifetime non-combat events than their combat-unexposed co-twins. Combat-exposed twins with PTSD had more severe alcoholism histories than the other three groups. Combat-exposed twins with PTSD also reported more depression than the other three groups. The Pearson correlation between total CAPS score and SCL-90-R Depression was very large (r=0.86) in the PTSD pairs but negligible (r=−0.06) in the non-PTSD pairs.
TABLE 1.
Group means (standard deviations) of combat-exposed Vietnam veterans with and without PTSD and their combat-unexposed, identical co-twins
| ---------PTSD pairs*-------- | -------Non-PTSD pairs†------ | --------------------Mixed Model-------------------- | ---Independent t-tests--- | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed (high-risk) |
Exposed | Unexposed (low-risk) |
Diagnosis | Exposure | Interaction | Exposed | Unexposed | ||||||||||
| (n =18) | (n = 18) | (n = 23) | (n = 23) | t(39) | p | t(40) | p | t(39) | p | t(39) | p | t(39) | p | |||||
| Age (years)‡ | 52.8 | (3.4) | 52.8 | (3.4) | 51.8 | (2.3) | 51.8 | (2.3) | - | - | - | - | - | - | 1.1 | 0.27 | 1.1 | 0.27 |
| CAPS§ | 73.3 | (16.9) | - | - | 6.2 | (7.3) | - | - | - | - | - | - | - | - | 17.2 | <0.001 | - | - |
| Combat severity¶ | 7.7 | (2.1) | - | - | 3.5 | (2.6) | - | - | - | - | - | - | - | - | 5.6 | <0.001 | - | - |
| Traumatic events╢ | 8.1 | (2.6) | 5.2 | (3.7) | 5.1 | (4.0) | 4.2 | (3.0) | 2.6 | 0.01 | 2.4 | 0.02 | 1.3 | 0.20 | 2.8 | 0.009 | 1.0 | 0.34 |
| MAST** | 19.1 | (17.6) | 6.4 | (10.2) | 2.4 | (4.5) | 2.5 | (4.0) | 4.5 | <0.001 | 2.8 | 0.007 | 2.8 | 0.007 | 4.4 | <0.001 | 1.7 | 0.10 |
| Depression†† | 2.5 | (0.9) | 0.3 | (0.5) | 0.6 | (0.7) | 0.4 | (0.5) | 4.6 | <.001 | 5.3 | <0.001 | 6.9 | <0.001 | 7.4 | <0.001 | 0.7 | 0.51 |
As determined by the presence of current, combat-related PTSD in the combat-exposed twin
As determined by the absence of current or past, combat-related PTSD in the combat-exposed twin
As of October 1, 2000
Clinician-Administered PTSD Scale (range 0–136)
18-item measure (range 0–18)
Number of potentially traumatic lifetime non-combat events
Michigan Alcoholism Screening Test (range 0–25)
Symptom Check List-90-Revised Depression Subscale (range 0–4)
Gray matter density
Table 2 presents the results of the contrasts between combat-exposed twins with vs. without PTSD. For the sake of a complete exposition of these data, all results statistically significant at the liberal threshold of uncorrected p<0.001 with spatial extent of k>10 voxels are shown. Of the seven peaks that met this threshold, four were located in predicted brain regions, viz., right hippocampus, pregenual ACC, right mid insula, and left anterior insula (shown in Figure 1). Each of these four peaks also met the statistical significance threshold of p<0.05 with small volume correction for the a priori size of the structure (as shown in the second column of Table 3). No voxels in non-predicted brain regions met the threshold of p<0.05 corrected for whole brain in these, or any other, analyses.
Table 2.
Loci showing gray matter density reductions in combat-exposed twins with vs. without combat-related PTSD that were significant at uncorrected p<0.001
| z | psvc | k | [x y z] | Brain Region |
|---|---|---|---|---|
| 4.50 | 0.001 | 248 | [44 −2 −14] | right mid insula |
| 4.47 | 145 | [−62 −56 −4] | left middle temporal gyrus | |
| 4.39 | 0.001 | 27 | [34 −28 −16] | right hippocampus |
| 3.95 | 0.005 | 236 | [−36 10 −4] | left anterior insula |
| 3.71 | 0.004 | 185 | [0 46 10] | pregenual anterior cingulate cortex |
| 3.52 | 35 | [−46 42 −14] | left inferior frontal gyrus | |
| 3.47 | 22 | [−64 −44 10] | left superior temporal gyrus |
psvc = significance level with small volume correction based upon the a priori size of the structure
k = cluster size
[x y z] = MNI coordinates of peak voxel
Predicted areas appear in boldface.
Figure 1. Brain regions showing diminution in gray matter density in combat-exposed twins with PTSD versus without PTSD.
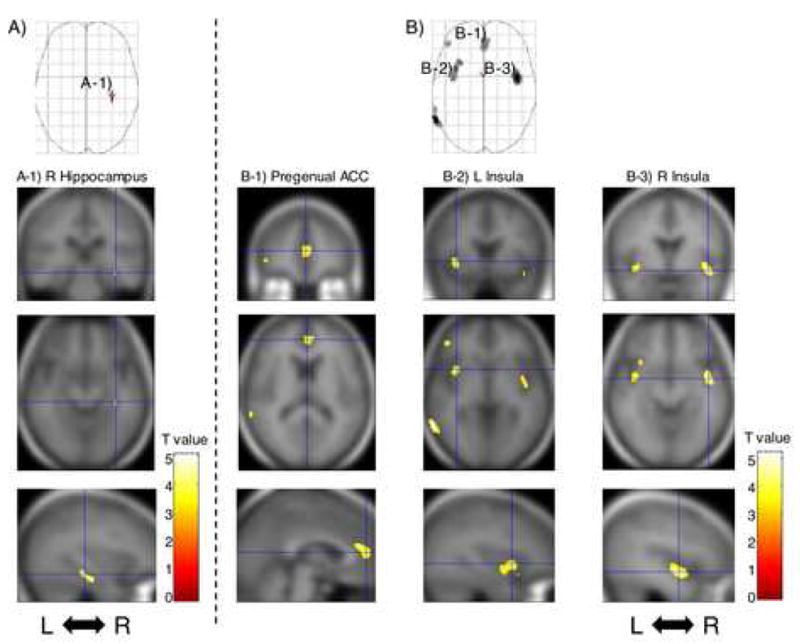
A.) SPM analysis with 4-mm Gaussian smoothing kernel revealed statistically significant reduced gray matter density shown in the axial projection. A-1.) Regional gray matter density reduction in the right hippocampus is rendered onto orthogonal slices of the averaged magnetic resonance image of the present study’s subjects. B.) SPM analysis with 12-mm Gaussian smoothing kernel revealed statistically significant reduced gray matter density shown in the axial projections. Regional gray matter density reductions in the following areas are rendered: B-1.) Pregenual anterior cingulate cortex; B-2.) Left insula; B-3.) Right insula. Abbreviations: L: left hemisphere, R: right hemisphere, ACC: anterior cingulate cortex.
Table 3.
Results adjusted for potentially confounding variables
| Unadjusted z (psvc) | Age z (psvc) | Combat Severitya z (psvc) | Traumatic Eventsb z (psvc) | MAST Scorec z (psvc) | Childhood Abused z (psvc) | SCL-90-R Depressione z (psvc) | Brain Region |
|---|---|---|---|---|---|---|---|
| Combat-Exposed Twins: PTSD vs. non-PTSD | |||||||
| 4.50 (0.001) | 4.43 (0.001) | 3.23 (0.05) | 3.98 (0.005) | 3.39 (0.02) | 3.19 (0.04) | 2.07 (0.49) | right mid insula [44 −2 −14] |
| 4.39 (0.001) | 4.36 (0.001) | 3.05 (0.11) | 4.11 (0.004) | 4.63 (<0.001) | 3.56 (0.04) | 1.52 (0.99) | right hippocampus [34 −28 −16] |
| 3.95 (0.005) | 3.98 (0.005) | 3.32 (0.03) | 3.06 (0.07) | 3.26 (0.04) | 3.63 (0.01) | 2.15 (0.44) | left anterior insula [−36 10 −4] |
| 3.71 (0.004) | 3.59 (0.009) | 2.50 (0.18) | 2.79 (0.08) | 2.40 (0.14) | 2.92 (0.03) | 0.93 (0.99) | pregenual anterior cingulate cortex [0 46 10] |
| Diagnosis × Combat Exposure Interaction | |||||||
| 3.32 (0.02) | 3.39 (0.02) | 3.32 (0.02)f | 2.76 (0.10) | 2.77 (0.10) | 3.42 (0.01) | 1.81 (0.53) | pregenual anterior cingulate cortex [8 50 12] |
psvc = significance level with small volume correction
18-item measure (range 0–18)
Number of potentially traumatic lifetime non-combat events
Michigan Alcoholism Screening Test (range 0–25)
Deleting 6 PTSD pairs and 5 non-PTSD pairs within which either twin had a history of childhood abuse
Depression subscale of Symptom Check List 90-Revised
Covariate is value in combat-exposed twin
At the right hippocampus, pregenual ACC, left anterior insula, and right mid insula loci shown in Table 2, within the 18 PTSD combat veterans, we examined the correlations between gray matter density and total CAPS score, as well as SCL-90-R Depression score. Because these analyses involved single voxels, the significance threshold was p<0.05 uncorrected. None of these correlations were significant. We also performed the same correlations for CAPS A (re-experiencing), B (avoidance/numbing) and C (hyperarousal) symptom cluster subscores; for these analyses we applied a Bonferroni correction to the significance threshold, viz. p<0.017 (0.05/3). The only significant correlations were between symptom cluster B (re-experiencing) and gray matter density in pregenual ACC (r=−0.57, p=0.008), left anterior insula (r=−0.53, p=0.0130 and right mid insula (r=−0.59, p=0.006).
The contrasts between the high-risk, combat-unexposed co-twins of the combat-exposed twins with PTSD vs. the low-risk, combat-unexposed co-twins of the combat-exposed twins without PTSD did not identify any voxels that met the statistical significance threshold of p<0.05, even with small volume corrections. The only statistically significant Diagnosis × Exposure interaction was found in pregenual ACC ([8 50 12], z=3.32, k=16, p=0.02 corrected for the a priori size of this structure). The location of this cluster is shown in Figure 2. Scatterplots of individual subjects’ values at the [8 50 12] pregenual ACC locus are shown in Figure 3. There were no significant correlations between gray matter density in the 18 PTSD combat veterans minus gray matter density in their combat-unexposed co-twins, and total CAPS score, SCL-90-R Depression, or (with Bonferroni-corrections) any of the CAPS symptom cluster subscores.
Figure 2. Brain region showing PTSD Diagnosis × combat Exposure interaction.
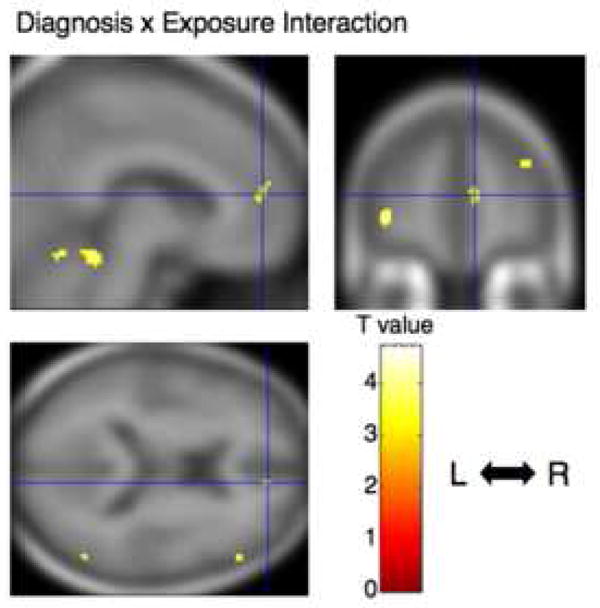
Regional interaction for gray matter density in pregenual anterior cingulate cortex is rendered onto orthogonal slices of the averaged magnetic resonance image of the present study’s subjects. Cross hairs indicate the peak coordinate of the interaction ([ 8 50 12]). Abbreviations: L: left hemisphere, R: right hemisphere.
Figure 3. Scatterplots of individual subjects’ adjusted VBM responses.
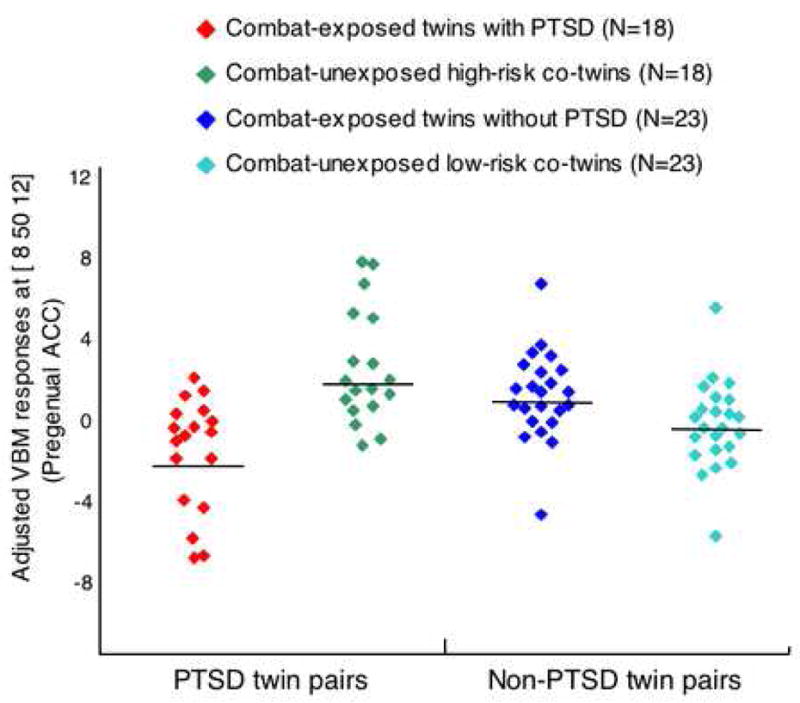
Shown at the site of the PTSD Diagnosis × Combat Exposure interaction in pregenual anterior cingulate cortex ([ 8 50 12]). Means are represented by solid horizontal lines drawn on each group’s distribution.
The mixed model and t-test analyses that yielded the statistically significant results described above were repeated entering the following possibly confounding variables into the respective models as covariates: age, combat severity (in the exposed twin), number of potentially traumatic lifetime non-combat events, MAST score, and SCL-90-R Depression score. To control for a possibly confounding role of childhood physical or sexual abuse, the data were re-analyzed deleting pairs within which either member had such a history. All these results are shown in Table 3.
DISCUSSION
Of the seven loci at which combat-exposed twins with PTSD had lower gray matter density than combat-exposed twins without PTSD at a liberal threshold of p<0.001, four were located in predicted brain regions, viz., right hippcocampus, pregenual ACC, and left anterior and right mid- insulae, even though the predicted brain regions occupy less than 10% of total gray matter volume. This regional specificity supports the validity of the present results and implicates limbic and paralimbic structures as the major sites of gray matter density reductions in combat-related PTSD. Gray matter reductions in pregenual ACC and both insulae significantly correlated only with the cluster B “re-experiencing” symptoms of PTSD.
The ACC, especially its pregenual, or “affective,” division, and insula are components of the anterior “paralimbic belt,” are strongly interconnected to each other and to the amygdala, and are highly involved in emotional aspects of brain function (30–32). Impaired pregenual ACC function is one of the most robust neuroimaging findings in PTSD (4–5). A neurocircuitry model of PTSD posits that the ventromedial prefrontal cortex, including pregenual ACC, inhibits the expression of classically conditioned fear responses by the amygdala (33). Thus impairment in this brain region might be expected to most affect the DSM-IV symptoms that are putatively most closely related to conditioned fear, viz., the cluster B symptoms (especially B.4 and B.5, viz., intense psychological distress [and/or] physiological reactivity on exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event). To the extent that diminished structure implies diminished function, reduced pregenual ACC gray matter density is consistent with this neurocircuitry model.
Functional neuroimaging studies of the hippocampus in PTSD are less common, but they too support impairment in this brain region (34–36). Hippocampal impairment may contribute to PTSD by reducing the ability to construct declarative narratives that bind the affect associated with the traumatic event (37), or by the ability to recognize safe contexts (33), or by other unknown mechanisms. The reduced gray matter density found in bilateral insulae is paradoxical in light of studies that have generally found hyperactivity in this brain region in PTSD (38) and other anxiety conditions (39). One model of anterior insula function posits that this structure detects the difference between an observed and expected body state and generates an interoceptive prediction signal that triggers anxiety (39). A structurally compromised insula may be less inhibited in generating such signals in PTSD, but this is in the realm of speculation.
The most interesting result from the present study is the significant Diagnosis × Exposure interaction in the pregenual ACC, with combat-exposed PTSD twins having lower gray matter density than their own combat-unexposed co-twins as well as than the combat-exposed twins without PTSD and their co-twins, supporting the inference that pregenual ACC gray matter reduction is an acquired sign of PTSD. In animals, exposure to chronic stress has been shown to damage not only the hippocampus in rodents (40) and primates (41), but also the ACC in rodents (42–43) and primates (44). It has been hypothesized that such damage may provide a basis for structural changes observed in PTSD (42,45). A recent study of mentally healthy persons that used automated segmentation found that those who reported early life stressors had smaller ACCs than those who did not (46). However, causal inferences are difficult to draw from the cross-sectional study of non-twins.
When the Diagnosis × Exposure interaction at [8 50 12] was adjusted for MAST score, its statistical significance level was reduced to corrected p=0.10, which falls short of statistical significance. We did not obtain data regarding recent alcohol consumption. This is a limitation considering that imaging findings related to alcohol may be more sensitive to recent as opposed to more remote intake. On the other hand, the likelihood that increased alcohol use by the PTSD veterans accounts for the gray matter density reduction in their ACCs is diminished by the consideration that if the PTSD subjects studied here had consumed enough alcohol to damage their brains, evidence for this should have been found in other brain regions known to be affected by alcohol, including superior, motor, and other areas of the frontal cortex and cerebellum (47–48), none of which (except for a small cluster in left inferior frontal cortex) showed volumetric reduction in the PTSD compared to the non-PTSD combat veterans at even the liberal threshold of uncorrected p<0.001. Thus, the specificity of volumetric diminution to our predicted brain regions argues against a global effect such as alcohol-induced brain damage. Finally, a recent manual tracing study found comparably (and significantly) reduced ACC volume in subgroups of PTSD veterans with and without a history of lifetime alcohol abuse or dependence, in comparison to non-PTSD veterans (49).
When the Diagnosis × Exposure interaction in pregenual ACC was adjusted for number of potentially traumatic lifetime non-combat events, its statistical significance level also was reduced to corrected p=0.10. This means we cannot be fully confident that stressful events other than military combat do not account for the reduced ACC gray matter density in the PTSD veterans. However, even if such events did contribute, this would still not be inconsistent with stress-induced diminution of this structure. When the Diagnosis × Exposure interaction in pregenual ACC was adjusted for depression, it was no longer significant. This is not surprising given the high association between depression and PTSD in the present sample, in which self-reported depression appears to have been acquired along with PTSD, making the two likely facets of the same post-traumatic psychopathology.
A limitation of the design employed here is that it cannot identify the specific environmental difference(s) between the combat-exposed PTSD twins and their non-combat-exposed, identical co-twins that is responsible for an acquired abnormality. However, because the most salient, common difference in the present study was the presence of combat-related PTSD in the former, and because as noted above the observed effects remained significant or nearly significant after considering the contributions of several important potentially confounding variables, it is reasonable to attribute this lower gray matter density to the presence of combat-related PTSD.
Combat-exposed twins with PTSD also had lower gray matter density than combat-exposed twins without PTSD in right hippocampus and left anterior and right mid- insulae, as well as at another site within pregenual ACC, replicating previous studies. These results could not be explained by group differences in age, combat severity, number of potentially traumatic lifetime non-combat events, alcoholism, or child abuse. Unfortunately, the analyses that included the data from the combat-unexposed co-twins were unable to shed light on the origin of these gray matter reductions in the combat-exposed twins with PTSD, because they failed to yield either a significant difference between high- and low-risk combat-unexposed co-twins (which would support a pre-trauma vulnerability factor) or a significant Diagnosis × Exposure interaction (which would support an acquired abnormality). Finally, the present results were unable to replicate previously reported segmentation and voxel-based morphometric findings of gray matter reduction in subcallosal cortex and dorsal ACC.
In the same twin sample studied here, we previously found manual tracing evidence that diminished hippocampal volume represents a pre-trauma vulnerability factor for PTSD, rather than an acquired PTSD sign (15). In contrast the present VBM results suggest that diminished volume in pregenual ACC is acquired as a result of the combat exposure that led to PTSD and/or the PTSD itself. We have no ready explanation as to why diminutions in these two structures should have different origins. As noted, above, however, the origin of gray matter density reduction in the pregenual ACC site other than the one that showed the significant interaction could not be explicated by the present data; it is possible that it represents a PTSD vulnerability factor. Additional techniques that may help to clarify this uncertainty in future studies include cortical parcellation (segmentation) and magnetic resonance spectroscopy.
It has been suggested that VBM may not detect very small, localized gray matter volume reductions, since false-negative VBM findings may arise from the changes in the shape or displacement of structures in the course of spatial normalization (7). Additionally, VBM may be biased against finding group differences in areas that are spatially complex (50). Inversely, we cannot rule out the possibility that the abnormalities detected by VBM in the present study reflected group differences in the shape of brain structures, rather than their volume (11), although even shape differences may have functional consequences. The failure of VBM to find a significant hippocampal gray matter reduction in the high- vs. low-risk, combat-unexposed co-twins contrasts with our positive result in the same sample using manual segmentation of hippocampus (15) suggests that the latter technique may be more sensitive to reduced volume in this structure than the voxel-based approach. Similarly, we are unable to rule out the possibility that subtle group differences in other brain regions in this study remained below the sensitivity of VBM, or the detection power conferred by our sample.
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Government of Japan to Dr. Kasai; a U.S. Department of Veterans Affairs Merit Review Grant to Dr. Gilbertson; USPHS Grant #R01MH54636 to Dr. Pitman; and USPHS Grant #K05MH01110 to Dr. Shenton. Prof. Karl Friston provided helpful suggestions regarding the SPM analyses. The authors also thank H. Matsuda, T. Ohnishi, M. Macklin, S. Williston, L. Paulus, and H. Croteau for assistance. The U.S. Department of Veterans Affairs provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Through their support of the VET Registry, numerous other U.S. organizations also provided invaluable assistance, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; and Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families, without whose contribution this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 3.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res. 2002;55:41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 9.Luders E, Gaser C, Jancke L, Schlaug G. A voxel-based approach to gray matter asymmetries. Neuroimage. 2004;22:656–664. doi: 10.1016/j.neuroimage.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbo V, Clément MH, Armory JL, Pruessner JC, Brunet A. Size vs. shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with PTSD. Biol Psychiatry. 2005;58:119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, Yan L, Zhang J, Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM. Atrophy of the hippocampus in posttraumatic stress disorder: how and when? Hippocampus. 2001;11:90–91. doi: 10.1002/hipo.1026. [DOI] [PubMed] [Google Scholar]
- 14.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 17.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston K. Multimodal image coregistration and partitioning--a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 21.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–21. [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 28.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR. SCL-90-R: Administration, scoring, and procedures: Manual II. Baltimore, MD: Clinical Psychometric Research; 1992. [Google Scholar]
- 30.Gloor P. The Temporal Lobe and Limbic System. New York: Oxford University Press; 1997. [Google Scholar]
- 31.Mesulam MM, Mufson EJ. The insula of Reil in man and monkey: architectonics, connectivity, and function. In: Salloway SP, Malloy PF, Duffy JD, editors. Cerebral Cortex. Vol. 4. Association and Auditory Cortices: Plenum Press; 1985. pp. 179–226. [Google Scholar]
- 32.Mega MS, Cummings JL. Frontal subcortical circuits: anatomy and function. In: Salloway SP, Malloy PF, Duffy JD, editors. The Frontal Lobes and Neuropsychiatric Illness. Washington, D.C: American Psychiatric Publishing; 2004. pp. 15–32. [Google Scholar]
- 33.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 35.Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 36.Astur RS, St Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 37.Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- 38.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 39.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 41.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 44.Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Perera T, Lisanby SH, Rosenblum LA, Gorman JM, Coplan JD. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biol Psychiatry. 2003;54:727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 45.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 46.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 48.Spampinato MV, Castillo M, Rojas R, Palacios E, Frascheri L, Descartes F. Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse. Top Magn Reson Imaging. 2005;16:223–230. doi: 10.1097/01.rmr.0000192175.26243.a7. [DOI] [PubMed] [Google Scholar]
- 49.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]


