Abstract
Many anterior cruciate ligament (ACL) reconstructions have increased laxity postoperatively. We hypothesized that enhancing an ACL graft with a collagen-platelet composite (CPC) would improve knee laxity and graft structural properties. We also hypothesized the platelet concentration in the CPC would affect these parameters. Twelve goats underwent ACL reconstruction with autologous patellar tendon graft. In six goats, a collagen-platelet composite was placed around the graft (CPC group). In the remaining six goats, the collagen scaffold only was used (COLL group). Three goats were excluded due to complications. After 6 weeks in vivo, anterior–posterior (AP) laxity and tensile properties of the ACL reconstructed knees were measured and normalized against the contralateral intact knee. At a knee flexion angle of 30°, the average increase in AP laxity was 40% less in the CPC group than the COLL group (p = 0.045). At 60°, the AP laxity was 30% less in the CPC group, a difference that was close to statistical significance (p = 0.080). No differences were found between treatment groups with respect to the structural properties (p > 0.30). However, there were significant correlations between serum platelet concentration and AP laxity (R2 = 0.643; p = 0.009), maximum load (R2 = 0.691; p = 0.006), and graft stiffness (R2 = 0.840; p < 0.001). In conclusion, use of a CPC to enhance healing of an allograft ACL reconstruction inversely correlated with early sagittal plane laxity and the systemic platelet count was highly predictive of ACL reconstruction graft strength and stiffness at 6 weeks. These findings emphasize the importance of further research on delineating the effect of platelets in treating of ACL injuries.
Keywords: ACL, platelet, wound healing, ACL reconstruction, animal models
Each year, an estimated 200,000 patients have ACL injuries in the United States. The current gold standard of treatment, ACL reconstruction, is a reproducible procedure that approximates normal sagittal plane [anterior–posterior (AP)] stability of the knee. However, the normal kinematics of the knee are not restored and a clinically relevant percentage of patients have increased AP laxity postoperatively.1–3 Translational animal models demonstrate that graft strength decreases and postoperative laxity increases during the first 4 to 12 weeks after graft implantation.4 If the analogous situation occurs in patients, this time period may represent a window of particular vulnerability of the graft to stress, thereby contributing to increased AP laxity as a patient's activity level increases. The early healing of the ACL reconstruction graft with decreased structural properties could explain in part previous work in humans showing the majority of the increase in postoperative knee laxity occurs in the first several months after surgery.3 Thus, strategies that could reduce the load–displacement response of the tibiofemoral joint (AP laxity), and/or enhance the structural properties of the graft (strength and stiffness) in the early postoperative healing phase could potentially improve ACL reconstruction outcomes.
Histological evaluation of a biomechanically stable partial ACL injury model where wound healing was stimulated with implantation of a collagen–platelet composite (CPC) demonstrated a similar growth factor expression profile to normal healing extraarticular ligaments like the medial collateral ligament (MCL).5 The expression and timing of growth factor expression in a healing extra-articular ligament was qualitatively reproduced by placement of a CPC in the partial ACL injury model. This is in contrast to the lack of healing and severely limited growth factor expression in the partial ACL injury without CPC. Exogenous growth factors (PDGF, TGF, EGF, and VEGF) have also been applied to ACL reconstruction models in large animal models,6–8 two of which are normally concentrated in platelets (PDGF and TGF). Their exogenous application demonstrated improved load and stiffness at 12 weeks postoperatively.6,8 However, the application of a growth factor to stimulate revascularization (VEGF) weakened the graft at 12 weeks.7 The ACL reconstruction graft healing response at earlier time points (6 weeks), optimal dosage, or appropriate combination or application is unknown. The 6-week time point is one of the most vulnerable periods of the graft healing phase because it is biomechanically at its weakest.9 Thus, a strategy to apply our own endogenous growth factors, which are concentrated in platelets, has potential clinical application.
One strategy of endogenous growth factor application using a collagen hydrogel supplemented with platelet-rich plasma has recently been shown to have some promise both enhancing suture repairs of the ACL and healing partial ACL laceration.5,10,11 Our overall hypothesis is that a CPC can provide a stable intraarticular provisional scaffold to facilitate wound healing and tissue growth. In this study, we hypothesized that placement of a CPC around an ACL graft at the time of surgery would decrease postoperative AP laxity and enhance the early structural properties of the graft (failure load and linear stiffness) 6 weeks after surgery. Given that the platelet concentration of the goat was highly variable, we also hypothesized the platelet concentration would correlate with the structural properties of the graft and the AP laxity of the knee after 6 weeks of healing.
Materials and Methods
Model of ACL Reconstruction
Twelve 4-year-old castrated male Nubian-cross goats underwent unilateral ACL reconstruction using a bone–patellar tendon–bone autograft. In the experimental group, six goats had the graft enhanced with a CPC (CPC group), while in the control group six had enhanced with a collagen hydrogel only (COLL group). The procedures were alternately performed in right and left knees in both the experimental and control groups. The animals were selected at random. Postoperatively the animals were allowed unrestricted cage activity during the 6 weeks of graft healing. They were then euthanized with an overdose of pentobarbital solution (Euthasol: 1 cc/10 lbs). At the time of euthanasia, both the ACL reconstructed knees and the contralateral intact knees were harvested and stored at −20°C prior to mechanical testing.
Surgical Technique
The animals were tranquilized preoperatively using acepromizine (10 mg i.m.). Anesthesia was induced with sodium pentothal (5–8 mg/kg i.v.) and maintained with isoflurane during surgery.
A scalpal was used to make an incision from the top of patella to below the tibial tubercle just medial of midline. The prepatellar bursa was cut in line with the skin incision to expose the paratenon. A longitudinal cut was made centrally in the paratenon to expose the patellar tendon. The medial and lateral borders of the patellar tendon were palpated and a 6-mm central graft was harvested. The patellar block was 15 × 6 mm, and the tibial bone block was 10 × 6 mm; both were trimmed to fit within a 6-mm diameter tube. Drill holes (1.5 mm) were then placed in the bone block on each side. Because the length of the patellar tendon is greater than the ACL, the tibial bone block was folded over onto the patellar tendon and sutured in place to make an 8-mm bone–tendon block on this side to shorten it.12 Two #2 Ethibond sutures were placed in each bone block. The intracondylar notch was exposed through the central defect in the patellar tendon by sectioning the fat pad. The intermeniscal ligament was not cut. A Lachman test was checked for baseline stability of the knee. A scalpel was used to release the ACL from the back of the notch, and the ACL was removed. A manual Lachman test verified complete functional loss of the ACL. The tibial tunnel was drilled using the tibial aiming guide set at 65°. The pin was overdrilled with an 8-mm drill and all soft tissue removed. A notchplasty was performed using a curette. The knee was hyperflexed and a 6-mm offset femoral guide (Arthrex Inc., Naples, FL) was placed into the back of the notch at the 10:30 position. The passing pin was drilled through the femur and then overdrilled with a 7-mm bit to a depth of 20 to 25 mm. Integrity of the back wall of the femoral tunnel was verified in all cases. The graft was placed into the femoral tunnel first using the Ethibond sutures, and then secured in the femur using a 5 × 20 mm metal interference screw (Arthrex Inc.). The graft was then pulled retrograde into the tibial tunnel and secured in the tibial tunnel using a 6 × 20 mm metal interference screw (Arthrex Inc.). The graft was firmly tensioned with the knee at 60° flexion while pulling the graft through the exit site of the tibia while stabilizing the femur and fixing it with an interference screw, as is done for humans. Tibial fixation was augmented with sutures to the periosteum if the tibial fixation was not deemed stable enough. For the experimental group, the graft was augmented with a collagen sponge placed between the ACL and lateral femoral condyle. The sponge was rectangular, measuring 1 × 2 cm. It was positioned adjacent to the graft, lying anteriorly and lateral to it. Two cubic centimeters of CPC was placed over the sponge. The control group was identical except no platelets were added to the collagen hydrogel. After 10 min, the knee was closed in layers. The animals were kept under anesthesia for 1 h after gel placement to maintain the knees in the resting position and allow complete gelation.
Postoperative analgesia was controlled using Buprenorphine (0.1 mg/kg i.m., twice daily) and Ketoprofen (1 mg/kg i.m., once daily) for 5 days. Ampicillin (10 mg/kg s.q.) was administered twice daily for 10 days to reduce the risk of infection.
COLL and CPC Preparation
Rat tail collagen was acid solubilized as previously described.5,10 For the COLL group, the collagen was neutralized to a pH of 7.4 and added to the surgical site just after neutralization. To add platelets to the gel, initially the production of platelet-rich plasma was attempted; however, due to the similarity in size and weight of the caprine platelet and red blood cell,13 centrifugation protocols using 150 to 250 g between 20 and 30 min were all ineffective at producing adequate platelet enrichment, despite previous reports of efficacy of this technique at producing reasonable platelet yields.13 The platelet counts in this study were less than 100% that of whole blood in all attempts using these centrifugation parameters. Therefore, we elected to use whole caprine autologous blood for the CPC group. We measured the platelet concentration in the peripheral blood for all animals in both the experimental and control groups. Blood (54 cc) was drawn from each animal into a syringe containing 6 cc of acid–citrate–dextrose as an anticoagulant. At the time of gel placement, the collagen was neutralized and mixed with the blood in a 4:1 collagen:blood ratio and the CPC added to the graft. This resulted in the platelet count in the CPC consistently being 20% of the systemic blood platelet concentration. Previous studies combining platelet-rich plasma and collagen have demonstrated gelation of the combination within 10 min on warming the mixture to body temperature (37°C).10,11
Mechanical Testing
The knees were thawed and prepared for laxity and failure testing. The soft tissues surrounding the tibia and femur were dissected free leaving the joint capsule intact. The distal tibia and proximal femur were then potted in PVC pipes using a potting material (SmoothCast 300; Smooth-On Inc., Easton, PA) so that they could be mounted on the material testing platform.
The AP load–displacement responses of the experimental and contralateral intact joints were measured using a custom-designed fixture with the knee locked at 30° and 60° of flexion.12 Anterior and posterior directed shear loads of ±60 Newtons were applied to the femur with respect to the tibia using an MTS 810 Materials Testing System (MTS, Prairie Eden, MN) while the AP displacement was measured. The knee flexion angle was prescribed, axial rotation of the tibia was locked in the neutral position, and all other motions (coronal plane translations and varus–valgus angulation) were left unconstrained. Following clinical convention, AP laxity was reported as motion of the tibia with respect to the femur.
After completing the AP laxity tests, the tibia and femur were positioned so that the mechanical axis of the ACL was collinear with the load axis of the material test system.14 The knee flexion angle was initially set at 30°. The tibia was mounted to the base of the MTS via a sliding X–Y platform while the femur was unconstrained to rotations.14 This enabled the specimen to seek its own position so that the load was distributed over the cross section of the healing graft when the tensile load was applied. The joint capsule, menisci, collateral ligaments, and the PCL were dissected from the joint leaving the ACL graft and scar mass intact. The femur–graft–tibia complexes were then loaded in tension to failure at 20 mm/min,10,15 while the failure load–displacement data were recorded. Identical protocols were performed on the contralateral ACL-intact knees. From the load–displacement tracing, the failure load, failure displacement, and the linear stiffness were determined.
Exclusions
Twelve animals underwent ACL reconstruction. The first animal was assigned to the CPC group. There were technical difficulties with the graft harvest and it was decided at the time of surgery to exclude this animal from the analysis. A second animal died from cardiovascular disease in the immediate postoperative period. In addition, one of the animals in the COLL group had a graft failure strength more than three times higher than any other animal in either group (probably as a result of incomplete transection of ACL at the time of ACL reconstruction), and was considered an outlier and thus excluded from the analyses due to the three-sigma rule. The same outlier criteria were applied to all animals. None of the excluded animals were represented in any group statistic nor are they graphically depicted in any figure. Thus, the final sample size consisted of nine animals: five experimental and four controls. Given the small sample sizes and therefore low power for detecting group differences, a lack of group differences could represent a high probability of a Type II (false negative) error. Assuming that the sample effects represent true population values, our observed power levels were between 20 and 55% for the structural and laxity variables compared (nQuery Advisor 7.0, Statistical Solutions, Boston, MA).
Statistical Analysis
Systemic platelet counts, AP laxity, failure strength, and linear stiffness were compared between CPC (experimental) and COLL (control) groups and assessed for normality using the Kolmogorov-Smirnov test. All variables conformed to a normal (Gaussian-shaped) distribution and were analyzed with parametric methods and summarized using the mean ± standard deviation. Systemic platelet counts between the experimental and control groups were assessed using the two-sample Student t-test. Differences in AP laxity between ACL reconstructed knees and contralateral ACL intact knees were compared using two-way repeated-measures mixed analyses of variance with treatment (experimental vs. control) as the between-subjects factor, knee as the repeated measures factor (using a compound symmetry covariance structure), and animal as a random subject factor. For the structural properties, the ratio of the reconstructed knee to the contralateral intact knee expressed as a percent was compared using unpaired Student t-tests. Pearson correlations were used to measure linear association between systemic platelet count, AP knee laxity, and failure properties of the graft 6 weeks after healing with the strength of relationships summarized by the coefficient of determination (R2). To compare correlations in the two treatment groups, Z-scores were computed to standardize the dependent variables and the covariate (platelet counts) within groups and then one-way analysis of covariance (ANCOVA) models were run with interactions between the groups and the covariate to test the equality of slopes between the CPC versus COLL groups. Statistical analysis was performed with SPSS software (version 16.0, SPSS Inc., Chicago, IL). A two-tailed value of p < 0.05 was used as the criterion for statistical significance.
Results
Surgical Outcomes
Eleven of the 12 animals recovered well from the surgery. One goat from the control group died within 1 day of surgery. Autopsy revealed extensive atherosclerosis, and the cause of death was thought to be cardiac related. At the time of euthanasia all remaining animals appeared to be walking normally.
Systemic Platelet Counts
No significant difference in systemic platelet count was found between CPC and COLL groups (4566 ± 1885 k/μL vs. 3519 ± 1077 k/μL, p = 0.358; Table 1).
Table 1.
Raw Laxity Data for Each Animal
| Goat # | Tx | Platelet Count |
AP Laxity | Structural Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACLR (mm) | NL (mm) | Delta (ACLR—NL) (mm) |
Max Load (N) |
Linear Stiffness (N/mm) |
||||||||
| AP60° | AP30° | AP60° | AP30° | AP60° | AP30° | ACLR | NL | ACLR | NL | |||
| 1348 | CPC | 5714 | 16 | 11 | 6 | 7 | 10 | 4 | 145 | 2350 | 38 | 31 |
| 1477 | COLL | 2771 | 22 | 19 | 6 | 5 | 16 | 14 | 130 | 1300 | 15 | 86 |
| 567 | CPC | 5078 | 17 | 11 | 3 | 3 | 14 | 8 | 135 | 1800 | 31 | 103 |
| 1483 | COLL | 2639 | 24 | 20 | 3 | 3 | 21 | 17 | 50 | 1550 | 6 | 101 |
| 1479 | CPC | 2608 | 21 | 19 | 3 | 4 | 18 | 15 | 100 | 1650 | 16 | 86 |
| 554 | COLL | 3692 | 26 | 24 | 6 | 5 | 20 | 19 | 90 | 1700 | 30 | 129 |
| 1014 | CPC | 2623 | 15 | 16 | 6 | 7 | 9 | 9 | 111 | 2000 | 10 | 95 |
| 381 | COLL | 4974 | 17 | 13 | 4 | 3 | 12.5 | 10 | 160 | 1884 | 38 | 160 |
| 1066 | CPC | 6807 | 12 | 10 | 3 | 4 | 9 | 5 | 206 | 1833 | 40 | 114 |
| Avg CPC (SD) | 4566 (1884.9) | 16.2 (3.3) | 13.4 (3.9) | 4.2 (1.6) | 5.0 (1.9) | 12.0 (3.9) | 8.3 (4.2) | 139 (41.4) | 1926 (267.4) | 27 (13.4) | 86 (32.3) | |
| Avg COLL (SD) | 3519 (1077.2) | 22.3 (3.9) | 19.0 (4.5) | 4.8 (1.5) | 4.0 (1.2) | 17.4 (3.9) | 15.0 (3.9) | 107 (47.9) | 1608 (246.9) | 22 (14.4) | 119 (32.6) | |
AP = anterior–posterior laxity (in mm); 60° = knee in 60° of flexion; 30° = knee in 30° of flexion; CPC = collagen–platelet composite; COLL = collagen hydrogel only; NL = contralateral control (normal); SD = standard deviation.
Gross Appearance
There was no difference between the CPC and COLL groups on gross appearance in terms of rate of reformation of the ligamentum mucosum, rate of scar or adhesion formation from the notch scar mass to the harvest defect of the patellar tendon, or amount of joint adhesions observed (Fig. 1). There was no difference between the groups in whether the scar mass infiltrated only the most cranial section of the graft or whether it bridged from femur to tibia; both findings were seen in grafts of both groups. Observers were blinded to group when harvesting and grading the specimens.
Figure 1.
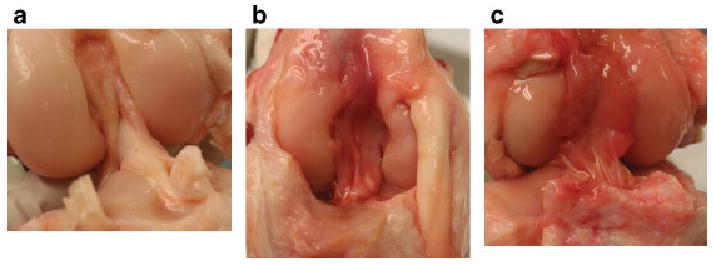
Anterior view of the intact and ACL reconstructed knees with and without collagen-platelet composite (CPC). (a) Normal ACL; (b) an ACL reconstruction with collagen hydrogel (COLL) encased by a scar mass; (c) ACL reconstruction enhanced with CPC also encased in a scar mass. No gross anatomical differences were observed between the two ACL reconstructed groups.
Biomechanical Testing
At a knee flexion angle of 30°, the mean difference in AP laxity was significantly (p = 0.045) decreased in the CPC group (8.3 ± 4.2 mm) when compared to the COLL group (15.0 ± 3.6 mm; Table 1). However, no significant decrease (p = 0.080) was observed at a knee flexion angle of 60° between the CPC group (12.0 ± 3.9 mm) and the COLL group (17.4 ±3.5 mm; Table 1). In addition, a higher systemic platelet count correlated with decreased AP laxity at both 30° (R2 = 0.628; Fig. 2a) and 60° (R2 = 0.44; Fig. 2b) of knee flexion. No significant interactions were observed between z-scores for the collagen gel treatments and systemic platelet count regarding joint laxity at 30° (p = 0.531) or 60° (p = 0.971), indicating no differences in slopes between treatments.
Figure 2.
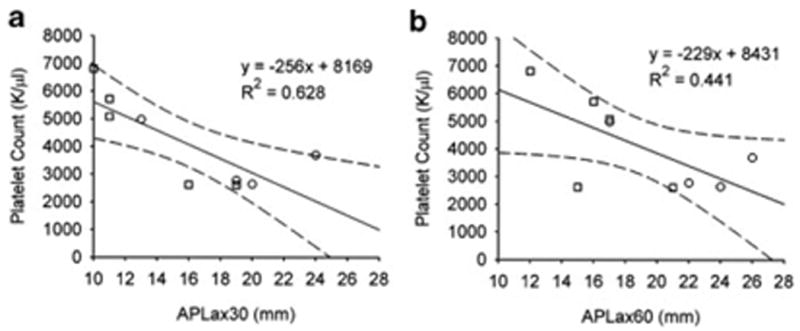
Inverse correlation between systemic platelet concentration and AP laxity with the knee at (a) 30° and (b) 60° of flexion for both treatment groups. Squares represent the points from the animals treated with CPC while the circles represent the points for the COLL group. The linear regression lines (solid) and 95% confidence intervals (dashed lines) are shown.
There was no significant difference in the failure loads of the CPC and the COLL groups (146 ± 54 N vs. 108 ± 48 N, respectively; p = 0.307; Table 1). The values in both groups were approximately 10% of the intact ACL strength.
There was no significant difference in linear stiffness between groups (COLL group: 22 ± 15 N/mm vs. CPC group 27 ± 14 N/mm; p = 0.628; Table 1). Both groups were less than one-third of that of the intact ligament (90 ± 34 N/mm).
When considering both groups combined, systemic platelets were positively correlated with ACL graft failure load (R2 = 0.691; Fig. 3a). The slopes in the group treated with CPC compared to the group treated with collagen scaffold only were 0.025 and 0.031, respectively (interaction test, p = 0.758). When considering both groups combined, systemic platelets were positively correlated with linear stiffness (R2 = 0.840; Fig. 3b). The slopes in the group treated with the platelet hydrogel compared to the group treated with collagen hydrogel were −0.002 and −0.003, respectively (interaction test, p = 0.840).
Figure 3.
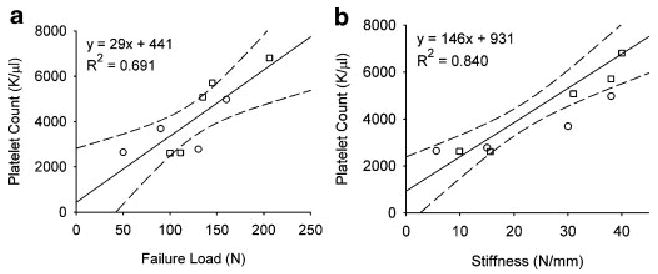
Positive correlation between systemic platelet concentration and (a) maximum ACL graft failure load and (b) graft stiffness for the collagen-platelet composite (CPC) and collagen only (COLL) groups. Squares represent the points from the animals treated with CPC while the circles represent the points for the COLL group. The linear regression line (solid) and 95% confidence intervals (dashed lines) are shown (failure load: R2 = 0.69, p = 0.006; stiffness: R2 = 0.84, p < 0.001).
Discussion
The addition of blood platelets to the collagen scaffold (CPC group) resulted in significant reduction in AP knee laxity at 6 weeks after autograft patellar tendon ACL reconstruction but did not appear to have an effect on the structural properties of the graft. The large reduction (∼50%) in early postoperative AP laxity warrants continued study. In addition, the systemic platelet count of the animals correlated directly with the maximum load for both the experimental group (CPC) and the control group (COLL) and inversely correlated with AP laxity. These findings were consistent across groups, which could be caused by intraarticular bleeding. Intraartciular bleeding could potentially have introduced platelets into the control hydrogel before it set. Nonetheless, these findings highlight the important role blood platelets may play in ACL reconstruction graft healing.
The improvement in AP laxity was most evident when the knees were tested in 30° of flexion (approximately full extension in the caprine knee). At this position, ACL reconstructed knees treated with CPC were on average approximately half the AP laxity at 30° versus the COLL (8.3 vs. 15.0 mm; Table 1). At 60° of knee flexion the CPC had less AP laxity; however, the difference between groups was smaller and not significant (12.0 vs. 17.4 mm for the CPC and COLL groups, respectively). The large increases in AP laxity seen in the COLL group are consistent with those of previous studies of ACL reconstruction with autogenous patellar tendon grafts using the goat model.16–19 After 6 weeks of healing, Papageorgiou et al. reported laxity values of 13.2 mm and 16.2 mm with the ACL reconstructed knee at 30° and 60° of flexion, respectively, compared to the contralateral control (3.9 mm).18 The improvement seen by the addition of CPC is also a marked improvement from results published previously where the AP laxity of the knee increased from 2.0 ± 0.7 mm in the intact ovine knee to 8.3 ± 2.3 mm in the ACL reconstructed ovine knee after 6 weeks.4 The mechanism causing the decrease in AP laxity was not determined in our experiment. Some plausible explanations include recruitment of myofibroblasts into the healing ACL graft, “tightening” of the knee capsule, scarring within the joint, and flexion contractures. However, no adhesions to the capsule other than the scar mass surrounding the graft were apparent upon dissection, and there were no perceived differences in the range of flexion–extension motion in either group.
When platelets come into contact with collagen in a wound site in vivo, they are known to degranulate and release growth factors in the local wound environment. This same effect has been noted previously using a CPC in both in vitro and in vivo studies. In vitro, PDGF-AB has been found to be released in relevant quantities from a similar CPC, suggesting platelet activation and cytokine release.20 In vivo, high levels of FGF-2, PDGF-AB, and TGF-b are found to be localized in the area of CPC implantation for up to 3 weeks after gel implantation, suggesting sustained presence of these platelet-related growth factors in the wound site after platelet activation.5
To our knowledge, this is the first report of a correlation between systemic platelet count and ACL graft strength in an animal model. This finding is supported by other studies detailing the role of specific growth factors released by platelets, such as IGF-1, TGF-β1 VEGF, PDGF, and FGF-2, in the healing of tendon and ligament.21 ACL cell migration in vitro has been stimulated by TGF-β1, while PDGF-AB and FGF-2 can stimulate ACL cell proliferation in a 3D collagen scaffold.22 Platelet-rich plasma has also been reported to stimulate healing of bone both in animal models23 and humans.24 Platelet gels have been used to improve the strength of the abdominal wall fascia after hernia repair in rats.25 However, the use of platelets to stimulate tendon and ligament healing directly has been less widely reported,26 and is a relatively new field of inquiry.
Previous attempts to accelerate the revascularization phase with VEGF in a sheep model found increased vessels but decreased linear stiffness and increased laxity.27 By applying platelets in a stabilized collagen hydrogel we have simulated a more “natural” environment with the natural occurrence of platelets deposited in a fibrin clot as in extraarticular healing. Further, platelets contain a multitude of growth factors in addition to TGF-β1 and PDGF seemingly through natural selection in the appropriate concentration for extraarticular healing. Highly relevant to clinical application is that the cost to apply autologous platelets from peripheral blood is much less than a recombinant TGF, PDGF, or EGF.
The 6-week time point is well recognized as a nadir of strength in ACL reconstruction for animal models. Failure load values in both our experimental and control ACL graft groups were approximately 10% of the intact ACL, which is slightly higher than previous reports of 3% in the sheep model,4 and typical for the goat model after 6 weeks of healing.16,18 Although this time point was useful in determining differences in the revascularization phase of healing of the ACL graft, longer term studies are required to evaluate the effects of platelets on graft strength and AP knee laxity later in the healing phase. As previous translational models of ACL graft healing with TGF-β/EGF6 and PDGF-BB8 demonstrated increased strength at 12 weeks, additional investigations examining higher platelet yields and the 12-week postoperative time point are planned.
One limitation of this study was that we were unable to perform platelet enrichment of the caprine blood. Centrifuge protocols that systematically changed the centrifugal force from 50 to 250 × g, with various cycle times and combinations proved ineffective, and limited our ability to evaluate the role of a two- or fourfold platelet concentration on ACL graft healing, levels that have been shown to enhance primary ACL repair in canine and porcine models.10,11 This was likely due to the similarities in the sizes of the caprine erythrocytes and platelets.13 Although prior studies have reported the use of caprine PRP, three do not report platelet counts in both the whole blood and “PRP;”26,28,29 thus, platelet enrichment levels actually achieved in those studies cannot be evaluated. A fourth study reported TGF-β release from goat “PRP” at levels far below that for human PRP, making it unclear if they had actually produced functional goat PRP.13 An additional potential problem with the caprine model is that with the similar size of the erythrocyte and platelet, overlap between the platelet and erythrocyte cell populations when cell size is used to determine cell numbers is more likely to occur. Therefore, the platelet count measure that correlated with mechanical properties in this study is likely a combination of platelet and the smallest erythrocyte fraction. It is likely that the platelets in this fraction were the more active cellular component; however, further studies to evaluate the relative contributions of platelets and small erythrocytes would be needed to verify this.This problem could be avoided by using the canine or porcine models, which have a greater distinction in cell size and thus have cells that are more easily sorted. With the difficulties in reliably producing caprine PRP, we elected to use the more consistent whole blood for the CPC preparations. Unfortunately, we did not anticipate the great degree of difficulty in obtaining complete hemostasis at the operative site for the goats (which had been easy to do in our prior experience with ACL repair in the pig model), and thus, we observed some blood mixing with the injected gel in the COLL group, which likely added platelets to the hydrogel in the control animals. This makes it difficult to know if the observed effects were due to the systemic platelets, the collagen sponge alone, or the collagen sponge-infused platelet composite. Thus, to fully answer the questions regarding the effect of platelet concentration on ACL graft properties and knee laxity, a different animal model (either porcine or canine) where platelet and erythrocyte size are more distinct and thus the platelets can more easily be concentrated. Additional experiments using collagen scaffold only and controlling for systemic platelets will be necessary.10,11 Nonetheless, the findings reported here suggest that platelets may have an important role in intraarticular graft healing.
It is possible that the sponge, which was wedged between the graft and the notch, migrated after implantation. The sponge was relatively large so we would expect that it would remain in the proper position. Also, once the sponge was implanted and saturated with plasma, it was allowed to congeal for a minimum of 1 h before knee motion was allowed. Certainly, future studies that utilize a sponge that is wrapped around the graft might be worth pursuing given the results in this paper.
A final limitation of the study was the inability to control rehabilitation in the goat model (present in all large animal models of ACL reconstruction). Ruminants need to be standing within the first few hours of surgery, and therefore it is difficult to protect the graft during the initial healing stage. Bandaging and immobilization are effective with this animal model. Although slings could be used, this would result in complete nonweight bearing, which is not relevant to modeling the human postoperative rehabilitation scenario where patients are typically partially weight bearing or weight bearing as tolerated after surgery.
In summary, use of a CPC to enhance healing of an allograft ACL reconstruction correlated with early sagittal plane laxity and the systemic platelet count was highly predictive of ACL reconstruction graft strength and stiffness after 6 weeks of healing in the caprine model. We were not able to separate the relative importance of platelets in the sponge on the graft, platelet concentration in the sponge, and systemic platelet levels in affecting graft and joint properties. Therefore, further work investigating the possible role of a CPC on improving ACL reconstruction outcomes is warranted.
Acknowledgments
The authors would like to acknowledge Megan Bowers, Koosha Aslani, Scott McAllister, Jessica Hootnick, Eduardo Abreu, and Matthew Palmer for their assistance with this project, and Lynn Cain for editorial assistance. Funding was received from Vanderbilt's Kenneth D. Schermerhorn Endowed Professorship, the RIH Orthopaedic Foundation and NIH Grants AR049199 (B.C.F.) and AR052772 (M.M.M.).
References
- 1.Tashman S, Kolowich P, Collon D, et al. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 2.Fleming BC, Brattbakk B, Peura GD, et al. Measurement of anterior-posterior knee laxity: A comparison of three techniques. J Orthop Res. 2002;20:421–426. doi: 10.1016/S0736-0266(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Uh BS, Fleming BC, et al. Rehabilitation following anterior cruciate ligament reconstruction; a prospective, randomized, double-blind comparison of accelerated versus non-accelerated rehabilitation. Am J Sports Med. 2005;33:347–359. doi: 10.1177/0363546504268406. [DOI] [PubMed] [Google Scholar]
- 4.Hunt P, Scheffler SU, Unterhauser FN, et al. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg. 2005;125:238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda K, Tomita F, Yamazaki S, et al. The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med. 2004;32:870–880. doi: 10.1177/0363546503261695. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Tohyama H, Katsura T, et al. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34:1918–1925. doi: 10.1177/0363546506294469. [DOI] [PubMed] [Google Scholar]
- 8.Weiler A, Forster C, Hunt P, et al. The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:881–891. doi: 10.1177/0363546503261711. [DOI] [PubMed] [Google Scholar]
- 9.Butler DL, Grood ES, Noyes FR, et al. Mechanical properties of primate vascularized vs. nonvascularized patellar tendon grafts; changes over time. J Orthop Res. 1989;7:68–79. doi: 10.1002/jor.1100070110. [DOI] [PubMed] [Google Scholar]
- 10.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 11.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 12.Fleming BC, Abate JA, Peura GD, et al. The relationship between graft tensioning and the anterior-posterior laxity in the anterior cruciate ligament reconstructed goat knee. J Orthop Res. 2001;19:841–844. doi: 10.1016/S0736-0266(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 13.Clemmons RM, Bliss EL, Dorsey-Lee MR, et al. Platelet function, size and yield in whole blood and in platelet-rich plasma prepared using differing centrifugation force and time in domestic and food-producing animals. Thromb Haemost. 1983;50:838–843. [PubMed] [Google Scholar]
- 14.Woo SLY, Hollis JM, Adams DJ, et al. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effect of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 15.Sakai T, Yasuda K, Tohyama H, et al. Effects of combined administration of transforming growth factor-beta1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res. 2002;20:1345–1351. doi: 10.1016/S0736-0266(02)00065-7. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitch SD, Papageorgiou CD, Withrow JD, et al. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J Orthop Res. 2003;21:707–715. doi: 10.1016/S0736-0266(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 17.Cummings JF, Grood ES. The progression of anterior translation after anterior cruciate ligament reconstruction in a caprine model. J Orthop Res. 2002;20:1003–1008. doi: 10.1016/S0736-0266(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 18.Papageorgiou CD, Ma CB, Abramowitch SD, et al. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620–626. doi: 10.1177/03635465010290051501. [DOI] [PubMed] [Google Scholar]
- 19.Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson M, Fufa D, Abreu EL, et al. Platelets, but not erythrocytes significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regeneration. 2008 doi: 10.1111/j.1524-475X.2008.00376.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Meaney Murray M, Rice K, Wright RJ, et al. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 23.Tozum TF, Demiralp B. Platelet-rich plasma: a promising innovation in dentistry. J Can Dent Assoc. 2003;69:664. [PubMed] [Google Scholar]
- 24.Lowery GL, Kulkarni S, Pennisi AE. Use of autologous growth factors in lumbar spinal fusion. Bone. 1999;25:47S–50S. doi: 10.1016/s8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 25.Zieren J, Castenholz E, Baumgart E, et al. Effects of fibrin glue and growth factors released from platelets on abdominal hernia repair with a resorbable PGA mesh: experimental study. J Surg Res. 1999;85:267–272. doi: 10.1006/jsre.1999.5608. [DOI] [PubMed] [Google Scholar]
- 26.Anitua E, Andia I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–286. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa T, Tohyama H, Enomoto H, et al. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:804–810. doi: 10.1007/s00167-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 28.Nikolidakis D, van den Dolder J, Wolke JG, et al. The effect of platelet-rich plasma on the bone healing around calcium phosphate-coated and non-coated oral implants in trabecular bone. Tissue Eng. 2006;12:2555–2563. doi: 10.1089/ten.2006.12.2555. [DOI] [PubMed] [Google Scholar]
- 29.Brehm W, Aklin B, Yamashita T, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14:1214–1226. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]


