Abstract
Background
Random effects models were used to explore how the shape of CD4 cell count responses after commencing combination antiretroviral therapy (cART) develop over time, and in particular the role of baseline and follow-up covariates.
Methods
Patients in APHOD who first commenced cART after January 1, 1997, and who had a baseline CD4 cell count and viral load measure and at least one follow-up measure between 6 and 24 months, were included. CD4 cell counts were determined at every 6-month period following the commencement of cART for up to 6 years.
Results
1638 patients fulfilled the inclusion criteria with a median follow up time of 58 months. Lower post-cART mean CD4 cell counts were found to be associated with increasing age (p<0.001), pre-cART hepatitis C co-infection (p=0.038), prior AIDS (p=0.019), baseline viral load ≤ 100,000 copies/ml (p<0.001), and the Asia-Pacific region compared with Australia (p=0.005). A highly significant 3-way interaction between the effects of time, baseline CD4 cell count and post-cART viral burden (p<0.0001) was demonstrated. Higher long-term mean CD4 cell counts were associated with lower baseline CD4 cell count and consistently undetectable viral loads. Among patients with consistently detectable viral load CD4 cell counts appeared to converge for all baseline CD4 levels.
Conclusion
Our analyses suggest that the long-term shape of post-cART CD4 cell count changes depends only on a 3-way interaction between baseline CD4 cell count, viral load response and time.
Keywords: antiretroviral therapy, long-term CD4 response, viral load response
INTRODUCTION
The introduction of combination antiretroviral therapy (cART) has been effective in decreasing morbidity and mortality rates due to HIV infection in Australia [1, 2] and other developed countries [3, 4]. Similar rate reductions have also been observed in HIV patients treated with cART in developing countries [5–7]. The use of cART has proven to effectively reduce viral replication, enabling increases in CD4 cell count and restoration of immune function[8]. The extent of immunological response following the initiation of cART has significant clinical implications.
Pre-treatment predictors of higher mean CD4 cell counts following the commencement of cART have been reported previously, and include younger age [9–13], female sex [12], higher pre-cART viral loads [11, 14] and mode of HIV exposure other than injecting drug use [12]. In addition, the effect of pre-cART (baseline) CD4 cell counts on subsequent CD4 cell counts has been extensively studied [10–24]. Some of these studies have found that baseline CD4 cell counts influence subsequent CD4 cell count increments [20, 21], whilst others reported either little or no evidence of such an association [22–24].
Previous research has demonstrated greater increases in mean CD4 cell counts if post-cART viral load suppression is completely or even partially sustained [16, 25] However, opinion remains divided as to the extent of long term immune system restoration in the presence of sustained virological suppression. A number of studies have reported a plateau in the growth of mean CD4 cell counts within 2 to 3 years of the commencement of cART in the presence of sustained virological suppression [11, 12, 15, 16, 18], whilst others have shown continued increases well beyond 3 years of effective cART [10, 14].
The objective of this paper was to use random effects models to explore statistically how the shape of CD4 cell count responses after commencing cART develop over time, and in particular the role of baseline and follow-up covariates.
METHODS
The Asia-Pacific HIV Observational Database (APHOD) is part of the National Institutes of Health (NIH) International Epidemiological Databases Evaluating AIDS (IeDEA) collaboration. APHOD consists of two adult cohorts, the Australian HIV Observational Database (AHOD) and the Treat Asia HIV Observational Database (TAHOD), and one paediatric cohort, the Treat Asia Paediatric HIV Observational Database (TApHOD). The current analyses include patients recruited to the adult cohorts AHOD and TAHOD. AHOD collects data from 27 sites throughout Australia and TAHOD currently collects data from 17 sites throughout 10 countries in the Asia-Pacific Region. Ethics approval for the two cohorts was granted to all participating sites by relevant institutional review boards (IRB). Written informed consent was obtained for all AHOD patients at recruitment. Unless required by local IRBs, written informed consent was wavered for TAHOD patients. Study methodologies are similar for AHOD and TAHOD. Data are transferred electronically to the National Centre in HIV Epidemiology and Clinical Research (NCHECR), and updated every six months. Core data variables collected include: sex, date of birth, date of most recent visit, HIV exposure category, HBV surface antigen status (HBV), anti-HCV antibody status (HCV) (note: missing equals negative), CD4 and CD8 T lymphocyte counts, HIV viral load measurements, antiretroviral and opportunistic infection prophylaxis treatments, AIDS defining illness and date and cause of death. Detailed descriptions of these cohorts have been provided elsewhere [26, 27].
Inclusion Criteria
Analyses include patients who commenced cART (defined as 3 or more antiretroviral drugs) after January 1 1997, and who have a baseline CD4 cell count and viral load measurement within 6 months prior to and up to 1 month after commencing cART. The measurements that were nearest to the date cART commenced were chosen as the baseline values. Patients were also required to have at least one follow-up CD4 cell count measurement and viral load measurement between six months and 24 months after the commencement of cART. Only CD4 cell measurements with a corresponding viral load measurement were used in the analysis. The recorded time at each CD4 cell count measurement after the commencement of cART was rounded to the nearest bi-annual anniversary date of the commencement of cART. Where patients had more than one CD4 cell count measurement within a six month period, the measurement closest to the bi-annual anniversary date was chosen. An intention to treat approach (ITT) was used in all the analyses. This assumes that once patients have commenced cART then all analyses include all patients with follow-up data regardless of their treatment status at each time-point.
Statistical Analysis
Linear random effects models were used to investigate the predictors of long-term changes in CD4 cell counts up to 6 years after the commencement of cART. Random effects models were chosen to analyse these data specifically because these models are relatively robust to missing value bias when the probability of an observation being missing depends only on the surrounding responses and/or covariates included in the model (i.e. missing at random (MAR) data) [28]. In the full multivariate model, post-cART CD4 cell count was defined as the dependent variable, and baseline CD4 cell count was treated as a continuous covariate and not included in the dependent variable, so as to determine predictors adjusted for baseline CD4 cell count. Categorical covariates included sex (male versus female), cohort (TAHOD versus AHOD), pre-cART viral load (≤ 100,000 versus >100,000 copies/ml), pre-cART HBV and/or HCV co-infection (yes versus no), prior AIDS (yes versus no), and reported mode of HIV exposure (homosexual, heterosexual, injecting drug use, other). Other continuous covariates included age at the commencement of cART and time after the commencement of cART. The proportion of time from 6 months after the commencement of cART that a subject’s viral load was above 400 copies/ml was included as a continuous time-dependent covariate (referred to hereafter as ‘post-cART viral burden’). Viral burden levels were categorised into the following groups: continuously sustained viral suppression (burden level =0), intermittent viral suppression (burden level=0.5) and continuous viral load greater than 400 copies/ml (burden level=1), representing 0%, 50% and 100% of post-cART viral load measurements > 400 copies/ml. Post-cART viral burden was updated using each new viral load measurement regardless of whether there was a corresponding CD4 cell count measurement.
Based on exploratory analyses, the following potential interactions were tested by adding interaction terms one at a time to the full random effects model. The interaction terms that were assessed (at the α =0.01 level) were cohort with a) time b) baseline CD4 cell count and c) post-cART viral burden, and time with a) age, b) sex, c) pre-cART HCV co-infection, d) pre-cART HBV co-infection, e) pre-cART HIV-1 RNA load and f) the reported mode of HIV exposure. The effects of significant covariates that did not interact with time were, by implication of the modelling structure, deemed to have occurred in the first six months, and therefore described as set predictors. The covariance structure for the full model was also chosen using Akaike’s information criterion. A reduced multivariate model was then obtained from the full model by eliminating non-significant covariates (p>0.05 for main effects, p>0.01 for interaction effects) that were not substantial confounders of other effects (<20% change in other effects). The covariance structure chosen for the full model was retained for the reduced model. All analyses were performed using SAS version 9.1 and STATA version 10.
RESULTS
Demographic and pre-cART clinical characteristics
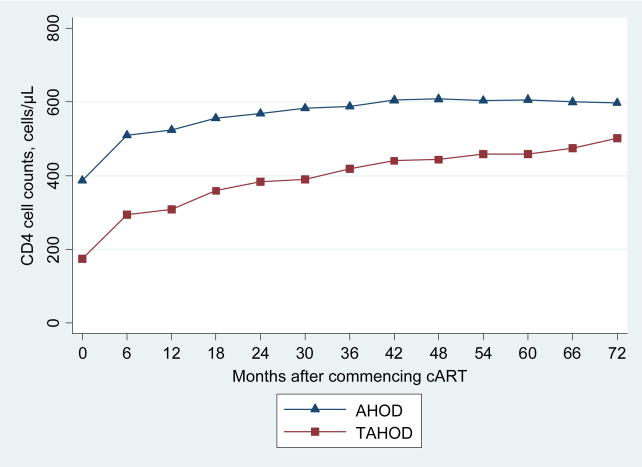
A total of 1638 patients fulfilled the inclusion criteria; 917 (56%) were from AHOD and 721 (44%) were from TAHOD. The median follow-up time was 58 months. Overall there were proportionally more males (86%) and patients whose exposure category was male homosexual contact (54%); and the majority of patients had no prior AIDS related illness reported (73%), and pre-cART viral load was ≤ 100,000 copies/ml (65%) (Table 1). TAHOD had a higher proportion of female patients (24% versus 6%), patients with a prior AIDS related illness (44% versus 13%) and patients with pre-cART HIV viral load greater than 100,000 copies/ml (40% versus 32%). The distribution of reported mode of HIV exposure also differed considerably between these cohorts. A greater proportion of patients in TAHOD reported mode of HIV exposure as heterosexual contact (58% versus 10%), while the reported mode of HIV exposure for the majority of patients in AHOD was homosexual contact (73% versus 28%). TAHOD patients were significantly younger at the time of commencing cART (37.6 versus 40.1 years) and had a lower mean baseline CD4 cell count (173 versus 386 cells/μL). The proportions of patients with pre-cART HCV coinfection were similar for both cohorts (TAHOD: 3%; AHOD 4%). Figure 1 shows the observed mean CD4 cell counts (cells/μL) over time for TAHOD and AHOD separately. Patients in TAHOD commenced cART at substantially lower CD4 cell counts (<200 cells/μL) compared to AHOD patients. However, both cohorts demonstrate increasing mean CD4 cell counts for up to 6 years.
Table 1.
Demographic and pre-cART clinical characteristics of patients, by cohort.
| Characteristic | AHOD N=917 | TAHOD N=721 | All Patients N=1638 |
|---|---|---|---|
| Age, mean years [95% CI] | 40.1[39.4, 40.7] | 37.6[36.8, 38.4] | 39.0[38.5, 39.5] |
| Sex | |||
| Female | 55 (6) | 170 (24) | 225 (14) |
| Male | 862 (94) | 551 (76) | 1413 (86) |
| Pre-cART HCV coinfection | |||
| No | 876 (96) | 700 (97) | 1576 (96) |
| Yes | 41 (4) | 21 (3) | 62 (4) |
| Pre-cART HBV coinfection | |||
| No | 898 (98) | 680 (94) | 1578 (96) |
| Yes | 19 (2) | 41 (6) | 60 (4) |
| Pre-cART HIV-1 RNA load | |||
| ≤ 100,000 copies/ml | 626 (68) | 431 (60) | 1057 (65) |
| > 100,000 copies/ml | 291 (32) | 290 (40) | 581 (35) |
| Pre-cART AIDS related illness | |||
| No | 795 (87) | 405 (56) | 1200 (73) |
| Yes | 122 (13) | 316 (44) | 438 (27) |
| Reported mode of HIV exposure | |||
| Male Homosexual contact | 676 (73) | 200 (28) | 876 (53) |
| Injecting drug use | 26 (3) | 16 (2) | 42 (3) |
| Heterosexual contact | 89 (10) | 420 (58) | 509 (31) |
| Other | 126 (14) | 85 (12) | 211 (13) |
| Baseline CD4 cell count, mean cells/μl [95% CI] | 386[369, 403] | 173[162, 185] | 292[281, 304] |
Note: Data are frequencies and within cohort region percentages in parentheses, unless otherwise indicated
Figure 1.
Observed mean CD4 cell counts (cell/μL) over time for TAHOD and AHOD.
Set predictors of post-cART mean CD4 cell counts
The set predictors of post-cART mean CD4 cell counts were covariates that did not significantly interact with time, including cohort, age, sex, pre-cART HCV and HBV status, pre-cART viral load and HIV exposure. Interactions between cohort and baseline CD4 cell count or post-cART viral burden were also assessed and were not significant. Table 2 presents the estimated effects from the full and reduced random effects models for the set predictors. Lower post-cART mean CD4 cell counts were found to be associated with increasing age (−14 cells/μL per 10-year increase in age 95%CI [−21, −7], p<0.001), pre-cART HCV co-infection (−41 cells/μL 95%CI [−79,−2], p=0.038), prior AIDS (−23 cells/μL 95%CI [−42, −2], p=0.019) and pre-cART HIV viral load ≤ 100,000 copies/ml (−40 cells/μL 95%CI [−56, −25], p=0.000). There was no evidence that either sex or pre-cART HBV co-infection affected post-cART CD4 cell counts (p=0.56 and p=0.36 respectively). Reported mode of HIV exposure was also not associated with post-cART CD4 cell counts overall (p=0.27). However, the exposure covariate was kept in the final model as the removal of this covariate produced a 28% change in the estimated effect of cohort primarily as a result of the larger proportion of heterosexuals in TAHOD. The final model indicated that, when adjusted for all other predictors (including time, post-cART viral burden and baseline CD4 cell count), patients from TAHOD had a mean post-cART CD4 cell count 27 cells/μL (95%CI [8, 46], p=0.005) lower than AHOD patients.
Table 2.
Set predictors a on CD4 T-cell counts from linear mixed model analyses. Models are also adjusted for time, post-cART viral burden and baseline CD4 cell count.
| Full Modelb | Reduced Modelc | ||||||
|---|---|---|---|---|---|---|---|
| N | Difference in mean CD4 count cells/μL (95% CI) | p- value | p-trend | Difference in mean CD4 count, cells/μL (95% CI) | p-value | p-trend | |
| Age at commencement of cART per 10 year increase | 1638 | −14(−21, −7) | 0.000 | −14(−21, −7) | 0.000 | ||
| Cohort | |||||||
| AHOD | 917 | Ref. group | Ref. group | ||||
| TAHOD | 721 | −26(−45, −7) | 0.007 | −27(−46, −8) | 0.005 | ||
| Sex | |||||||
| Female | 225 | Ref. group | |||||
| Male | 1413 | 7(−17, 32) | 0.564 | … | |||
| Pre-cART HCV coinfection | |||||||
| No | 1576 | Ref. group | Ref. group | ||||
| Yes | 62 | −40(−77, −1) | 0.043 | −41(−79, −2) | 0.038 | ||
| Pre-cART HBV coinfection | |||||||
| No | 1578 | Ref. group | |||||
| Yes | 60 | −18(−58, 21) | 0.357 | … | |||
| Pre-cART HIV-1 RNA load | |||||||
| ≤ 100,000 copies/ml | 1057 | Ref. group | Ref. group | ||||
| > 100,000 copies/ml | 581 | 40(24, 56) | 0.000 | 40(25, 56) | 0.000 | ||
| Pre-cART AIDS related illness | |||||||
| No | 1200 | Ref. group | Ref. group | ||||
| Yes | 438 | −23(−43,−4) | 0.016 | −23(−42,−2) | 0.019 | ||
| Reported mode of HIV exposure | |||||||
| Homosexual contact | 876 | Ref. group | Ref. group | ||||
| Injecting drug use | 42 | −22(−68, 24) | 0.352 | 0.273 | −22(−68, 24) | 0.352 | 0.274 |
| Heterosexual contact | 509 | −20(−41, 1) | 0.065 | −22(−42, −2) | 0.030 | ||
| Other | 211 | −5(−29, 18) | 0.636 | −7(−30, 16) | 0.544 | ||
Table omits parameter estimates corresponding to the interacting effects of the covariates- time after the commencement of cART, post-cART viral burden and baseline CD4 cell count.
Model containing all covariates.
Model containing significant covariates and/or substantial confounders of other effects.
The effects of time, post-cART viral burden levels and baseline CD4 cell counts on post-cART mean CD4 cell counts
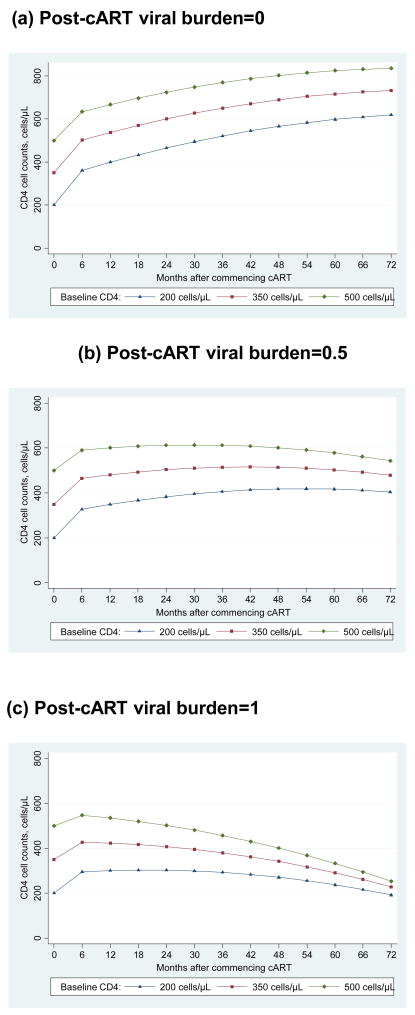
The final random effects model revealed a highly significant 3-way interaction between the effects of time, baseline CD4 cell count and post-cART viral burden (p<0.0001). The disadvantage of fitting a third order interaction model is that it does not provide a simple summary for the effects of the interacting covariates. To assist in the interpretation of these interaction effects, we present them graphically using selected estimates for the clinically relevant baseline CD4 cell count levels of 200, 350 and 500 cells/μL and post-cART viral burden levels of 0, 0.5, 1 (representing 0%, 50% and 100% of post-cART viral load measurements > 400 copies/ml). Figure 2a, b and c demonstrates this 3-way interaction by illustrating the shape of mean CD4 cell increase predicted by the final random effects model for subjects with the following set predictor characteristics: 45 years old, AHOD cohort, no pre-cART HCV co-infection, pre-cART viral load ≤ 100,000 copies/ml, no prior AIDS and homosexual contact as the reported mode of HIV exposure. Whilst varying the values for the set predictors would produce a vertical shift in the curves from months 6 to 72, the rates of change during this period would remain the same, so overall shapes would not be altered.
Figure 2.
Predicted mean CD4 cell counts (cell/μL) according to post-cART viral burden levels (a) 0 (b) 0.5 (c) 1 and baseline CD4 cell count levels 200, 350, 500 cells/μl for subjects with the following set predictor characteristics; 45 years old, AHOD cohort, no pre-cART hepatitis C coinfection, pre-cART viral load less than 100,000 copies/ml, no pre-cART AIDS related illness and with homosexual contact as the reported mode of HIV exposure.
For example, as shown in Figure 2a, a patient with the following set predictor characteristics: 45 years old, AHOD cohort, no pre-cART HCV co-infection, pre-cART viral load ≤ 100,000 copies/ml, no prior AIDS and homosexual contact as the reported mode of HIV exposure, and with viral load remaining below 400 copies/ml, will experience a continuous increase in CD4 cell counts beyond 6 years. This increase occurs across all baseline CD4 cell strata, and at year 6 the mean absolute CD4 cell count is above 500 cells/μL for all baseline CD4 cell strata. For viral burden levels of 0.5 (intermittently below 400 copies/ml), there is an initial increase in CD4 cell count for all baseline CD4 cell strata. After 4 years the mean absolute CD4 cell count begins to decrease for the higher baseline CD4 cell strata and plateau for patients whose baseline CD4 cell count was 200 cells/μL. However, for all groups at 6 years of follow-up mean absolute CD4 cell counts remain above baseline CD4 cell levels, and all greater than 300 cells/μL. For viral loads continuously above 400 copies/ml (viral burden=1), mean absolute CD4 cell count begins to decrease very soon after 6 months for the higher baseline CD4 cell strata, and for patients in the lowest baseline CD4 cell strata values begin to decrease after approximately 2 years. At year 6 mean CD4 cell count for all strata are below baseline values, yet remain around 200 cells/μL or greater (Figure 2c). Further, the mean absolute CD4 cell count for each baseline CD4 cell strata appear to converge by year 6.
DISCUSSION
In this study, patients with sustained virological response demonstrate increasing CD4 cell counts for up to 6 years following the commencement of cART, with mean absolute CD4 cell counts at year 6 above 500 cells/μL for all baseline CD4 cell strata. Patients with intermittent virological response also demonstrated increases in CD4 cell count for several years post cART, with CD4 cell increases continuing longer among patients in the lowest baseline CD4 cell strata. At year 6, mean absolute CD4 cell counts among patients with intermittent virological response were above 350 cells/μL for all baseline CD4 cell strata, and among patients with consistently detectable viral load (>400 copies/ml) mean CD4 cell counts at 6 years were approximately 200 cells/μL or greater.
Our results are broadly consistent with the literature demonstrating greater increases in mean CD4 cell counts if viral load suppression is sustained or partially sustained [16, 21, 24, 25, 29–31]. To our knowledge, this study is the first to qualitatively explore the shape of long-term CD4 cell responses by modelling the three way interaction between CD4 cell count, viral load and time. Several studies have reported a biphasic CD4 cell increase, demonstrated as a rapid increase in CD4 cell count change in the first few months with continued yet slower increase for up to several years of follow-up with a plateau by approximately the third year of follow-up [11, 12, 17, 19, 20, 32, 33], while other studies have shown continual increases in CD4 cell count response with sustained low viral loads; even after the fourth year following the commencement of cART [10, 31, 34–36]. Our study suggests that age, cohort, HCV status, baseline viral load and prior AIDS act as set predictors on CD4 cell count changes, producing a vertical shift in the long-term CD4 cell count response. The long-term shape of the CD4 cell count response was determined in our analyses by a 3 way interaction between baseline CD4 cell count, detectable viral load and time.
As reported previously by others we also observed greater increases in CD4 cell counts for patients in the lowest baseline CD4 cell strata in the continuous or intermittent virological response groups (Figures 2a and 2b). In the MACS study, long term significant increases in CD4 cell count were observed among ART naïve patients whose baseline CD4 cell count was below 400 cells/μl, and among intermittent antiretroviral users, with baseline CD4 cell counts below 200 cells/μL [37]. In contrast, another study found no significant difference in response to highly active antiretroviral therapy among those patients with higher pre-treatment CD4 cell counts once adjusted for age and nadir CD4 cell count [38]. Further, our study showed that patients who commenced cART at CD4 cell counts greater than 350 cells/μL achieved CD4 cell levels similar to HIV negative patients. This has been reported by Mocroft et al (2007), who also proposed that with longer period of time with suppressed viral load, similar levels may also be achieved among patients commencing cART at lower CD4 cell counts. Our results broadly concur with this as we also have shown that despite the lower absolute CD4 cell counts at year 6 for patients commencing cART at lower CD4 cell counts, if viral load suppression is sustained, these patients continue to experience CD4 growth. Also suggesting that with longer duration of time with suppressed viral load, CD4 levels may also normalise for these patients [36]. Moore and Keruly (2007) have also reported normalised CD4 cell levels after 6 years of sustained virological response, but only among patients with baseline CD4 cell counts above 350 cells/μl [12].
Post-cART mean CD4 cell counts were not found to be significantly associated with sex, prior HBV co-infection or the reported mode of HIV exposure. Whilst we are not aware of any studies finding a significant effect for pre-cART HBV co-infection, there have been other studies that have found that injecting drug use as the mode of HIV infection was associated with lower post-cART mean CD4 cell counts [12, 39]. Yet with only 42 of the 1638 patients in this study having injecting drug use as the mode of HIV infection, there was low statistical power to detect a small to moderate effect. However, consistent with the findings of a number of other studies, we found that lower post-cART mean CD4 cell counts were associated with increasing age at commencement of cART [9–13], and prior AIDS [14]. Pre-treatment (baseline) viral load of 100,000 copies/ml or less [11, 14] was also associated with lower post-cART mean CD4 response. Although this may seem counter-intuitive initially, these findings demonstrate that among subjects with equal pre-treatment CD4 counts, those with higher baseline viral loads will have a greater absolute CD4 response following the initiation of effective treatment. These findings have also been reported in at least two other studies [11, 14]. We also found that pre-cART HCV co-infection was associated with lower mean CD4 cell counts. Whist this association is consistent with the findings of 2 studies that we are aware of [18, 40], others have not found such a link [10–12].
The long-term trends in CD4 cell counts were consistent for both the AHOD and TAHOD cohorts, despite the small, and questionably clinically relevant, benefit for AHOD in terms of mean CD4 cell counts post cART. The lower mean CD4 cell response observed in TAHOD compared with AHOD may be due to Asian versus primarily Caucasian populations. In a previous study comparing TAHOD patients with patients from the French Aquitaine cohort, patients from TAHOD had lower absolute CD4 cell count at every level of CD4% [41]. Reduced circulating CD4 cells counts have also been shown in African American versus Caucasian American populations [42]. However, this cohort difference between AHOD and TAHOD may also reflect differences in antiretroviral availability between the cohorts with some resource limited sites in TAHOD having reduced access to second and third line antiretrovirals [43].
There are important limitations to our analyses. First is survivorship bias, that is, the possible selective drop out of patients with poorer immunological responses. The group investigated may be biased toward being inherently better responders and therefore more likely to remain in follow-up than non-responding or non-adherent patients. However, we did not restrict our investigation to those with poor CD4 cell count at commencing cART or just patients with a sustained viral load below detection. We were able to describe CD4 response for all levels of baseline CD4 cell count and among those with and without sustained viral suppression. Further, random effects models were used in this study to minimise potential bias arising from missing at random data. Second, these analyses were in essence exploratory and not hypothesis testing. We considered many interaction terms in the random effects models, which may raise concerns about multiple comparisons. To limit false positive interactions, these were only considered if p-values were less than 0.01. Third, our analyses ignored ARV changes, assuming that ARV effects on CD4 cell count responses are entirely predicted by viral load response. Our modelling approach, using proportion of viral loads detectable, though simple, may not completely capture the relationship between viral load and CD4 cell count changes. However, specifically analysing ART regimens is notoriously difficult, and we preferred an intention to treat approach in this analysis. This approach also has the advantage that results are likely broadly applicable to all cART regimens.
In summary, our data have shown that patient’s with continuously or intermittently sustained virological response experience ongoing CD4 cell increases for several years after commencing cART, with higher increases among patients with lower baseline CD4 cell counts. Our findings illustrate the effect of baseline CD4 cell count, post-cART viral load and time on the shape of long-term CD4 cell response. The long-term CD4 cell increase observed in this study was similar for both the AHOD and TAHOD cohorts, despite the slightly lower overall absolute CD4 cell response in TAHOD over the six years. Future analyses should aim to investigate whether the potential regional differences in CD4 cell count responses identified in our study are clinically important.
Acknowledgments
The TREAT Asia HIV Observational Database and the Australian HIV Observational Database are part of the Asia Pacific HIV Observational Database and are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. U01AI069907), and from the Dutch Minisitry of Foreign Affairs through a partnership with Stichting AIDS Fonds. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
The Australian HIV Observational Database
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, T Franic, S Agrawal, M Fitzgibbins, Holdsworth House General Practice, Darlinghurst; D Allen, Holden Street Clinic, Gosford; D Smith, C Mincham, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, R Vale, 407 Doctors, Surry Hills; C O’Connor, D Templeton; Royal Prince Alfred Hospital Sexual Health, Camperdown; E Jackson, D Hunter, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Gotowski, S Taylor, L Stuart-Hill, Bligh Street Clinic, Tamworth; D Cooper, A Carr, R Norris, G Keogh, M Lacey, K Hesse, St Vincent’s Hospital, Darlinghurst; R Finlayson, I Prone, Taylor Square Private Clinic, Darlinghurst; MT Liang, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, N Skobalj, Illawarra Sexual Health Clinic, Warrawong; L Wray, H Lu, Sydney Sexual Health Centre, Sydney; Dubbo Sexual Health Centre, Dubbo; P Canavan*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; I Zablotska*, National Centre in HIV Social Research, University of NSW; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, K Falster*, Sadaf Marashi Pour*, C Bendall National Centre in HIV Epidemiology and Clinical Research, University of NSW.
Northern Territory: A Kulatunga, P Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin.
Queensland: J Chuah*, D Lester, W Fankhauser, B Dickson, M Ngieng, M Page, Gold Coast Sexual Health Clinic, Miami; D Russell, J Leamy, C Remington, Cairns Sexual Health Service, Cairns; D Sowden, A Walker*, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Sexual Health Service, Nambour; D Orth; D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, P Ambrose, J Murray, P Negus, H Magon, AIDS Medical Unit, Brisbane.
South Australia: W Donohue, A Lohmeyer, The Care and Prevention Programme, Adelaide University, Adelaide.
Victoria: R Moore, J Anderson, P Cortissos, S Edwards, P Locke, The Carlton Clinic, Carlton; NJ Roth*, J Nicholson, Prahran Market Clinic, South Yarra; T Read, J Silvers, Melbourne Sexual Health Centre, Melbourne; J Hoy, K Watson*, M Bryant, S Aitchison, A Mijch, The Alfred Hospital, Melbourne; I Woolley, Monash Medical Centre, Clayton.
Western Australia: D Nolan, S Mallal, C Forsdyke, J Skett, S Bulgannawar, Department of Clinical Immunology, Royal Perth Hospital, Perth.
*AHOD Steering committee members in 2006–2008.
The TREAT Asia HIV Observational Database
CV Mean*, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*†, HX Zhao and N Han, Beijing Ditan Hospital, Beijing, China;
PCK Li*‡ and MP Lee, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy* and S Saghayam, YRG Centre for AIDS Research and Education, Chennai, India;
S Pujari* and K Joshi, Institute of Infectious Diseases, Pune, India;
TP Merati* and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
S Oka*‡ and M Honda, International Medical Centre of Japan, Tokyo, Japan;
JY Choi* and SH Han, Division of Infectious Diseases, Dept. of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
C KC Lee* and R David, Hospital Sungai Buloh, Kuala Lumpur, Malaysia;
A Kamarulzaman* and A Kajindran, University of Malaya, Kuala Lumpur, Malaysia;
G Tau*, Port Moresby General Hospital, Port Moresby, Papua New Guinea
R Ditangco* and R Capistrano, Research Institute for Tropical Medicine, Manila, Philippines;
YMA Chen*, WW Wong and YW Yang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan;
PL Lim*, CC Lee and E Foo, Tan Tock Seng Hospital, Singapore;
P Phanuphak*, and M Khongphattanayothing, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Sungkanuparph* and B Piyavong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
T Sirisanthana* and W Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand;
J Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia;
K Frost*, J Smith* and B Nakornsri, The Foundation for AIDS Research, New York, USA;
DA Cooper*, MG Law*, K Petoumenos, R Oyomopito and J Zhou*, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.
*TAHOD Steering Committee member;
†Current Steering Committee chair; ‡co-chair.
References
- 1.Correll PK, et al. HIV disease progression in Australia in the time of combination antiretroviral therapies. Med J Aust. 1998;169(9):469–72. [PubMed] [Google Scholar]
- 2.Law MG, et al. Estimating the population impact in Australia of improved antiretroviral treatment for HIV infection. Aids. 2000;14(2):197–201. doi: 10.1097/00002030-200001280-00016. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352(9142):1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–64. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy N, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41(10):1525–8. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 7.Severe P, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353(22):2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg ES, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278(5342):1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 9.Florence E, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4(3):255–62. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 10.Hunt PW, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. Aids. 2003;17(13):1907–15. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann GR, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 12.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44(3):441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 13.Viard JP, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the EuroSIDA study. J Infect Dis. 2001;183(8):1290–4. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 14.Smith CJ, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190(10):1860–8. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 15.Viard JP, et al. Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. Aids. 2004;18(1):45–9. doi: 10.1097/00002030-200401020-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tarwater PM, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27(2):168–75. doi: 10.1097/00126334-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 17.Valdez H, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. Aids. 2002;16(14):1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 18.Le Moing V, et al. Long-term evolution of CD4 count in patients with a plasma HIV RNA persistently <500 copies/mL during treatment with antiretroviral drugs. HIV Med. 2007;8(3):156–63. doi: 10.1111/j.1468-1293.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith CJ, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. Aids. 2003;17(7):963–9. doi: 10.1097/00002030-200305020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Notermans DW, et al. Immune reconstitution after 2 years of successful potent antiretroviral therapy in previously untreated human immunodeficiency virus type 1-infected adults. J Infect Dis. 1999;180(4):1050–6. doi: 10.1086/315013. [DOI] [PubMed] [Google Scholar]
- 21.Staszewski S, et al. Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. Aids. 1999;13(8):951–6. doi: 10.1097/00002030-199905280-00011. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi Lepri A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. Aids. 2001;15(8):983–90. doi: 10.1097/00002030-200105250-00006. [DOI] [PubMed] [Google Scholar]
- 23.Phillips AN, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. Jama. 2001;286(20):2560–7. doi: 10.1001/jama.286.20.2560. [DOI] [PubMed] [Google Scholar]
- 24.Li TS, et al. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351(9117):1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 25.Le Moing V, et al. Predictors of long-term increase in CD4(+) cell counts in human immunodeficiency virus-infected patients receiving a protease inhibitor-containing antiretroviral regimen. J Infect Dis. 2002;185(4):471–80. doi: 10.1086/338929. [DOI] [PubMed] [Google Scholar]
- 26.Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3(1):28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diggle P, et al. Analysis of Longitudinal Data. 2. Oxford University Press; 2002. [Google Scholar]
- 29.Wood E, et al. Full suppression of viral load is needed to achieve an optimal CD4 cell count response among patients on triple drug antiretroviral therapy. Aids. 2000;14(13):1955–60. doi: 10.1097/00002030-200009080-00011. [DOI] [PubMed] [Google Scholar]
- 30.Deeks SG, et al. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. Aids. 2002;16(2):201–7. doi: 10.1097/00002030-200201250-00009. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Jama. 2006;296(12):1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 32.Renaud M, et al. Determinants of paradoxical CD4 cell reconstitution after protease inhibitor-containing antiretroviral regimen. Aids. 1999;13(6):669–76. doi: 10.1097/00002030-199904160-00007. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann GR, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. Aids. 2000;14(17):2643–51. doi: 10.1097/00002030-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann GR, et al. Long-term immunological response in HIV-1-infected subjects receiving potent antiretroviral therapy. Aids. 2000;14(8):959–69. doi: 10.1097/00002030-200005260-00007. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann GR, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41(3):361–72. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 36.Mocroft A, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370(9585):407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita TE, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. Aids. 2001;15(6):735–46. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann GR, et al. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. Aids. 2002;16(3):359–67. doi: 10.1097/00002030-200202150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Dronda F, et al. CD4 cell recovery during successful antiretroviral therapy in naive HIV-infected patients: the role of intravenous drug use. Aids. 2004;18(16):2210–2. doi: 10.1097/00002030-200411050-00018. [DOI] [PubMed] [Google Scholar]
- 40.Greub G, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, et al. Difference betwen Aisan and Caucasian HIV-infected patients in absolute CD4 count and CD4 percentage; AIDS 2006 - XVI International AIDS Conference: Abstract no. MOPE0116; 2006. [Google Scholar]
- 42.Cruse JM, et al. Contrasting quantitative alterations in CD4+ and CD8+ lymphocytes in HIV-infected African Americans compared with the Caucasian population. Pathol Immunopathol Res. 1989;8(5–6):300–13. doi: 10.1159/000157158. [DOI] [PubMed] [Google Scholar]
- 43.Srasuebkul P, et al. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD) AIDS Res Ther. 2007;4(1):18. doi: 10.1186/1742-6405-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]