Abstract
Background
Midregional pro-atrial natriuretic peptide (MR-proANP) is a newly described stable fragment of the N-terminal part of pro-atrial natriuretic peptide. We tested the hypothesis that in adults with essential hypertension, plasma levels of MR-proANP would be associated with systolic blood pressure (SBP), pulse pressure, and hypertension severity.
Method
Participants included 1034 African Americans (65±9 y, 72% women) and 880 non-Hispanic whites (61±9 y, 55% women) belonging to sibships ascertained on the basis of hypertension. MR-proANP was measured by an immunoluminometric assay. Hypertension severity was based on number of hypertension medication classes used and multiples of SBP and diastolic BP (DBP) deviations from 120/70 mm Hg. Generalized estimating equations (GEE) were used to assess whether plasma levels of MR-proANP were associated with SBP, pulse pressure, and hypertension severity independent of potential confounding variables.
Results
In African Americans, after adjustment for age, sex, body mass index, estimated glomerular filtration rate, smoking history, diabetes, total cholesterol, high density lipoprotein cholesterol, medication (beta-blocker, statin, and aspirin) use, and previous history of myocardial infarction or stroke, higher MR-proANP levels were significantly associated with greater SBP (P <0.0001), pulse pressure (P <0.0001), and hypertension severity (P = 0.0013). The associations were replicated in non-Hispanic whites; after adjustment for the above variables, higher MR-proANP levels were significantly associated with greater SBP (P = 0.013), pulse pressure (P = 0.0006), and hypertension severity (P = 0.028).
Conclusion
Plasma MR-proANP may be a marker of arterial stiffness and severity of hypertension in adults with hypertension.
Keywords: hypertension, blood pressure, pulse pressure, atrial natriuretic peptide
Introduction
Increased left ventricular and atrial wall stretch resulting from volume and pressure overload lead to increased circulating levels of cardiac natriuretic peptides, i.e., A-type (atrial) natriuretic peptide (ANP) and B-type (brain) natriuretic peptide (BNP), and the amino-terminal fragments of their prohormones (NT-proANP and NT-proBNP, respectively). Both ANP and BNP are vasodilators that also promote natriuresis and diuresis, and inhibit the renin-angiotensin-aldosterone axis, with ANP comprising up to 98% of the natriuretic peptides in the circulation.
Elevated blood pressure (BP) and arterial stiffness increase cardiac afterload. Whether increased levels of ANP are associated with BP indices in hypertensive adults has not been established. Higher levels of natriuretic peptides have been reported in patients with essential hypertension than in normotensives,1, 2 but this observation has not been confirmed in other studies.3, 4 In a large (n=1338) population-based study, Flickinger et al5 found no association between plasma levels of ANP and systolic BP (SBP), diastolic BP (DBP), or the presence of hypertension. However, in the Framingham Heart Study, higher levels of NT-ANP and NT-BNP were associated with higher SBP and lower DBP.6
A potential explanation for these conflicting results could be that conventional assays measure the mature ANP peptide which has a low half life. ANP is derived from the cleavage of its precursor pro-hormone, which is significantly more stable in the circulation than the mature peptide. A midregional fragment of the precursor hormone (amino acids 53–90 of NT-proANP), called midregional-proANP (MR-proANP), may be relatively resistant to degradation by exoproteases, unlike epitopes in the N- or C-terminals of proANP used in previous immunoassays.7, 8 Previous studies may, therefore, have underestimated the utility of ANP/proANP as a biomarker.
We hypothesized that in adults with essential hypertension, plasma levels of MR-proANP would be associated with higher SBP, greater arterial stiffness and severity of hypertension. We therefore, examined the association of plasma levels of MR-proANP with SBP, pulse pressure, and hypertension severity in a bi-ethnic cohort of adults with essential hypertension. We sought to determine whether any detected association was independent of confounding variables, particularly age, body mass index (BMI), and renal function.
Methods
The study was part of the Proteomic Markers of Arteriosclerosis Study which is investigating the association of multiple markers in various etiologic pathway of vascular disease with several phenotypes of arteriosclerosis.9, 10 Participants belonged to the Genetic Epidemiology Network of Arteriopathy (GENOA) Study, a multicenter, community-based study that aims to identify genetic variants influencing BP levels and the development of target organ damage due to hypertension.11 Participants were enrolled if two or more members of a sibship had hypertension. The only exclusionary criterion at enrollment was the presence of a secondary cause of hypertension (such as documented renal artery stenosis or advanced renal insufficiency) in the index sibs. The sampling frame of the GENOA Rochester cohort was the Mayo Clinic diagnostic index and medical record linkage system of the Rochester Epidemiology Project. It was used to identify non-Hispanic white residents of Olmsted County MN with a diagnosis of essential hypertension made before age 60. The Jackson MS cohort of the Atherosclerosis Risk in Communities study,12 which had originally been a probability sample of persons with driver’s licenses, was used to ascertain African-American sibships. If the eligible proband had at least one sibling with hypertension, all available full biologic siblings of the index hypertensive including normotensive siblings were invited to participate in interviews, physical examinations, and phlebotomy. The study was approved by the Institutional Review Boards of the University of Mississippi Medical Center, Jackson MS and Mayo Clinic, Rochester MN. Written informed consent was obtained from each participant. The present study included 1914 participants (1034 African Americans and 880 non-Hispanic whites) who had hypertension.
Height was measured by stadiometer, weight by electronic balance, and BMI was calculated as weight in kilograms divided by the square of height in meters. Diabetes was considered present if the participant was being treated with insulin or oral agents or had a fasting glucose level ≥126 mg/dL. ‘Ever’ smoking was defined as having smoked >100 cigarettes. Information about the use of medications was obtained from the participants at the time of the study visit. Each prescription drug recorded at the study visit was assigned a code number corresponding to the first six digits of the Medi-Span Generic Product Identifier.13 This number identifies pharmacologically equivalent drug products and was used to categorize agents with a similar therapeutic action.13 BP-lowering medications were classified as: diuretics, beta-blockers, calcium-channel blockers, or renin-angiotensin-aldosterone system (RAAS) inhibitors.
Blood was drawn by venipuncture after an overnight fast. Serum total cholesterol and high-density lipoprotein (HDL) cholesterol were measured by standard enzymatic methods. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation as previously described.14 Resting SBP and DBP were measured by random zero sphygmomanometer (Hawskley and Sons, London, UK) after participants had rested for at least 10 min in the supine position. Three measures at least 2 min apart were taken and the average of the second and third measurements was used. The diagnosis of hypertension was established based on BP levels measured at the study visit (≥140/90 mm Hg) or a prior diagnosis of hypertension and current treatment with antihypertensive medications. Pulse pressure was calculated as the difference between SBP and DBP. We used a measure of hypertension severity that accounted for medication use.15 The hypertension severity score was based on the number of anti-hypertensive medications and the BP as follows: number of hypertension medication classes a patient was taking + (DBP − 70)/30 + (SBP − 120)/60.15
Plasma levels of mid regional atrial natriuretic peptide (MR-proANP)
Plasma was collected at the time of blood sampling in plastic vials containing ethylenediaminetetraacetic acid (EDTA). These were placed on ice and then centrifuged at 3000 × g and frozen at −80°C until assayed. MR-proANP was detected using a novel commercial sandwich immunoassay in the chemiluminescence-coated tube format (MR-proANP LIA, B.R.A.H.M.S, Hennigsdorf/Berlin, Germany) as previously described.8 Briefly, patient samples (1:40 dilution of 5 μl plasma in incubation buffer) or standards were added in duplicate to antibody-coated tubes (affinity purified sheep polyclonal antibodies directed against proANP peptide 73 to 90) and incubated for 30 min at room temperature. After washes with 1 ml washing buffer, 200 μl tracer was added, containing acridinium ester-labeled anti-proANP antibody (affinity purified sheep polyclonal antibodies directed against proANP peptide 53 to 72), followed by 30 min incubation at room temperature. Tubes were washed with 1 ml washing buffer, and detection was performed in a LB952T luminometer (Berthold, Bad Wildbad, Germany; 1 s detection time per sample). Relative light units of the chemiluminescence assay were expressed in pmol/l MR-proANP, as calculated from a calibration curve (4 to 1,800 pmol/L) that was included in every analytical run. The lower detection limit of the assay is 4.3 pmol/L and the functional sensitivity of the assay is 11 pmol/L MR-proANP. The inter-assay coefficient of variation (CV) within the range of plasma measurements was under 10% (8.0% CV at 100 pmol/L; 6.5% CV at 400 pmol/L). Participants (n=13) with MR-proANP levels > 400 pmol/L were excluded from the analyses as such levels may be due to left ventricular dysfunction.
Statistical Methods
Statistical analyses were carried out using SAS v 9.1 (SAS Institute, Cary NC) software package. Because of the presence of sibships in the sample, regression analyses were performed using generalized estimating equations (GEE).16 Continuous data were summarized as either mean ± SD or median and quartiles and categorical data were expressed as percentages. Because of significant differences in age and the proportion of women between the two ethnic groups, participant characteristics were compared after adjustment for age and sex. The P value for the trends across quartiles of plasma MR-proANP levels were assessed using ANOVA and Kruskal Wallis test for continuous variables and likelihood ratio tests for categorical data. Plasma MR-proANP and serum creatinine were log transformed to reduce skewness. In each ethnic group, we constructed multiple regression models including age, sex, BMI, total cholesterol and HDL cholesterol, smoking history, diabetes, previous history of myocardial infarction (MI) or stroke, medication (BP-lowering, statin, and aspirin) use, eGFR, and MR-proANP. Backward elimination was performed to identify the set of variables independently associated with BP measures. In each model, the mean BP indices in each quartile of plasma levels of MR-proANP were estimated using least squares means. We also checked for interactions between conventional risk factors and MR-proANP in the prediction of BP indices and incorporated interactions significant at P <0.01 in the models. In addition, to evaluate whether any association between MR-proANP levels and BP indices was modified by ethnicity, we performed analyses including all participants, using ethnicity as a covariate in the regression models. A two-sided P-value of <0.05 was deemed statistically significant.
Results
African Americans were older and there was a greater proportion of women in both African American and non-Hispanic white cohorts (Table 1). After adjustment for age and sex, African Americans had a higher prevalence of diabetes, lower use of statins, and higher eGFR than their non-Hispanic white counterparts. Although SBP, pulse pressure, and hypertension severity were greater in African Americans, MR-proANP levels were similar in both ethnic groups (Table 1).
Table 1.
Participant characteristics
| African Americans (n = 1034) | Non-Hispanic whites (n = 880) | P value* | |
|---|---|---|---|
| Age, years | 64.8±8.6 | 61.1±9.3 | <0.001 |
| Women, n (%) | 729 (72.5) | 490 (55.7) | <0.001 |
| BMI, kg/m² | 32.0±6.6 | 31.4±6.2 | 0.008 |
| Total cholesterol, mg/dL | 201.5±41.7 | 196.6±33.7 | 0.004 |
| HDL cholesterol, mg/dL | 57.6±17.8 | 50.8±14.7 | <0.001 |
| Plasma glucose level, mg/dL | 115.6±51.0 | 107.9±26.3 | <0.001 |
| SBP, mm Hg | 142.3±20.8 | 135.3±17.1 | <0.001 |
| DBP, mm Hg | 79.9±11.4 | 74.6±9.7 | <0.001 |
| Pulse pressure, mm Hg | 62.4±17.5 | 60.7±15.8 | 0.001 |
| Hypertension severity score | 2.46±1.13 | 2.06±0.94 | <0.001 |
| Heart rate, bpm | 68±12 | 65±11 | 0.001 |
| Serum creatinine, mg/dL | 0.91±0.33 | 0.91±0.26 | NS |
| eGFR, ml/min | 97.9±31.2 | 83.3±22.8 | <0.001 |
| Smoking, n (%) | 418 (40.4) | 430 (48.9) | <0.001 |
| Diabetes, n (%) | 346 (33.5) | 159 (18.1) | <0.001 |
| Previous history of MI or stroke, n (%) | 135 (13.0) | 122 (13.9) | NS |
| Statin, n (%) | 222 (21.5) | 307 (34.9) | <0.001 |
| Aspirin, n (%) | 371 (35.9) | 332 (49.1) | 0.003 |
| Beta-blocker, n (%) | 211 (20.4) | 374 (42.5) | <0.001 |
| Calcium-channel blocker, n (%) | 367 (35.5) | 174 (19.8) | <0.001 |
| Diuretic, n (%) | 585 (56.6) | 445 (50.6) | 0.004 |
| RAAS inhibitor, n (%) | 506 (48.9) | 419 (47.6) | NS |
| MR-proANP, pmol/L | 62.3 (43.7–93.0) | 63.1 (44.1–95.0) | NS |
Continuous variables are presented as means ± standard deviation or median and inter-quartile range, whereas categorical variables are presented as counts and percentages.
P values are for ethnic differences after adjustment for age and sex.
BMI, body mean index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; BMI, body mean index; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin-aldosterone system; MR-proANP, midregional pro-atrial natriuretic peptide.
For each ethnic group, participant characteristics in quartiles of MR-proANP levels are presented in Table 2. Older age, lower BMI, higher HDL cholesterol, greater SBP, pulse pressure, and hypertension severity (Fig 1. A), lower eGFR, previous history of MI or stroke, and use of beta-blockers were each significantly associated with higher plasma MR-proANP levels in African Americans. After adjustment for age and sex, African Americans in the highest quartile of MR-proANP levels had significantly greater SBP (10.1 mm Hg increase, P<0.0001), pulse pressure (8.4 mm Hg increase, P<0.0001), and hypertension severity (0.53 unit increase, P<0.0001) than in those in the bottom quartile (Table 3). In separate multivariable linear regression models, after adjustment for age, sex, BMI, total and HDL cholesterol, eGFR, smoking history, diabetes, previous history of MI or stroke, and medication (beta blocker, statin, and aspirin) use, higher MR-proANP levels were significantly associated with greater SBP (P <0.0001), pulse pressure (P <0.0001), and hypertension severity (P = 0.0013). African Americans in the highest quartile of MR-proANP levels had significantly greater SBP (10.5 mm Hg increase, P<0.0001), pulse pressure (9.0 mm Hg increase, P<0.0001), and hypertension severity (0.42 unit increase, P = 0.0002) than in those in the bottom quartile (Table 3).
Table 2.
Participant characteristics in quartiles of MR-proANP levels
| African Americans |
Non-Hispanic whites |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of MR-proANP (pmol/L) | Quartiles of MR-proANP (pmol/L) | |||||||||
| I | II | III | IV | I | II | III | IV | |||
| <44 | 44–62 | 62–93 | >93 | P trend | <44 | 44–63 | 63–95 | >95 | P trend | |
| Age, years | 60.5 | 63.9 | 65.6 | 69.2 | <.0001 | 56.5 | 58.8 | 62.3 | 66.7 | <.0001 |
| Women, n (%) | 177 (69) | 190 (73) | 196 (76) | 187 (72) | 0.34 | 104 (47) | 110 (50) | 133 (61) | 143 (64) | 0.0003 |
| BMI, kg/m² | 32.9 | 31.8 | 31.5 | 31.5 | 0.043 | 32.0 | 31.8 | 30.6 | 31.3 | 0.11 |
| Total cholesterol, mg/dL | 198.4 | 199.1 | 203.1 | 205.0 | 0.22 | 196.3 | 196.6 | 200.0 | 193.6 | 0.27 |
| HDL cholesterol, mg/dL | 55.0 | 55.1 | 60.0 | 60.4 | <.0001 | 48.8 | 47.8 | 52.6 | 54.0 | <.0001 |
| Plasma glucose level, mg/dL | 117.6 | 115.3 | 112.1 | 118.0 | 0.055 | 110.3 | 107.8 | 104.2 | 109.7 | 0.077 |
| SBP, mm Hg | 136.1 | 140.8 | 143.5 | 148.6 | <.0001 | 129.4 | 134.9 | 137.3 | 139.5 | <.0001 |
| DBP, mm Hg | 80.6 | 80.0 | 79.6 | 79.4 | 0.63 | 76.1 | 75.8 | 75.1 | 71.4 | <.0001 |
| Pulse pressure, mm Hg | 55.5 | 60.8 | 63.9 | 69.3 | <.0001 | 53.3 | 59.1 | 62.3 | 68.1 | <.0001 |
| Hypertension severity score | 2.31 | 2.36 | 2.36 | 2.81 | <.0001 | 1.84 | 2.0 | 2.11 | 2.30 | <.0001 |
| Heart rate, bpm | 71 | 67 | 65 | 64 | <.0001 | 70 | 66 | 62 | 62 | <.0001 |
| Serum creatinine, mg/dL | 0.82 | 0.86 | 0.88 | 1.05 | <.0001 | 0.87 | 0.89 | 0.90 | 0.97 | 0.0003 |
| eGFR, ml/min | 110.0 | 101.4 | 97.3 | 81.2 | <.0001 | 89.4 | 87.2 | 82.1 | 74.7 | <.0001 |
| Smoking, n (%) | 109 (42) | 98 (38) | 99 (38) | 112 (43) | 0.47 | 112 (51) | 101 (46) | 102 (47) | 115 (52) | 0.52 |
| Diabetes, n (%) | 90 (35) | 88 (34) | 75 (29) | 95 (37) | 0.27 | 41 (19) | 39 (18) | 29 (13) | 50 (23) | 0.090 |
| History of MI/stroke, n (%) | 19 (7) | 31 (12) | 27 (10) | 61(24) | <.0001 | 27 (12) | 21 (10) | 27 (12) | 47 (21) | 0.0037 |
| Statin, n (%) | 55 (21) | 52 (20) | 46 (18) | 71 (28) | 0.051 | 78 (35) | 78 (35) | 75 (34) | 76 (34) | 0.99 |
| Aspirin, n (%) | 86 (33) | 90 (35) | 94 (36) | 101 (40) | 0.56 | 96 (44) | 98 (45) | 107 (49) | 131 (59) | 0.0042 |
| Beta-blockers, n (%) | 29 (11) | 40 (15) | 57 (22) | 86 (33) | <.0001 | 56 (25) | 77 (35) | 101 (46) | 140 (63) | <.0001 |
| Calcium-channel blocker, n (%) | 90 (35) | 101 (39) | 84 (32) | 91 (35) | 0.48 | 40 (18) | 38 (17) | 39 (18) | 58 (26) | 0.061 |
| Diuretic, n (%) | 152 (59) | 139 (54) | 132 (51) | 162 (63) | 0.031 | 113 (51) | 107 (49) | 114 (52) | 111 (50) | 0.88 |
| RAAS inhibitor, n (%) | 141 (55) | 123 (47) | 112 (43) | 130 (50) | 0.066 | 115 (52) | 107 (49) | 98 (45) | 99 (45) | 0.33 |
BMI, body mean index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; BMI, body mean index; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin-aldosterone system; MR-proANP, midregional pro-atrial natriuretic peptide.
Figure 1.
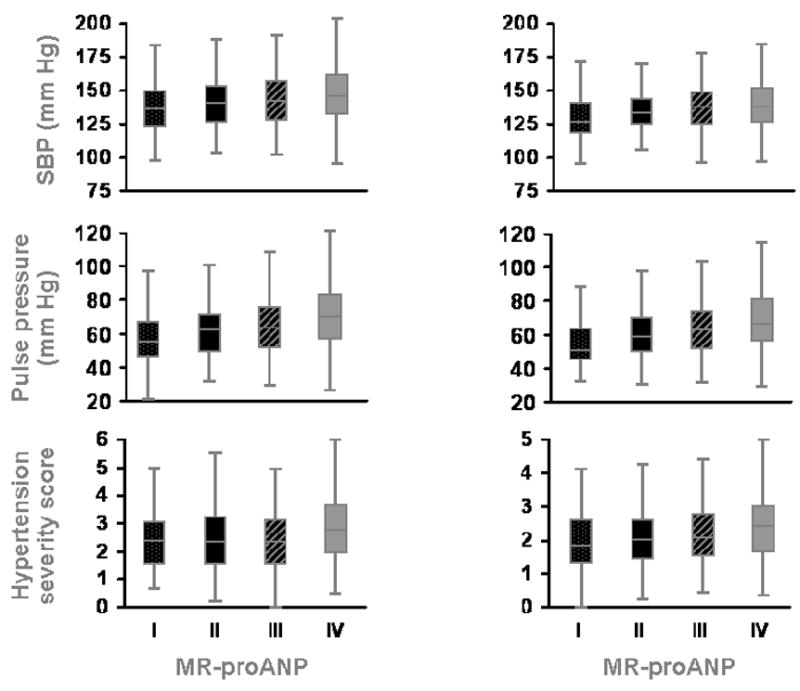
Box plots (median and inter-quartile range) of systolic blood pressure, pulse pressure and hypertension severity in quartiles of MR-proANP. Left column, African Americans; Right column, Non-Hispanic whites
P <0.001 for all associations shown.
Table 3.
Mean±SE SBP, pulse pressure, and hypertension severity in quartiles of plasma MR-proANP levels
| African Americans |
Non-Hispanic whites |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of MR-proANP (pmol/L) | Quartiles of MR-proANP (pmol/L) | |||||||||
| I | II | III | IV | I | II | III | IV | |||
| <44 | 44–62 | 62–93 | >93 | P trend | <44 | 44–63 | 63–95 | >95 | P trend | |
|
Age- and sex-adjusted | ||||||||||
| SBP, mm Hg | 136.8±1.2 | 140.5±1.3 | 142.2±1.2 | 146.9±1.5 | <.0001 | 131.7±1.1 | 136.0±1.0 | 136.1±1.1 | 136.4±1.3 | 0.0098 |
| 0.026 | 0.0007 | <.0001 | 0.002 | 0.005 | 0.012 | |||||
| Pulse pressure, mm Hg | 56.8±0.9 | 60.0±1.0 | 61.4±1.0 | 65.2±1.2 | <.0001 | 56.8±0.9 | 60.6±0.8 | 60.5±0.9 | 63.4±1.1 | 0.0002 |
| 0.011 | 0.0004 | <.0001 | 0.0009 | 0.003 | <.0001 | |||||
| Hypertension severity score | 2.31±0.07 | 2.38±0.07 | 2.37±0.07 | 2.84±0.08 | <.0001 | 1.91±0.05 | 2.05±0.06 | 2.10±0.07 | 2.21±0.07 | 0.016 |
| 0.47 | 0.57 | <.0001 | 0.11 | 0.034 | 0.002 | |||||
|
Adjusted for age, sex, conventional risk factors and medication use | ||||||||||
| SBP, mm Hg | 137.3±1.2 | 140.8±1.2 | 141.7±1.2 | 147.8±1.5 | <.0001 | 131.8±1.1 | 135.7±0.9 | 136.0±1.2 | 136.8±1.3 | 0.013 |
| 0.027 | 0.006 | <.0001 | 0.003 | 0.010 | 0.006 | |||||
| Pulse pressure, mm Hg | 57.0±1.0 | 60.4±0.9 | 61.3±1.0 | 66.0±1.2 | <.0001 | 57.0±0.09 | 60.5±0.08 | 60.4±1.0 | 63.2±1.1 | 0.0006 |
| 0.006 | 0.001 | <.0001 | 0.002 | 0.009 | <.0001 | |||||
| Hypertension severity score | 2.36±0.07 | 2.42±0.07 | 2.41±0.07 | 2.78±0.08 | 0.0013 | 1.92±0.05 | 2.05±0.06 | 2.11±0.07 | 2.18±0.07 | 0.028 |
| 0.51 | 0.60 | 0.0002 | 0.086 | 0.025 | 0.005 | |||||
SBP, systolic blood pressure; MR-proANP, midregional pro-atrial natriuretic peptide.
Similar findings were noted in non-Hispanic whites. Older age, female sex, higher HDL cholesterol, greater SBP, pulse pressure, and hypertension severity (Fig 1. B), lower DBP, lower eGFR, previous history of MI or stroke, and use of beta blockers and aspirin were associated with higher plasma MR-proANP levels. After adjustment for age and sex, non-Hispanic whites in the highest quartiles of MR-proANP levels had significantly greater SBP (4.7 mm Hg increase, P = 0.012), pulse pressure (6.6 mm Hg increase, P<0.0001), and hypertension severity (0.30 unit increase, P = 0.002) than in those in the bottom quartile (Table 3). In separate multivariable linear regression models, after adjustment for age, sex, BMI, total and HDL cholesterol, eGFR, smoking history, diabetes, previous history of MI or stroke, and medication (beta-blocker, statin, and aspirin) use, higher MR-proANP levels were significantly associated with greater SBP (P = 0.013), pulse pressure (P = 0.0006), and hypertension severity (P = 0.028) (Table 3). Non-Hispanic whites in the highest quartiles of MR-proANP levels had significantly greater SBP (4.6 mm Hg increase, P = 0.006), pulse pressure (6.2 mm Hg increase, P<0.0001), and hypertension severity (0.26 unit increase, P = 0.005) than in those in the bottom quartile (Table 3).
The regression coefficients for the association between MR-proANP and BP indices were greater in African Americans. However, in the pooled sample, after adjustment for potentially confounding variables, we did not find a statistically significant interaction between ethnicity and MR-proANP levels in predicting SBP (P = 0.14), pulse pressure (P = 0.31), or hypertension severity (P = 0.89) (analysis not shown).
Discussion
To the best of our knowledge, this study is the first to report an independent association of MR-proANP, a stable fragment of the N-terminal part of pro-atrial natriuretic peptide, with SBP, pulse pressure, and severity of hypertension in adults with hypertension. Our findings suggest that plasma MR-proANP may be a marker of arterial stiffness and severity of hypertension in adults with hypertension. These associations remained significant even after adjustment for age, sex, conventional risk factors, eGFR, and medication use, and were present in both African Americans and non-Hispanic whites.
The natriuretic peptides have several physiologic roles including regulation of BP, salt and water excretion, cell proliferation, and vasodilator tone.17, 18 The natriuretic peptide axis is influenced by multiple pathophysiological signals, including left and right ventricular dysfunction, cardiac hypertrophy, elevated intracardiac filling pressure, diastolic dysfunction, valvular disease, cardiac ischemia, and renal disease.19, 20 Knocking out the ANP gene in mice results in salt sensitive hypertension and significant cardiac enlargement,21 whereas over-expression of the gene in transgenic mice results in lower BP.22 Moreover, administration of recombinant ANP lowers BP levels in humans and in animal models.23, 24 Chronic hypertension eventually leads to volume and pressure overload and ultimately left ventricular hypertrophy; left ventricular compliance decreases, leading to increased atrial work and atrial stretch, a major stimulus for ANP release. In a separate study, we found a significant association between MR-proANP levels and left ventricular mass in African Americans.25 The association between MR-proANP levels and BP indices may reflect a feedback mechanism to increase sodium excretion and lower BP in the presence of hypertension. Patients with more severe hypertension therefore have higher ANP levels and ANP levels may serve as a marker of elevated BP and increased left ventricular and atrial work in hypertensive adults. Elevated levels of MR-proANP (in the absence of heart failure) may indicate a need for intensive pharmacologic therapy to reduce BP.
Levels of MR-proANP were significantly associated with pulse pressure in both ethnic groups, highlighting that arterial stiffness increases ANP secretion likely by increasing left ventricular and atrial stretch. The association of MR-proANP with pulse pressure was predominantly due to its association with increased SBP in African-Americans. In non-Hispanic whites, higher MR-proANP was associated with higher SBP as well as a lower DBP. In a report from Framingham Heart Study (n=1962, 43% hypertensive) NT-proANP was associated with carotid pulse pressure in men but not in women.26 In contrast, in this study, we did not find any interaction between MR-proANP and sex in the prediction of pulse pressure. It has been reported that omapatrilat demonstrated enhance reduction of central and peripheral pulse pressure.
In investigating the association between MR-proANP and BP indices, we considered the confounding effect of several variables, most importantly age, sex, BMI, eGFR, and medication use. Consistent with previous reports, age was significantly, independently associated with higher MR-proANP levels in both ethnic groups.6, 27 This could be due to increased myocardial fibrosis, greater arterial stiffness, and a reduction in renal and non-renal clearance mechanisms in the elderly. An inverse relationship between NT-proANP and BMI was described in the Framingham Heart Study28 and by Khush et al.29 We noted a weak inverse association with BMI that was no longer significant after adjustment for age (analyses not shown).
Women had higher levels of MR-proANP than men, although in African Americans the association with sex was weak and significant only after adjustment for other variables. Lower eGFR was significantly associated with higher MR-proANP levels, a finding also noted by Codognotto et al30 and indicating that renal clearance is an important determinant of plasma levels of ANP. Beta-blocker use was significantly associated with higher MR-proANP levels independent of heart rate and other confounding variables. Luchner et al31 also reported a similar finding in a population-based sample (n=672). The mechanism underlying this association remains to be delineated.
Hypertension is highly prevalent in African Americans and tends to be more severe and more often associated with target organ damage compared to non-Hispanic whites.32 The attributable risk of death due to hypertension in African Americans is ~30%, double that in non-Hispanic whites.32 Although hypertension was more severe in African Americans than in non-Hispanic whites in this study, MR-proANP levels were similar in the two ethnic groups after adjustment for age and sex, or even after adjustment for other conventional risk factors and medications (analysis not shown). This result implies that either African Americans have lower baseline level of ANP or a blunted neurohumoral reflex compared to non-Hispanic whites. Whether this contributes to the susceptibility of African Americans to hypertension and target organ damage mediated by hypertension, merits further investigation.
A strength of the present study is the inclusion of a large bi-ethnic cohort of adults with hypertension and the use of uniform protocols including questionnaires, anthropometric, and laboratory measurements. In addition, plasma levels of NT-proANP were measured using a novel immunoassay covering midregional epitopes, allowing measuring a more reliable assessment of ANP release. Limitations of this study include its cross sectional nature. The majority of patients in this study were on BP-lowering medications at the time of measuring BP indices and MR-proANP levels. However, this would tend to lessen the ability to find associations between ANP and BP indices. The hypertension severity score we used needs to be validated in other studies.
Hypertension affects ≥65 million adult Americans and is associated with subclinical target organ damage, a major cause of morbidity and mortality in hypertension. Midregional pro-atrial natriuretic peptide (MR-proANP) is a newly described fragment of the N-terminal part of pro-atrial natriuretic peptide that is relatively resistant to degradation by exoproteases and is significantly more stable in the circulation than the mature peptide. Our results suggest that plasma levels of MR-proANP are independently associated with greater SBP, pulse pressure, and hypertension severity in adults with hypertension. The fact that MR-proANP was associated with BP indices independent of age, sex, eGFR, conventional risk factors, and medication use indicates the potential use of MR-proANP as a marker of arterial stiffness and severity of hypertension in adults with hypertension. In a 12-week double blind, randomized clinical trial, a greater reduction in central and peripheral pulse pressure was reported in hypertensive subjects treated with omapatrilat, a vasopeptidase inhibitor that inhibits both angiotensin converting enzyme and neutral endopeptidase (an enzyme that inactivated several vasodilatory peptides, including the natriuretic peptides), compared to enalapril, suggesting a potential role for pharmacological modulation of natriuretic peptides in the treatment of hypertension.33 Further investigation is needed to assess the utility of measurement of plasma MR-proANP for early detection of target organ damage or for novel approach in adults with hypertension.
Acknowledgments
This work was supported by grant HL-81331 from the NHLBI.
Footnotes
Disclosure: Dr. Bergmann holds ownership in BRAHMS AG, patent rights to the midregional proatrial natriuretic peptide (MR-proANP) assay, and is a member of the board of directors of BRAHMS AG. Dr. Struck holds patent rights to the MR-proANP assay and is an employee of BRAHMS AG. Dr. Morgenthaler is an employee of BRAHMS AG.
References
- 1.Sagnella GA, Markandu ND, Shore AC, MacGregor GA. Raised circulating levels of atrial natriuretic peptides in essential hypertension. Lancet. 1986;1:179–181. doi: 10.1016/s0140-6736(86)90653-7. [DOI] [PubMed] [Google Scholar]
- 2.Arendt RM, Gerbes AL, Ritter D, Stangl E, Bach P, Zahringer J. Atrial natriuretic factor in plasma of patients with arterial hypertension, heart failure or cirrhosis of the liver. J Hypertens Suppl. 1986;4:S131–135. [PubMed] [Google Scholar]
- 3.Nilsson P, Lindholm L, Schersten B, Horn R, Melander A, Hesch RD. Atrial natriuretic peptide and blood pressure in a geographically defined population. Lancet. 1987;2:883–885. doi: 10.1016/s0140-6736(87)91373-0. [DOI] [PubMed] [Google Scholar]
- 4.Zachariah PK, Burnett JC, Jr, Ritter SG, Strong CG. Atrial natriuretic peptide in human essential hypertension. Mayo Clin Proc. 1987;62:782–786. doi: 10.1016/s0025-6196(12)62331-3. [DOI] [PubMed] [Google Scholar]
- 5.Flickinger AL, Burnett JC, Jr, Turner ST. Atrial natriuretic peptide and blood pressure in a population-based sample. Mayo Clin Proc. 1995;70:932–938. doi: 10.4065/70.10.932. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, Sutherland P, Omland T, Vasan RS. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 7.Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, Vuolteenaho O. Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A- and B-type natriuretic peptides. Clin Chem. 2004;50:1576–1588. doi: 10.1373/clinchem.2004.032490. [DOI] [PubMed] [Google Scholar]
- 8.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten RE, Accurso FJ, Bernard GR, Caprioli RM, Klee EW, Klee GG, Kullo IJ, Laguna TA, Roth FP, Sabatine M, Srinivas P, Wang TJ, Ware LB. Challenges in Translating Plasma Proteomics from Bench to Bedside: Update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.00044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granger CB, Van Eyk JE, Mockrin SC, Anderson NL. National heart, lung, and blood institute clinical proteomics working group report. Circulation. 2004;109:1697–1703. doi: 10.1161/01.CIR.0000121563.47232.2A. [DOI] [PubMed] [Google Scholar]
- 11.Boerwinkle E. Multi-Center Genetic Study of Hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39:3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Medi-Span, Proprietary Concepts. Master Drug Data Base Documentation Manual Indianapolis. Indiana: Medi-Span, Inc; 1996. pp. 2.1–2.8. [Google Scholar]
- 14.Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ. Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension. 2007;50:708–714. doi: 10.1161/HYPERTENSIONAHA.107.095257. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Mosley TH, Jr, Bailey KR, Jack CR, Jr, Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci. 2008;271:53–60. doi: 10.1016/j.jns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 17.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992;10:907–912. [PubMed] [Google Scholar]
- 18.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. II: Natriuretic peptide receptors. J Hypertens. 1992;10:1111–1114. doi: 10.1097/00004872-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Burnett JC. Natriuretic peptides in the pathophysiology of congestive heart failure. Curr Cardiol Rep. 2000;2:198–205. doi: 10.1007/s11886-000-0069-3. [DOI] [PubMed] [Google Scholar]
- 21.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 22.Steinhelper ME, Cochrane KL, Field LJ. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 23.Hunt PJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG. Differing biological effects of equimolar atrial and brain natriuretic peptide infusions in normal man. J Clin Endocrinol Metab. 1996;81:3871–3876. doi: 10.1210/jcem.81.11.8923831. [DOI] [PubMed] [Google Scholar]
- 24.Charles CJ, Espiner EA, Richards AM. Cardiovascular actions of ANF: contributions of renal, neurohumoral, and hemodynamic factors in sheep. Am J Physiol. 1993;264:R533–538. doi: 10.1152/ajpregu.1993.264.3.R533. [DOI] [PubMed] [Google Scholar]
- 25.Khaleghi M, Al-Omari MA, Kondragunta V, Morgenthaler N, Struck J, Bergmann A, Mosley TH, Jr, Kullo IJ. Relation of Plasma Midregional Pro-Atrial Natriuretic Peptide to Target Organ Damage in Adults with Systemic Hypertension. American Journal of Cardiology. 2009 doi: 10.1016/j.amjcard.2009.01.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy D, Hwang SJ, Kayalar A, Benjamin EJ, Vasan RS, Parise H, Larson MG, Wang TJ, Selhub J, Jacques PF, Vita JA, Keyes MJ, Mitchell GF. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation. 2007;115:3079–3085. doi: 10.1161/CIRCULATIONAHA.106.652842. [DOI] [PubMed] [Google Scholar]
- 27.Loke I, Squire IB, Davies JE, Ng LL. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail. 2003;5:599–606. doi: 10.1016/s1388-9842(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 29.Khush KK, Gerber IL, McKeown B, Marcus G, Vessey J, Foster E, Chatterjee K, Michaels AD. Obese patients have lower B-type and atrial natriuretic peptide levels compared with nonobese. Congest Heart Fail. 2006;12:85–90. doi: 10.1111/j.1527-5299.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- 30.Codognotto M, Piccoli A, Zaninotto M, Mion M, Plebani M, Vertolli U, Tona F, Ruzza L, Barchita A, Boffa GM. Renal dysfunction is a confounder for plasma natriuretic peptides in detecting heart dysfunction in uremic and idiopathic dilated cardiomyopathies. Clin Chem. 2007;53:2097–2104. doi: 10.1373/clinchem.2007.089656. [DOI] [PubMed] [Google Scholar]
- 31.Luchner A, Burnett JC, Jr, Jougasaki M, Hense HW, Riegger GA, Schunkert H. Augmentation of the cardiac natriuretic peptides by beta-receptor antagonism: evidence from a population-based study. J Am Coll Cardiol. 1998;32:1839–1844. doi: 10.1016/s0735-1097(98)00478-1. [DOI] [PubMed] [Google Scholar]
- 32.Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich) 2003;5:5–11. doi: 10.1111/j.1524-6175.2003.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]


