Abstract
Tobacco use is one of the leading preventable causes of death in developed countries. Since existing medications are only partially effective in treating tobacco smokers, there is a great need for improved medications for smoking cessation. It has been recently proposed that cannabinoid CB1 receptor antagonists represent a new class of therapeutic agents for drug dependence, and, notably, nicotine dependence. Here, we will review current evidence supporting the use of this class of drugs for smoking cessation treatment. Preclinical studies indicate that nicotine exposure produces changes in endocannabinoid content in the brain. In experimental animals, Rimonabant (SR141716) and AM251, two cannabinoid CB1 receptor antagonists, block nicotine self-administration behavior, an effect that may be related to the blockade of the dopamine-releasing effects of nicotine in the brain. Rimonabant also seems efficacious in decreasing the influence of nicotine-associated stimuli over behavior, suggesting that it may act on two distinct neuronal pathways, those implicated in drug-taking behavior and those involved in relapse phenomena. The utility of Rimonabant has been evaluated in several clinical trials. It seems that Rimonabant is an efficacious treatment for smoking cessation, although its efficacy doesn’t exceed that of nicotine replacement therapy and its use may be limited by emotional side effects (nausea, anxiety and depression, mostly). Rimonabant also appears to decrease relapse rates in smokers. These findings indicate significant, but limited, utility of Rimonabant for smoking cessation.
Introduction
Smoking is currently responsible for the death of one in ten adults worldwide (about 5 million deaths each year). Half the people that smoke today -that is about 650 million people- will eventually be killed by tobacco (Tobacco Advisory Group of the Royal College of Physicians, 2000) and this morbidity and mortality can be reduced if subjects stop smoking (Department of Health and Human Services, 1990). The use of tobacco is on the rise in developing countries and consequently, tobacco use is one of the few causes of mortality that is increasing (http://www.who.int/tobacco/en/). Although considerable progress has been made in decreasing the prevalence of tobacco smoking in developed countries, the problem is not going away. Levels of tobacco smoking are still very high in developing countries like China and India and, even in a developed country like Canada, 18% of the population age 15 years and older are current smokers (Survey, 2006) (over 4.5 million people).
Drug dependence is a chronic, relapsing disorder in which compulsive drug-seeking and drug-taking behavior persists despite serious negative consequences (American Psychiatric Association, 2000). Addictive substances, such as cannabinoids, opioids, ethanol and psychostimulants, including nicotine, induce pleasant states or relieve distress, effects that contribute to their recreational use. After repeated exposure, adaptive changes occur in the central nervous system that lead to drug dependence (American Psychiatric Association, 2000). Although addictive drugs produce their effects through actions at various receptors in the brain, it is thought that their common effects on activity of dopaminergic brain reward pathways is primarily responsible for their addictive properties (Koob, 1992; Wise, 2004). Notably, the mesocorticolimbic system, which projects from the ventral tegmental area to the nucleus accumbens, cortical areas and the amygdala, is implicated in the rewarding effects of psychostimulants and other drugs of abuse, as well as the effects of non-drug natural rewards such as food (Wise, 1982). The involvement of dopamine in the rewarding effects of drugs of abuse is suggested by findings that most drugs abused by humans increase levels of dopamine in the nucleus accumbens (Imperato et al., 1986; Pidoplichko et al., 1997) and that blockade of dopamine transmission reduces the rewarding effects of psychostimulants (Koob, 1992). However, the role of dopamine seems more complex than simply mediating the primary reinforcing effects of drugs of abuse (Salamone et al., 2003; Wise, 2004). Recent evidence suggests that dopamine is strongly implicated in learning and conditioning processes (Schultz, 2002; Schultz et al., 1997) and in drug seeking-behavior (Phillips et al., 2003).
Nicotine is the principal component of tobacco smoke that leads to addiction. Nicotine activates several subtypes of neuronal nicotinic acetylcholine (nACh) ion channel receptors, formed by the combination of five subunits (α and/or β). Hetero-oligomeric receptors containing β2 and α4 subunits seem to play pivotal roles in the addictive effects of nicotine (Grottick et al., 2000; Maskos et al., 2005; Picciotto et al., 1998; Walters et al., 2006). Although nicotine directly activates dopaminergic transmission through actions on nAChRs, recent studies suggest that this activation is modulated by the endocannabinoid system. Consequently, the potential therapeutic utility of drugs acting on the endocannabinoid system for the treatment of tobacco dependence has received considerable attention (De Vries and Schoffelmeer, 2005; Le Foll and Goldberg, 2005a).
There are currently two identified and cloned forms of cannabinoid receptors, CB1 and CB2 receptors (Gerard et al., 1991; Matsuda et al., 1990; Munro et al., 1993). The CB1 receptor and its splice variant the CB1A receptor are predominantly found in the brain, with the highest density in the globus pallidus, substantia nigra pars reticulata, hippocampus, cerebellum, cortex and striatum. CB2 receptors are predominantly located peripherally, principally associated with the immune system (Howlett et al., 2002), but they also been identified in the brain (Onaivi et al., 2006; Van Sickle et al., 2005). Several investigators have obtained clear evidence that some cannabinoid effects are not mediated by either CB1 and CB2 receptors (Begg et al., 2005; Wilson and Nicoll, 2002). It has been recently proposed that the orphan receptor GPR55 is a novel cannabinoid receptor (Pertwee, 2007; Ryberg et al., 2007), although its functional role is still unclear. The transient receptor potential vanilloid 1 (TRPV1) has been also proposed as a potential target of cannabinoid ligands (Begg et al., 2005).
This review focuses on the development of cannabinoid CB1 receptor antagonists for the treatment of nicotine addiction. The behavioral studies performed so far have used Rimonabant (SR141716) and AM251, which are inverse agonists/antagonists for the CB1 receptors (see (Howlett et al., 2002; McPartland et al., 2007; Vemuri et al., 2007) for information on pharmacology of compounds). For simplicity, we will present in this review those compounds as antagonists, although they are inverse agonists and it appears clear that their behavioral effects may be slightly different from pure neutral antagonists (Salamone et al., 2007; Sink et al., 2008). We will first summarize the main animal models used to assess subjective and rewarding/reinforcing effects of drugs of abuse and then present the preclinical and clinical findings related to the effects of cannabinoid CB1 receptor blockade on the subjective and rewarding/reinforcing effects of nicotine in these models. We will also review the various clinical trials that have been conducted to assess the efficacy of Rimonabant in smokers and summarize results of these trials that have been presented at scientific meetings.
Effects of CB1 antagonists in preclinical models of nicotine dependence
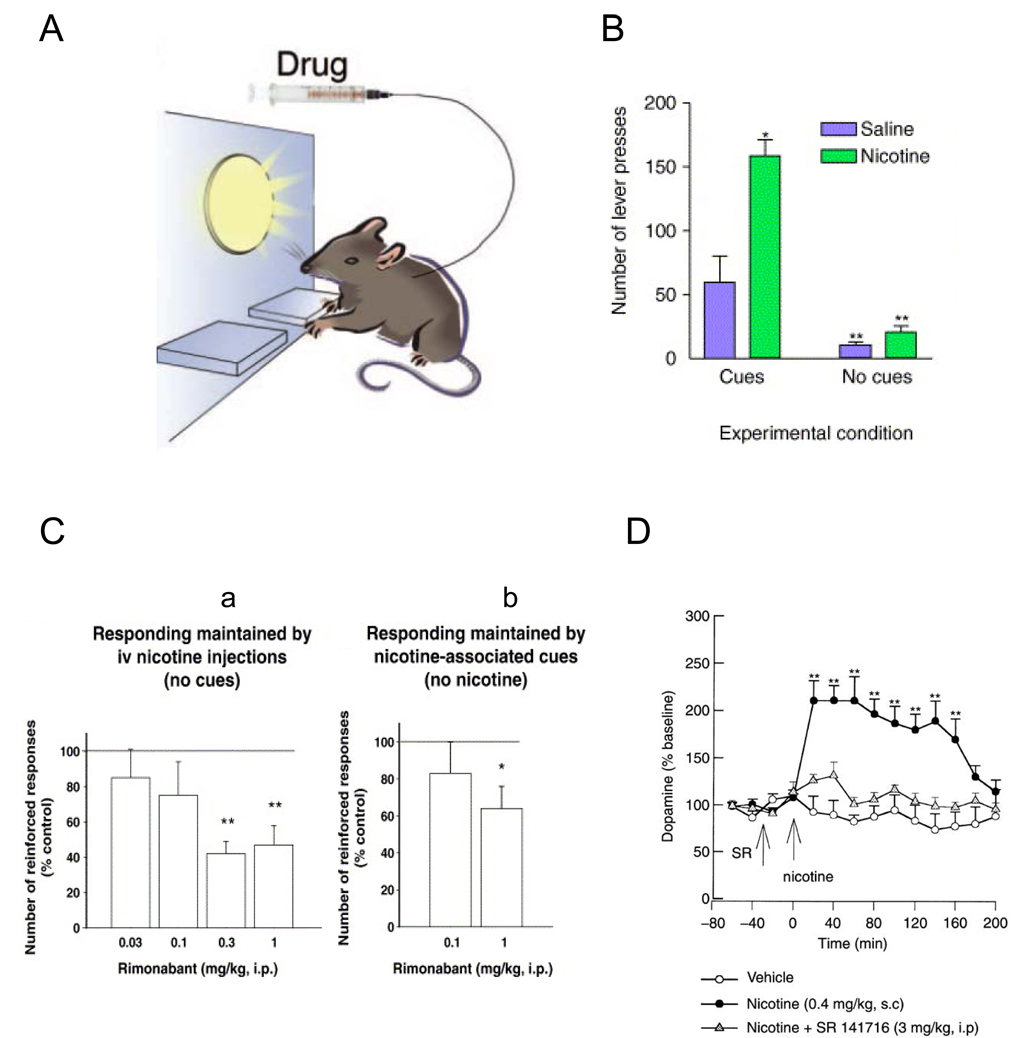
Intravenous nicotine self-administration (IVSA) (figure 1A)
Figure 1. Rimonabant decreases nicotine self-administration and cue-maintained responding in rats.
A) During repeated sessions, animals learned to press a lever to obtain intravenous injections of nicotine and a brief light stimulus was associated with each nicotine injection. This light stimulus progressively gained motivational properties. B) Removing the environmental light stimuli associated with nicotine injection (no-cues condition) dramatically reduced nicotine self-administration by rats, as compared to situation in which nicotine delivery is associated with presentation of these light stimuli (cues condition). Adapted from (Donny et al., 2003) and reproduced with permission from (Le Foll and Goldberg, 2005a). Results are expressed as mean (±SEM) of number of lever-press for saline or nicotine injection per session. C) Rimonabant (SR 141716) administration decreased intravenous nicotine self-administration (a) and responding maintained by nicotine-associated stimuli (b) in rats. Reproduced with permission from (Cohen et al., 2005). D) Rimonabant (SR 141716) administration decreased nicotine-induced dopamine in the shell of the nucleus accumbens, as assessed with microdialysis in awake rats. Reproduced with permission from (Cohen et al., 2002).
Intravenous drug self-administration procedures are generally considered the most direct measures of the reinforcing effects of drugs. With these procedures, a catheter is chronically implanted in a jugular vein and this allows the animal to repeatedly intravenously self-administer small amounts of drug by making an operant response, such as pressing a lever. Administration of drug constitutes the event that positively reinforces the operant behavior and reward is inferred if the frequency of responding subsequently increases (thus, defining reinforcement). With these behavioral procedures, some stimuli (visual and/or auditory) are repeatedly associated with drug administration. These stimuli, or cues, can progressively gain motivational value that allows them to induce and maintain drug-seeking behavior and to reinstate drug-seeking behavior after extinction (Arroyo et al., 1999; de Wit and Stewart, 1981; Goldberg, 1975; Goldberg et al., 1975; Goldberg et al., 1983; Meil and See, 1996; Self and Nestler, 1988; Stewart, 1983), providing useful measures of the motivational effects of drug-related stimuli.
Various schedules of reinforcement of nicotine self-administration behavior have been studied. Under a fixed-ratio schedule of intravenous drug injection, a fixed number of lever-presses is required to obtain each injection of drug (e.g., five lever presses for a fixed-ratio 5, i.e. a FR5 schedule) (Fig. 1a). In contrast, under a progressive-ratio schedule, the number of lever-press responses required to obtain a nicotine injection progressively increases after each drug injection (Hodos, 1961). Thus, the number of responses the subject must make for each successive nicotine injection (the ratio value) increases progressively until the subject fails to emit the required number of responses: this highest ratio (the “breaking point”) is thought to reflect the reinforcing effectiveness of the nicotine injection (Donny et al., 1999; Le Foll et al., 2007). Once an animal has learned to self-administer nicotine, the influences of nicotine priming (Andreoli et al., 2003; Chiamulera et al., 1996; Lindblom et al., 2002), stressors (Buczek et al., 1999) or presentation of nicotine-associated cues (Caggiula et al., 2002; Caggiula et al., 2001; Lesage et al., 2004) on nicotine self-administration behavior or on relapse to extinguished nicotine-seeking behavior provide additional useful measures (Le Foll and Goldberg, 2006; Shalev et al., 2002). It should be noted that nicotine self-administration is critically influenced by the presence of nicotine-associated stimuli during the session (Fig. 1B) (Caggiula et al., 2002; Caggiula et al., 2001; Goldberg et al., 1981; Le Foll and Goldberg, 2005b, 2006).
The efficacy of the selective CB1 antagonist, Rimonabant, on nicotine intake was first systematically evaluated using a fixed-ratio nicotine self-administration procedure in rats (Cohen et al., 2002). In this study, Rimonabant (0.3 mg/kg and 1 mg/kg i.p.) significantly reduced the number of responses on the nicotine-associated lever and the number of nicotine infusions (0.03 mg/ kg/infusion) (Fig. 1Ca). This effect appeared related to the blockade of nicotine-induced dopamine release in the shell of the nucleus accumbens (Fig. 1D) (Cohen et al., 2002). In addition, pretreatment with Rimonabant (1 mg/kg i.p.) reduced responding maintained by nicotine-associated cues in rats, in the absence of nicotine (after 1 month of nicotine withdrawal) (Cohen et al., 2005)(Fig. 1Cb). In another study, Rimonabant reduced reinstatement of nicotine-seeking behavior induced by nicotine-associated cues after prolonged abstinence (De Vries et al., 2005). Similar results have been obtained with AM251, another CB1 antagonist, which also attenuates nicotine self-administration and nicotine-seeking behavior in rats (Shoaib, 2008). These experiments with two highly selective CB1 antagonists in rats suggest that CB1 receptor activity is necessary for nicotine to serve as a reinforcer of drug-seeking and drug-taking behavior, as well as for nicotine-associated cues to maintain drug-seeking behavior and to reinstate nicotine-seeking behavior after extinction.
However, conflicting results have been obtained in mice. CB1 receptor knockout mice did appear to learn to self-administer nicotine (Cossu et al., 2001), indicating that activation of CB1 receptors does not play an equivalent role in the processes controlling nicotine self-administration in the two species. It is possible that CB1 receptor knockout mice develop compensatory mechanisms during development that could explain such differences. In addition, the acquisition and maintenance of stable nicotine self-administration behavior has not been sufficiently evaluated in mice lacking CB1 cannabinoid receptors. More studies are needed to better understand differences which may exist between pharmacological blockade and genetic invalidation of CB1 receptors in mice.
Nicotine-induced conditioned place preferences
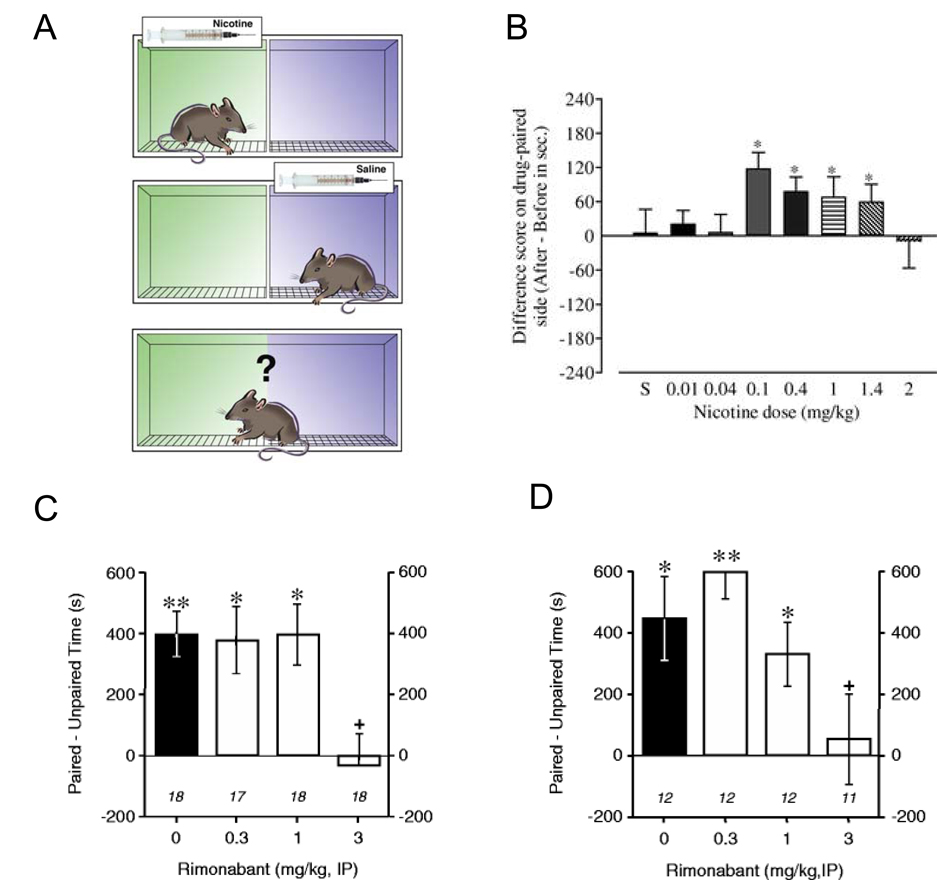
The conditioned place preference (CPP) procedure is an experimental animal model for exploring the rewarding effects of drugs of abuse (Fig. 2A). A distinctive environment (e.g., one compartment of a two- or three-compartment apparatus (Le Foll and Goldberg, 2005c) or an environment with a particular floor texture (Forget et al., 2005)) is paired repeatedly with administration of a drug, and a different environment is repeatedly associated with administration of vehicle. CPP occurs when repeated administration of a drug in this particular environment results in the ability of that environment to elicit approach behavior and increased time contact (place preference) in the absence of the previously administered drug. It has been argued that CPP, like drug self-administration and a number of related phenomena, is an example of dopamine-mediated incentive learning and that the approach behavior and increased time spent by animals in a drug-paired environment can be considered a measure of drug-seeking behavior (Bardo and Bevins, 2000; Le Foll and Goldberg, 2005c,b). CPP have been demonstrated for most drugs of abuse, including nicotine (Bardo and Bevins, 2000; Le Foll and Goldberg, 2005c), as well as natural rewards such as food. The development of a drug-induced CPP is likely to reflect the rewarding properties of a drug of abuse, whereas its expression reflects the influence on behavior of environmental stimuli previously associated with a drug’s effects.
Figure 2. Rimonabant blocks nicotine-induced conditioned place preferences (CPP).
A, In order to induce CPP, a box with two discrete chambers, or environments, is usually used. Rats were repeatedly injected with nicotine before being placed in one environment and with saline before being placed in the other environment. Then, in a nicotine-free state, the animals were allowed access to both environments and the amount of time spent in each environment was recorded. Reproduced with permission from (Le Foll and Goldberg, 2005a). B, Nicotine induced significant conditioned place preferences over a large range of doses in rats. Results are expressed as the difference in time in sec spent in the drug-paired side between the post- and the pre-conditioning session. * P <0.05. Adapted from (Le Foll and Goldberg, 2005c) C, Rimonabant (3 mg/kg) blocked the establishment of nicotine-induced conditioned place preference when administered before each conditioning session with nicotine. Reproduced with permission from (Forget et al., 2005). D, Rimonabant administered acutely before the test-session blocked the short term expression of nicotine-induced CPP when the test take place 24h after the last conditioning session, * P < 0.05; ** P<0.01 paired vs unpaired time. + P <0.05 vs nicotine conditioned group without Rimonabant (black diagrams). Reproduced with permission from (Forget et al., 2005). Similar results have been reported by (Le Foll and Goldberg, 2004).
Nicotine has been shown to induce CPPs in a number of studies (see (Le Foll and Goldberg, 2005c) for a review) (Fig. 2B), but successful demonstration of nicotine-induced CPPs is sensitive to procedural details, such as route of administration, the strain of rodent, the dose of nicotine used, the duration of individual conditioning sessions, the number of drug conditioning sessions and pre-exposure to the apparatus before conditioning sessions begin. Nicotine pre-exposure (Shoaib et al., 1994), use of ‘adolescent’ animals (Belluzzi et al., 2004; Shram et al., 2006; Vastola et al., 2002) and food restriction (Forget et al., 2005) appear to facilitate the development of nicotine-induced CPPs (Le Foll and Goldberg, 2005c). As in self-administration procedures, β2-containing nAChRs seem to primarily mediate the development of nicotine-induced CPPs (Grabus et al., 2006; Walters et al., 2006).
Recently, it has been proposed that CPP procedures can also be used to study reinstatement. This observation arose from the finding that once established, CPPs can be extinguished by repeated pairings of both drug-associated and vehicle-associated environments with saline (Mueller and Stewart, 2000). After extinction of CPPs, the same factors that reinstate extinguished drug-seeking behavior in a self-administration procedure are able to reinstate extinguished CPPs (Lu et al., 2002; Mueller and Stewart, 2000; Sanchez and Sorg, 2001), although only very limited research has been conducted with nicotine with such a reinstatement procedure (Biala and Budzynska, 2006).
The effect of CB1 blockade by Rimonabant has recently been evaluated with nicotine using a CPP procedure. When administered acutely, Rimonabant (3 mg/kg, IP) inhibited both the development (Fig. 2C) and the expression (Fig. 2D) of nicotine-induced CPPs in rats (Forget et al., 2005; Le Foll and Goldberg, 2004). However, inhibition of the expression of nicotine-induced CPP was only effective when Rimonabant was administered 24h after the last conditioning session and not when Rimonabant was given 3 or 12 weeks after the last conditioning session (Forget et al., 2005). These data suggest that the integrity of CB1 receptors is necessary for the perception by rats of the positive interoceptive effects of nicotine or for the development of conditioned associations between the interoceptive effects of nicotine and the associated environment in which nicotine’s effects are experienced. In addition, CB1 receptors seem to be differentially involved in short- versus long-term expression of conditioned place preferences induced by nicotine in rats (Forget et al., 2006). In line with these findings in rats, nicotine was not able to induce conditioned place preferences in CB1-receptor deficient mice, as compared to their wild-type littermates (Castane et al., 2002).
Nicotine discrimination
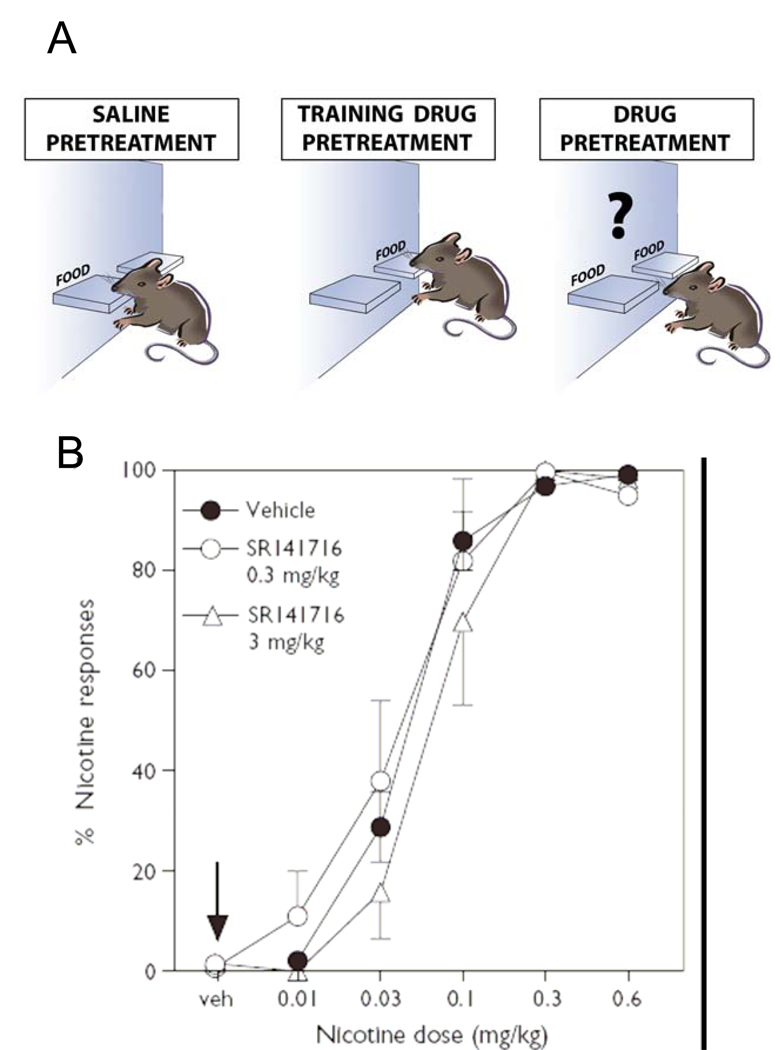
Drug discrimination procedures are frequently used as animal models for the subjective reports of drug effects in humans (Colpaert, 1999; Le Foll and Goldberg, 2005a; Solinas et al., 2006). An organism’s ability to perceive and identify characteristic interoceptive effects of drugs is thought to play a role in drug-seeking, encouraging the development of this behavior and directing it towards one substance rather than another, on the basis of relative potencies and effects (Stolerman and Shoaib, 1991). In animals, the interoceptive effects of drugs can serve as discriminative stimuli to indicate how to obtain a reinforcer such as a food pellet or how to avoid an electric shock (Fig. 3A). Nicotine can serve as a discriminative stimulus in animals (Rosecrans and Villanueva, 1991). Pharmacological blockade or genetic invalidation of different subtypes of nicotinic receptors indicated that nAChRs containing the β2 subunit are the main target of nicotine discriminative effects (Shoaib et al., 2002; Shoaib et al., 2000; Stolerman et al., 1997). Pharmacological blockade or genetic invalidation of receptors can also be used to assess involvement of cannabinoid CB1 receptors in the discriminative effects of nicotine.
Figure 3. Rimonabant does not affect subjective effects induced by nicotine in rats.
A) Description of the drug discrimination paradigm. Rats are trained to press on levers to get food pellets. Rats will be trained to respond on the lever after either saline or training drug injections. During some training sessions after the saline pretreatment, only one lever will allow the animal to get the food pellet (left graph). On other training sessions after the injection of the training drug (e.g nicotine 0.4 mg/kg in B), only lever-presses on the other lever will results in food pellets delivery (middle graph). After training, the rats will press only the saline-associated lever during the saline sessions and only the nicotine-associated lever after injection of nicotine at the training dose (see B). These rats are trained to discriminate the subjective effects induced by nicotine injections (right). B) Dose-effect functions for the discriminative-stimulus effects of nicotine in rats (n = 24) trained to discriminate 0.4 mg/kg nicotine from saline. The percentage of responses on the lever associated with nicotine administration is shown as a function of dose (mg/kg) during tests with various nicotine doses. SR141716 given acutely 60 min before the session did not modify the discrimination of nicotine (no shift of the curve). The arrow indicates that SR141716 alone, at doses of 0.3 and 3 mg/kg, did not produce responding on the nicotine-associated lever, i.e. did not produce any ‘nicotine-like’ effects in those rats. Reproduced with permission from (Le Foll and Goldberg, 2004).
Rimonabant administration did not block the discriminative stimulus effects of a high 0.4 mg/kg training dose of nicotine in two recent studies (Cohen et al., 2002; Le Foll and Goldberg, 2004) and also failed to change the discriminative stimulus effects of lower doses of nicotine ranging from 0.01 to 0.6 mg/kg in another recent study (Le Foll and Goldberg, 2004) (Fig. 3B). Moreover, Rimonabant did not produce any ‘nicotine-like’ effects in rats trained to discriminate nicotine from saline (Le Foll and Goldberg, 2004) (Fig. 3B). However, Rimonabant appeared to counteract the dopaminergic-component of the nicotine discrimination (Cohen et al., 2002). These results indicate that CB1 receptors are not directly involved in nicotine discrimination, suggesting that the effect of Rimonabant is specific to nicotine reward.
Summary of preclinical findings obtained so far with Rimonabant: effects on dopamine or glutamate?
The experiments reviewed above suggest that Rimonabant and AM251 can affect both nicotine self-administration behavior and reinstatement of extinguished nicotine-seeking behavior. This behavioral profile makes cannabinoid CB1 receptor antagonists a potentially valuable therapeutic tool for the treatment of tobacco dependence. However, place preference experiments in rats indicate that, although activation of CB1 receptors is likely necessary for the short-term expression of nicotine incentive learning, after longer time-intervals (2–3 weeks) the same function seems to be assumed by neurobiological processes independent of the endocannabinoid system (Forget et al., 2006). Therefore, we may question the efficacy of Rimonabant in a situation where subjects have been exposed chronically to nicotine and may have developed such endocannabinoid-independent adaptations. In agreement with this hypothesis, using a non-human primate model (Le Foll et al., 2007), we have recently found that Rimonabant is not effective in reducing responding maintained by nicotine-associated cues in monkeys with long histories of nicotine self-administration (unpublished findings).
The mechanisms underlying the effects of CB1 receptor blockade on drug-induced reinforcement/reward and relapse to drug-seeking behavior remain unknown, but animal data suggest some possible explanations. Cannabinoid agonists, when administered intravenously in either anesthetized or freely moving rodents, can increase the level of activity of dopaminergic neurons in the ventral tegmental area (French et al., 1997; Gessa et al., 1998; Wu and French, 2000) and this augmentation includes both an increase in baseline firing rates and an increase in the frequency of action potential bursting. This cannabinoid-induced increase in dopamine neuron activity is apparently responsible for increases in extracellular levels of dopamine observed in the nucleus accumbens after Δ9-tetrahydrocannabinol administration (Chen et al., 1990; Ng Cheong Ton et al., 1988; Tanda and Di Chiara, 1997). Since Rimonabant can dose-dependently block the nicotine-induced elevations in dopamine levels in the nucleus accumbens (Cohen et al., 2002), it is likely that Rimonabant reduces the reinforcing/rewarding effects of nicotine by decreasing nicotine-induced activation of dopaminergic neurotransmission.
Cannabinoid CB1 receptors are present in limbic structures, including the ventral tegmental area and the nucleus accumbens (Herkenham et al., 1991), and endocannabinoids may act at these two levels to facilitate the activation of mesolimbic dopaminergic neurons. Chronic administration of nicotine in rats results in an increase in endogenous levels of endocannabinoids such as anandamide in the limbic forebrain (Gonzalez et al., 2002). Increased levels of endocannabinoids may facilitate dopaminergic neuron activity in the ventral tegmental area (Cheer et al., 2007), an effect that could be reversed by CB1 receptor blockade with Rimonabant.
It has been shown that the endocannabinoids anandamide and 2-arachidonylglycerol (Mechoulam et al., 1998) act as synaptic retrograde messengers. They are released from postsynaptic neurons upon depolarization, and then act in a retrograde manner to activate CB1 receptors located on axon terminals, thus inhibiting neurotransmitter release. In the ventral tegmental area, nicotine activates dopamine neurons (Pidoplichko et al., 1997), a phenomenon that can induce endocannabinoid release in the nucleus accumbens or the ventral tegmental area (Riegel and Lupica, 2004). The activation of CB1 receptors on axon terminals of GABAergic neurons by endocannabinoids would result in inhibition of GABA release and disinhibition of dopaminergic neurons, supporting dopamine release in the nucleus accumbens. On the contrary, activation of CB1 receptors on glutamatergic axon terminals (e.g, from the prefrontal cortex) would result in inhibition of this excitatory afferent and less activation of mesolimbic dopamine neurons (Lupica and Riegel, 2005). In the nucleus accumbens, activation of medium spiny neurons by glutamatergic afferents coming from the cortex may also produce endocannabinoid release (Robbe et al., 2002). Stimulation of CB1 receptors on axon terminals of these glutamatergic neurons may inhibit the activation of medium spiny neurons by reducing glutamate release. This could result in a disinhibition of mesolimbic dopamine neurons and enhanced dopamine release in the nucleus accumbens (Robbe et al., 2001).
The effects of pharmacological blockade of CB1 receptors with cannabinoid antagonists such as Rimonabant or AM251 would be the opposite of the hypothetical mechanisms described above for endocannabinoid-induced facilitation of mesolimbic dopaminergic neuron activity and this would explain the inhibitory effect of Rimonabant on nicotine-induced dopamine release in the nucleus accumbens (Fig. 1D). Since dopamine release in the nucleus accumbens is thought to play a major role in the positive reinforcing effects of nicotine (Di Chiara, 2000), this would also explain the inhibitory effects of cannabinoid CB1 receptor antagonists on nicotine reinforcement/reward.
It is possible that the ability of cannabinoid CB1 receptor antagonists to reduce the effects of nicotine-associated cues on maintenance or reinstatement of nicotine seeking can also be explained by actions on the mesolimbic dopamine reward system. Indeed, environmental stimuli associated with self-administration of nicotine or other drugs of abuse can produce dopamine elevations in the nucleus accumbens (Bassareo et al., 2007; Di Ciano et al., 1998; Ito et al., 2000; Roitman et al., 2004; Weiss et al., 2000). A recent study by Kodas et al. (2007) showed that Rimonabant produced its effect on cue-induced nicotine seeking in rats via an action on key cortico-limbic structures, e.g. the shell of the nucleus accumbens, the prelimbic cortex and the basolateral amygdala. The basolateral amygdala and the prefrontal cortex (of which the prelimbic cortex is a part) play critical roles in the induction of drug seeking behavior by drug-associated cues. For example, basolateral amygdala lesions disrupt cue-elicited cocaine or heroin seeking behavior in rats (Fuchs et al., 2006; McLaughlin and See, 2003; Parkinson et al., 2001), and exposure to nicotine-, cocaine- or ethanol-associated stimuli increases neuronal activity of prefrontal cortical regions in rats (Ciccocioppo et al., 2001; Le Foll et al., 2002) and in humans (Brody et al., 2002; Grusser et al., 2004). The effects of injections of Rimonabant into the basolateral amygdala and the prelimbic cortex on cue-induced nicotine seeking could be explained by the fact that the prefrontal cortex and the basolateral amygdala may work in concert to modulate dopaminergic and/or glutamatergic neurotransmission in the shell of the nucleus accumbens (Phillips et al., 2003; Robbe et al., 2001).
All of these studies suggest that the effects of cannabinoid CB1 receptor antagonists could be mediated by the modulation of dopamine activity. However, recent studies indicate that glutamate may be involved in the effects of CB1 blockade. Pretreatment with AM251 (the selective CB1 antagonist) dose dependently inhibited cocaine-induced increases in nucleus accumbens glutamate but not increases in dopamine or GABA levels (Xi et al., 2006). Blockade of accumbens metabotropic glutamate mGluR2/3 receptors by LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid] slightly facilitated cocaine-enhanced glutamate release but blocked the antagonism of cocaine-induced reinstatement by AM251 (Xi et al., 2006). These data suggest that under cocaine-extinction conditions, cannabinoid CB1 receptors exert tonic inhibition over nucleus accumbens glutamate release. Therefore, some of the behavioral effects obtained in the relapse model by AM251 may be related to effects on glutamate and it has been proposed that activation of presynaptic mGluR2/3 autoreceptors secondary to AM251-induced increase (disinhibition) of nucleus accumbens glutamate release is implicated. Although this is still hypothetical, cannabinoid CB1 receptor antagonists may affect both glutamatergic and dopaminergic transmission to reduce the reinforcing/rewarding effects of nicotine and reinstatement.
Effects of CB1 antagonism on clinical trials in human smokers
Given the efficacy of the selective cannabinoid CB1 receptor antagonist Rimonabant in reducing nicotine self-administration (Cohen et al., 2002), as well as nicotine-induced dopamine release in the shell of the nucleus accumbens (Cohen et al., 2002) and the expression of nicotine- induced conditioned place preferences (Le Foll and Goldberg, 2004) in animals, Sanofi-Aventis developed a clinical program of Rimonabant for smoking cessation, called STRATUS (Studies with Rimonabant And Tobacco Use). Results from five (yet unpublished) phase III STRATUS trials were presented at various scientific meetings (Anthenelli and Despres, 2004; Cinciripini et al., 2006; Dale, 2004; Dale et al., 2004; Niaura, 2005; Rigotti et al., 2007). Two trials — one in Europe (STRATUS-EU) and one in the USA (STRATUS-US) — were identical in design and involved a double-blind 10-week treatment course with Rimonabant versus placebo followed by a 40-week post-treatment follow-up. A STRATUS-META trial was conducted in the USA and involved only a double blind Rimonabant versus placebo 10-week treatment course, with no extended follow-up. A STRATUS-WORLDWIDE (WW) trial was designed to assess the long-term efficacy and safety of Rimonabant for maintenance of abstinence in smokers who successfully quit smoking. Finally, the CIRRUS trial assessed the efficacy and safety of adding a nicotine patch to Rimonabant for smoking cessation. In all STRATUS trials, participants were males or females at least 18 years of age, and had to be smoking at least 10 cigarettes/day, be free of uncontrolled systemic/chronic illnesses or substance abuse and be motivated to quit smoking.
A pooled analysis of the three short term STRATUS –US/–EU/–META trials was presented at a scientific meeting (Cinciripini et al., 2006). In these trials, participants were randomised to placebo, Rimonabant 5 mg/day (STRATUS-US/-EU) or Rimonabant 20 mg/day (all studies) for 10 weeks. The target quit date was set at the end of the second week of treatment. Participants were seen on a weekly basis over the first 12 weeks. Brief behavioral counselling sessions on topics related to smoking behaviors (e.g., cravings and relapse prevention) were provided at each visit. No specific dietary restriction was provided during the course of the study, although diet and exercise were included as behavioral counselling topics. Smokers randomised to a 5 mg/day dose (in two of the three trials) were not taken into account in this pooled analysis. The primary efficacy endpoint was prolonged abstinence during the last 4 weeks of treatment, measured by self-reported smoking abstinence and expired carbon monoxide concentrations and confirmed by plasma cotinine levels. Body weight change from baseline to the end of treatment was a secondary efficacy endpoint. All analyses were performed in the intent-to-treat population. Smokers (n=1579) were assigned to Rimonabant 20 mg/day (n=789) or placebo (n=790). Rimonabant 20 mg/day resulted in a significantly higher prolonged abstinence rate compared with placebo (22% vs 15%, p<0.001; odds ratio [OR]: 1.6, 95% CI: 1.2–2.1). In participants achieving prolonged abstinence, Rimonabant significantly reduced post-cessation weight gain versus placebo: +0.7 vs +2.8 kg (p<0.001).
The aim of the STRATUS-WW trial was to assess the long-term efficacy and safety of Rimonabant for maintenance of abstinence in smokers who were abstaining at the end of an initial 10-week treatment. The STRATUS-WW was a randomised, double-blind, 5-arm, placebo-controlled 2-year clinical trial. The trial was carried out in Australia, Canada and the United States. A total of 5055 cigarette smokers were randomised to two treatment groups: 5mg or 20mg Rimonabant. At Week 10, 1672 successful quitters were re-randomised to either placebo or 5 mg (for those already receiving 5 mg) and placebo, 5 mg or 20 mg (for those already receiving 20mg). Active treatment continued for 42 weeks, followed by a 50-week off-drug period. Relapse was defined by seven or more consecutive days of smoking (even a puff) or two or more consecutive days with five or more cigarettes (even a puff) smoked per day. In the 5 mg stratum, patients who were re-randomised to continue the same treatment or placebo had similar relapse rates after 32 weeks of treatment. However, in smokers who had initially received 20 mg, a significantly higher proportion of participants treated with 5 and 20 mg Rimonabant were abstinent at 32 weeks compared to those receiving placebo (51.9%; p<.001 and 50.9%; p<.05 vs placebo, respectively). Post-cessation weight gain in patients that did not relapse to smoking was significantly lower in the 20 mg Rimonabant group (p<0.001 vs placebo). Post-cessation weight gain was similar in the placebo and 5 mg Rimonabant groups.
The aim of the STRATUS-CIRRUS trial was to assess the possible value of adding a nicotine patch to Rimonabant for smoking cessation. This randomised double blind placebo-controlled trial enrolled 755 smokers who were given Rimonabant (20 mg/day) open-label for 9 weeks. Target quit date was set at week 1, when participants were randomly assigned to receive nicotine patch (21 mg for 8 weeks, 14 mg for 1 week, and 7 mg for 1 week) or placebo for 10 weeks. Participants had brief cessation counselling at each visit, and were followed-up until week 26. The primary endpoint was sustained abstinence from week 6 to week 9, verified by carbon monoxide concentrations. Smokers receiving Rimonabant plus active nicotine patch had a higher rate of sustained abstinence compared to those receiving Rimonabant plus placebo patch (OR 2.36; 95% CI 1.71–3.27). They also had a higher 7-day point abstinence rate at the end of treatment and at week 26, more reduction in cigarette cravings at the end of treatment and no difference in body weight change from baseline among completers or sustained abstainers. Adding a nicotine patch to Rimonabant did not lead to increases in serious adverse events or in treatment discontinuation due to adverse events. Placebo-controlled trials showed a slightly higher percentage of treatment-emergent adverse events and of treatment discontinuations, and no change in serious adverse events incidence with 20 mg/day Rimonabant compared to placebo. Most adverse events were gastro-intestinal symptoms (Cinciripini et al., 2006).
Although these first reports, taken individually, suggested that the use of Rimonabant was relatively well tolerated, those studies reported trends to increase anxiety or depressive symptoms. This risk has been confirmed recently by two meta-analysis studies (Christensen et al., 2007; Rucker et al., 2007) and by a report by the US Food and Drug Administration (Food and Drug Administration Endocrinologic and Metabolic Advisory, June 13, 2007). Those studies concluded that the subjects in the RIO trials conducted for obesity were two to three times more likely to discontinue the treatment because of depressive mood or anxiety in the Rimonabant group compared to the placebo group. Since patients with serious mood disorders were not included in the RIO trials, it is possible that the incidence of these psychiatric disorders may be higher in general population and, possibly, in smokers, since the incidence of psychiatric disorders is higher in tobacco smokers compared to non smokers. For these reasons, the FDA Advisory Committee concluded that more safety information was needed to put Rimonabant in the US market. Further studies that are in progress will clarify the incidence of these psychiatric disorders and assess whether these conditions are associated with an increased risk of suicide.
CONCLUSION: is there a place for cannabinoid CB1 receptor antagonists in smoking cessation?
Evidence collected so far indicates that cannabinoid CB1 receptor antagonists have potential as a treatment for smoking cessation, but that their efficacy would not be greater than efficacies of the established medications, bupropion, nicotine replacement therapy and varenicline, all of which act to reduce both nicotine withdrawal symptoms and the motivation to take nicotine. There is no indication that cannabinoid CB1 receptor antagonists would be effective in decreasing nicotine withdrawal symptoms.
The rate of smoking cessation by subjects entering into clinical trials which combine effective medication and behavioral and cognitive therapy is around 30% at the end of the first year; the vast majority of subjects relapse (Fiore, 2000; Le Foll and George, 2007). Since cannabinoid CB1 receptor antagonists significantly increase the likelihood of maintaining abstinence, this class of drugs represents a potentially useful tool. However, their efficacy is limited. The significant effect of CB1 receptor antagonists on the control of weight gain indicates that they may be particularly useful for individuals that wish to avoid weight gain following smoking cessation. Post-cessation weight gain concerns may be an important deterrent to stopping smoking. The probability of attempting to stop smoking appears to be lower in subjects reporting smoking-related weight concerns (Jeffery et al., 2000; Klesges et al., 1988; Klesges and Shumaker, 1992; Meyers et al., 1997; Pomerleau et al., 2001). A CB1 antagonist could lower post-cessation weight concerns and increase the rate of smoking cessation attempts in some smokers. Interestingly, preclinical data obtained so far seems to implicate CB1 receptors in relapse to various drugs of abuse. Therefore, cannabinoid CB1 receptor antagonists may be of particular interest for treatment of subjects dependent on multiple drugs. One of the most appealing populations for evaluating the effects of Rimonabant or other CB1 antagonists would be subjects addicted both to tobacco and marijuana, since CB1 receptors clearly mediate the reinforcing effects of marijuana (Tanda et al., 2000).
The evidence collected in rats indicates that cannabinoid CB1 receptor antagonists may be particularly useful for the prevention of relapse. However, recent data obtained in preclinical experiments in rats and non-human primates and in human clinical trials suggests that the utility of cannabinoid CB1 receptor antagonists may be more limited than initially thought. However, clinical trials designed specifically to evaluate relapse prevention may reveal important effects of this class of drugs. It is also clear that the increased incidence of anxiety and depressive symptoms in subjects treated with Rimonabant is a concern and that clinicians should be using this drug carefully in that regard. Various agencies have established guidelines to reduce the risk. It will be interesting to compare the side effects of Rimonabant to those of other CB1 inverse agonists, such as taranabant (Addy et al., 2008; Addy et al., 2008; Kirkham, 2008). It should be noted that Rimonabant is an inverse agonist/antagonist. It has been recently demonstrated that a cannabinoid CB1 receptor neutral antagonist AM4113 can produce suppression of food intake and food-reinforced behavior in animals (Sink et al., 2008), without producing some unwanted side effects such as nausea and vomiting (Sink et al., 2008), (the main side effects in terms of frequency during clinical trials). Further studies are needed to confirm that such CB1 receptor neutral antagonists can block the motivation to take nicotine and have a good tolerability profile in humans.
Table 1.
Main effect of CB1 blockade on the subjective, discriminative and rewarding/reinforcing effects of nicotine in animals and human subjects
| Species | Model | Results | Reference |
|---|---|---|---|
| Humans | Smoking cessation trial | Rimonabant increased smoking cessation rates | (Anthenelli and Despres, 2004) (Cinciripini et al., 2006) |
| Rats | Self-administration of IV nicotine at a low ratio requirement (FR4) | Rimonabant and AM251 decreased self administration | (Cohen et al., 2002; Shoaib, 2008) |
| Rats | Expression of CPP (Stimulus-controlled behavior) | Rimonabant blocked preferences for nicotine-paired environment | (Forget et al., 2005; Le Foll and Goldberg, 2004) |
| Development of CPP | Blocked by rimonabant | (Forget et al., 2005) | |
| Rats | Nicotine discrimination | No effect of Rimonabant | (Cohen et al., 2002; Le Foll and Goldberg, 2004) |
| Mice | Nicotine-induced CPP | No CPP for nicotine in CB1 deficient mice | (Castane et al. 2002). |
| Mice | Self-administration of IV nicotine at a low ratio requirement (FR1) | No effect of CB1 receptor invalidation | (Cossu et al., 2001) |
Acknowledgements
Supported in part by the Intramural Research Program of NIDA, NIH, DHHS. BLF is supported by a Grant from CIHR –TUSP program and BF is a recipient of a fellowship from CIHR Strategic Training Program in Tobacco Research.
List of abbreviations
- SR 141716, Rimonabant
N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide
- AM-251
N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
References
- Addy C, Li S, Agrawal N, Stone J, Majumdar A, Zhong L, Li H, Yuan J, Maes A, Rothenberg P, Cote J, Rosko K, Cummings C, Warrington S, Boyce M, Gottesdiener K, Stoch A, Wagner J. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, for the treatment of obesity: results from a double-blind, placebo-controlled, single oral dose study in healthy volunteers. J Clin Pharmacol. 2008;48:418–427. doi: 10.1177/0091270008314467. [DOI] [PubMed] [Google Scholar]
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN, 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Ed. 4. Washington CD: American Psychiatric Association; 2000. revised version. [Google Scholar]
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Despres JP. Effects of Rimonabant in the reduction of major cardiovascular risk factors. Results from the STRATUS-US trial (smoking cessation in smokers motivated to quit). American College of Cardiology 53rd Annual Scientific Session; New Orleans, LA, USA. 2004. [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous acces to cocaine. Psychopharmacology. 1999;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B. Reinstatement of nicotine-conditioned place preference by drug priming: effects of calcium channel antagonists. Eur J Pharmacol. 2006;537:85–93. doi: 10.1016/j.ejphar.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 1996;127:102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Aubin HJ, Dale LC, Niaura R, Anthenelli RM Group atS. Pooled analysis of three short-term, randomised, double-blind, placebo controlled trials with rimonabant 20 mg/d in smoking cessation. 8th Annual Conference of the SRNT Europe; Kusadasi, Turkey. 2006. [Google Scholar]
- Cohen C, Kodas E, Griebel G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol Biochem Behav. 2005;81:387–395. doi: 10.1016/j.pbb.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-Associated Cues Maintain Nicotine-Seeking Behavior in Rats Several Weeks after Nicotine Withdrawal: Reversal by the Cannabinoid (CB(1)) Receptor Antagonist, Rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Dale L. Efficacy of rimonabant in smoking cessation and prevention of post-cessation weight gain: the STRATUS-US trial. 2004 Annual Meeting of the North American Association for the Study of Obesity (NAASO); Las Vegas, Nevada, USA. 2004. [Google Scholar]
- Dale L, Anthenelli R, Despres JP. Effects of rimonabant in the reduction of major cardiovascular risk factors: results from the STRATUS-US Trial (Smoking Cessation in Smokers Motivated to Quit) and the RIO-LIPIDS Trial (Weight Reducing and Metabolic Effects in Overweight/Obese Patients With Dyslipidemia). 53rd Annual Scientific Session of the American College of Cardiology (ACC); New Orleans, Louisiana, USA. 2004. Abstr. 409–401. [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–168. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacol. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. The health benefits of smoking cessation: a report of the Surgeon General. Washington, D.C.: Government Priting Office; 1990:90–8416. DHHS publication n.
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Fiore MC. US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care. 2000;45:1200–1262. [PubMed] [Google Scholar]
- Food and Drug Administration Endocrinologic and Metabolic Advisory. [assessed March 13, 2008];Briefing information, NDA 21–888 ZIMULTI (rimonabant)-Sanofi Aventis. 2007 June 13; http://wwwfdagov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounderpdf.
- Forget B, Barthelemy S, Saurini F, Hamon M, Thiebot MH. Differential involvement of the endocannabinoid system in short-and long-term expression of incentive learning supported by nicotine in rats. Psychopharmacology (Berl) 2006;189:59–69. doi: 10.1007/s00213-006-0525-x. [DOI] [PubMed] [Google Scholar]
- Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 2005;181:722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Stimuli associated with drug injections as events that control behavior. Pharmacol Rev. 1975;27:325–340. [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT, Morse WH. Second-order schedules of drug injection. Fed Proc. 1975;34:1771–1776. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav. 1983;19:1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive Ratio as a Measure of Reward Strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychol. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Taranabant cuts the fat: new hope for cannabinoid-based obesity therapies? Cell Metab. 2008;7:1–2. doi: 10.1016/j.cmet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol. 1988;7:575–589. doi: 10.1037//0278-6133.7.6.575. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Shumaker SA. Understanding the relations between smoking and body weight and their importance to smoking cessation and relapse. Health Psychol. 1992;11(Suppl):1–3. doi: 10.1037/h0090339. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dopamine, addiction and reward. Sem Neurosci. 1992;4:139–148. [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Francès H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005a;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005b;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005c;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology(Berl) 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Le Foll B, George TP. Treatment of tobacco dependence: integrating recent progress into practice. CMAJ. 2007;177:1373–1380. doi: 10.1503/cmaj.070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lindblom N, de Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration. 2002;69:254–260. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- Lu L, Xu NJ, Ge X, Yue W, Su WJ, Pei G, Ma L. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology (Berl) 2002;159:125–132. doi: 10.1007/s002130100885. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cue recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Niaura R. Long-term treatment with rimonabant for smoking cessation and the maintenance of abstinence: results from STRATUS-WorldWide trial. 11th Annual Meeting and the 7th Annual European Conference of the Society for Research on Nicotine and Tobacco (SRNT); Prague, Czech Republic. 2005. POS1-054. [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC. The role of the primate amygdala in conditioned reinforcement. J Neurosci. 2001;21:7770–7780. doi: 10.1523/JNEUROSCI.21-19-07770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: results from a national survey of women smokers. Nicotine Tob Res. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Gonzales D, Dale LG, Lawrence D Group ftCS. Efficacy and safety of adding a nicotine patch to rimonabant for smoking cessation: a randomized controlled trial. 2007 Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT); Austin, Texas, USA. 2007. Abstr. PA11–14. [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosecrans JA, Villanueva HF. Discriminative stimulus properties of nicotine: mechanisms of transduction. NIDA Res Monogr. 1991:101–116. doi: 10.1037/e496182006-007. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. Bmj. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Sorg BA. Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res. 2001;908:86–92. doi: 10.1016/s0006-8993(01)02638-5. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Dep. 1988;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-admininstration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;52:438–444. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology (Berl) 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Zubaran C, Stolerman IP. Antagonism of stimulus properties of nicotine by dihydro-beta-erythroidine (DHbetaE) in rats. Psychopharmacology (Berl) 2000;149:140–146. doi: 10.1007/s002139900348. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The Novel Cannabinoid CB(1) Receptor Neutral Antagonist AM4113 Suppresses Food Intake and Food-Reinforced Behavior but Does not Induce Signs of Nausea in Rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug-administration. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Survey CTUM. 2006 [Google Scholar]
- Tanda G, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common m1 opioid receptor mecanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tobacco Advisory Group of the Royal College of Physicians. Nicotine addiction in Britain A report of the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians of London; 2000. [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vemuri VK, Janero DR, Makriyannis A. Pharmacotherapeutic targeting of the endocannabinoid signaling system: Drugs for obesity and the metabolic syndrome. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Res. 1982;5:39–87. [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wu X, French ED. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]