SUMMARY
1. Artificial heart valves have been in use for over five decades to replace diseased heart valves. Since the first heart valve replacement performed with a caged-ball valve, more than 50 valve designs have been developed, differing principally in valve geometry, number of leaflets and material. To date, all artificial heart valves are plagued with complications associated with haemolysis, coagulation for mechanical heart valves and leaflet tearing for tissue-based valve prosthesis. For mechanical heart valves, these complications are believed to be associated with non-physiological blood flow patterns.
2. In the present review, we provide a bird’s-eye view of fluid mechanics for the major artificial heart valve types and highlight how the engineering approach has shaped this rapidly diversifying area of research.
3. Mechanical heart valve designs have evolved significantly, with the most recent designs providing relatively superior haemodynamics with very low aerodynamic resistance. However, high shearing of blood cells and platelets still pose significant design challenges and patients must undergo life-long anticoagulation therapy. Bioprosthetic or tissue valves do not require anticoagulants due to their distinct similarity to the native valve geometry and haemodynamics, but many of these valves fail structurally within the first 10–15 years of implantation.
4. These shortcomings have directed present and future research in three main directions in attempts to design superior artificial valves: (i) engineering living tissue heart valves; (ii) development of advanced computational tools; and (iii) blood experiments to establish the link between flow and blood damage.
Keywords: aortic, bileaflet, circulation, haemodynamics, heart valve, mechanical, mitral, prosthesis, trileaflet
INTRODUCTION
The heart is a muscular organ that pumps blood throughout the body. The contractions of the heart necessary to drive the blood are controlled by electrochemical impulses created by the pacemaker cells. These cells ensure rhythmic synchronized contractions of all cardiac muscles. The heart valves are the key components that turn the contracting chambers of the heart into pumps. Four heart valves, two on either side of the heart, ensure that each muscle contraction produces efficient, unidirectional flow. On the right side of the heart, the tricuspid and pulmonic valves regulate the flow of blood that is returned from the body to the lungs for oxygenation, whereas on the left side, the mitral and aortic valves control the flow of oxygenated blood to the body. For a detailed description of the physiology of a healthy heart, the reader is directed to the human physiology textbook by Silverthorn.1
The present article focuses on artificial heart valves that replace severely dysfunctional native valves. Since the first heart valve replacement in 1952, more than 50 prosthetic heart valve designs have been developed and nearly three million prosthetic heart valves have been implanted worldwide. Currently, over 290 000 heart valve procedures are performed annually worldwide and that number is estimated to triple to over 850 000 by 2050.2 Thus, the demand for artificial heart valves is expanding at a rate of 10–12% per year.3 Even though the first heart valve was designed by a surgeon, Dr Hufnagel, the science behind artificial heart valve function currently draws major branches of engineering and sciences together. In the present article, we present a bird’s-eye view of the fluid mechanics of artificial heart valves to the non-engineering readers and, in particular, attempt to highlight the engineering perspective towards the treatment of heart valve diseases. The article is intentionally non-technical from an engineering standpoint and is organized as follows: first we introduce basic measures of valve performance and a brief history of artificial heart valves. Second, we highlight the need to study fluid mechanics of heart valves as a necessary step for improving present-day prosthetic valves and then describe the fluid mechanics of major artificial heart valve types. Third, we provide the important directions present-day engineering approaches have taken to develop better artificial heart valves.
HEART VALVE PERFORMANCE MEASURES
One of the main afflictions of the cardiovascular system is heart valve disease, which is generally caused by congenital birth defects, ageing or diseases such as rheumatic fever. Such heart valve disease compromises the functionality of the valve by restricting the motion of the valve leaflets or by damaging its supporting structure. This leads to either valve stenosis (calcification of the leaflets associated with narrowing of the valve, resulting in greater resistance to blood flow and a greater cross-valvular pressure drop) or regurgitation (failure of the valve to close completely), both eventually leading to valve failure. Clinicians have therefore developed two parameters to quantify the degree of stenosis/regurgitation to better assess valve performance: (i) the effective orifice area (EOA), which is a measure of the effective valve opening during the forward flow phase; and (ii) the regurgitant volume, which is a measure of the back flow (or regurgitation) during the leakage flow phase.
A low EOA, commonly resulting from valve stenosis, is usually associated with a higher net blood pressure loss across the valve and therefore an increased workload for the pumping heart. The EOA is traditionally computed from measured flow and pressure drop using the Gorlin relation, which is based on the principle of energy conservation:
where Qrms is the root mean square systolic/diastolic flow rate (cm3/s) and Δp̄ is the mean systolic/diastolic pressure drop (mmHg).
Conversely, the regurgitant volume corresponds to the total volume of fluid that leaks back across after closure and is related to valve shape and leaflet closing dynamics. A high regurgitant volume indicates that the net cardiac output is reduced and the heart has to contract more to meet the demands of the body. Thus, a small regurgitant volume is preferable because it indicates a good coaptation of the valve.
More recently, turbulence stress levels, which are a surrogate measure of the shear stress experienced by blood cells and platelets in a turbulent flow environment, have been used to assess the potential of the valves towards causing thromboembolic complications. Turbulent stress levels from 10 to 100 Pa are considered to trigger platelet activation,4,5 with a more precise threshold known as Hellums criteria, which states that platelets will activate if the product of shear stress and its time duration is above 3.5 Pa.6 However, the threshold for haemolysis is much higher at 800 Pa.7 Note, the values cited in this paper are all in vitro measurements because these measures have not been quantified in vivo.
BRIEF HISTORY OF ARTIFICIAL HEART VALVES
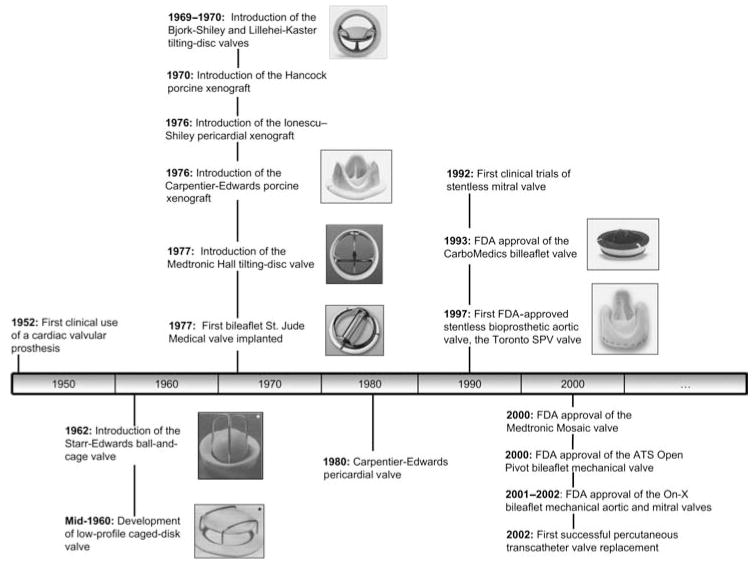
Artificial heart valves are divided in two major types, mechanical heart valves and bioprosthetic heart valves. Until recently, heart valve replacement was commonly performed using mechanical heart valves; however, bioprosthetic heart valves are currently implanted just as frequently as mechanical heart valves owing to their resemblance to the native valve and their freedom from anticoagulant therapy. Figure 1 shows the history of artificial heart valves from the 1950s to the present day, focusing on the most commonly used heart valves and the valves that have made notable contributions to heart valve replacement. A thorough description of the development of heart valve design may be found in the literature.8
Fig. 1.
Towards the ideal prosthetic heart valve. Timeline of significant milestones in the history of prosthetic heart valve development. (*Image from Ramstack et al.;5 all other valve images are obtained from manufacturers’ websites: http://www.edwards.com, http://www.medtronic.com and http://www.sjm.com, www.carbomedics.com).
Mechanical heart valves
The first heart valve replacement was performed in 1952 by Hufnagel, with the so-called Hufnagel caged-ball valve. The valve was not implanted in place of the diseased aortic valve; rather, it was inserted in the descending aorta in an open-chest procedure. The development of the heart valve design was fuelled by the introduction of the heart–lung bypass machine, which was first used successfully on humans in 1953 to perform an atrial septal repair. The use of the Hufnagel caged-ball valve became obsolete with the introduction of the Starr–Edwards ball-and-cage valve in 1962. The design of the ball-and-cage valve consists of a ball within a metal cage. The ball occludes the valve orifice and thus passively prevents backflow. During the forward flow phase, the high blood pressure pushes the ball downstream, thus opening the orifice, with the metal cage holding the ball distal to the orifice. Since 1962, the Starr–Edwards valve has undergone many modifications to improve its performance; however, the changes have focused on materials and construction techniques and have not altered the overall concept of the valve design. The ball-and-cage design, although not the most popular today, remains the valve of choice for some surgeons.
Similar to the ball-and-cage valves, caged-disc valves were developed in the mid-1960s. Examples of the caged disc designs are the Kay–Shiley and Beall prostheses, which were introduced in 1965 and 1967, respectively. The design of these types of valves is similar to that of the ball-and-cage valves, except that the valve occluder is no longer a ball, but a disc. However, owing to their inferior haemodynamic characteristics, caged-disc valves are not implanted anymore.
These two early artificial valve designs have two major drawbacks, namely a high-profile configuration and excessive occluder-induced turbulence in the flow through and distal to the valve, thus reducing the valve EOA. Such drawbacks have been overcome by the tilting disk and bileaflet heart valve designs.
The most significant development in mechanical heart valve design occurred in 1969 and 1970 with the introduction of the Bjork–Shiley and Lillehei–Kaster tilting-disc valves. These two tilting-disc valve designs are composed of a disc and a retaining strut mechanism. The disc totally occludes the valve orifice in the closed position and tilts to an angle in the open position. The disc can rotate during normal function, thus preventing excessive contact wear from the retaining strut components on one particular region of the disc. Design improvements made in subsequent generations of tilting disc valves have focused on the disc-retaining system, the disc tilting angles during both the open and closed phases and disc geometry. For example, the Björk–Shiley design is characterized by a convexo-concave disc, whereas the Medtronic Hall valve includes a flat disc.
Most recent mechanical heart valve designs are based on the bileaflet mechanical heart valve designed by St Jude Medical (SJM) Inc. (Minneapolis, MN, USA) and introduced in the market in 1978. This valve is composed of two semicircular occluders called leaflets. Within the annular housing, each leaflet has an extension region, known as leaflet ear, which pivots about a recessed hinge. When the leaflets are open, the blood flows through a central rectangular orifice and two semicircular lateral orifices. When the leaflets are fully closed, small gaps between the closed leaflets (b-datum gap) and between the leaflets and the housing (periphery gap) allow some degree of leakage flow. Leakage blood flow may also occur through the hinge region.
The introduction of the first bileaflet mechanical heart valve was associated with a major breakthrough: the identification of pyrolytic carbon as a suitable valve material by Bokros and Gott in 1966.8 This material has now become the material of choice to design mechanical heart valves because of its superior biocompatible, thromboresistant and wear resistant properties compared with previously used metals and plastics.
Since the introduction of SJM bileaflet valve, numerous other attempts have been made to improve upon that bileaflet valve design. For example, the CarboMedics (Sorin Group, Austin, TX, USA) bileaflet prosthesis, which gained US Food and Drug Administration (FDA) approval in 1993, differs from the SJM bileaflet heart valve by the opening angle of its leaflet and the shape of the hinge region (which has sharper features than the hinge region of the SJM valve). The ATS Open Pivot valve (Minneapolis, MN, USA), introduced in 2000, also features a change in the pivot design. This design inverts the traditional pivot mechanism by exposing the pivot to the bulk forward flow and using a protruding rather than a recessed hinge design. The On-X valve, marketed by the Medical Carbon Research Institute (Austin, TX, USA), is the most recent mechanical valve design introduced in the US. Some of the innovative features of this valve are a length-to-diameter ratio close to that of native heart valves, a smoothed pivot recess that allows the leaflets to open at an angle of 90° relative to the valve housing and a two-point landing mechanism during valve closure. The bileaflet mechanical heart valve is, at present, the most popular mechanical design and accounts for approximately 80% of implanted mechanical valves.
Bioprosthetic heart valves
Despite their widespread use, mechanical heart valves are plagued by the need for life-long anticoagulation therapy and the accompanying bleeding problems. An alternative approach in the development of prosthetic heart valves has been to use naturally occurring heart valves. This led to the approach of using the patient’s own pulmonary valve to replace the aortic valve (the so-called Ross procedure, first performed in 1967), followed by the use of a homograft to function as the pulmonary valve. Although this approach is primarily associated with aortic valve replacement, it has also been performed to replace diseased mitral valves. Another approach consists of using antibiotic- or cryo-treated human aortic valves (called homografts) for implantation in place of a patient’s own diseased valve. Such a procedure, first performed in 1962, is not yet ideal, despite the satisfactory clinical outcomes, because it depends on the cadaveric valve supply.
A major breakthrough in tissue valve substitutes was made by Carpentier in 1969 with the development of a tissue-fixation procedure using glutaraldehyde, which resulted in increased biological tissue stability and a decrease in biodegradation.10 This improvement in tissue preservation, which provided relatively inert biological tissue, and the use of flexible valve stents allowed for the development of a new valve design for cardiac valve replacement. Such a valve design, combining biological and mechanical structures to create a tissue-based valve with low thrombogenicity, was named ‘bioprothesis’ by Carpentier.11 Several tissues types, mainly porcine valve and pericardium tissues, have been used since then to design bioprosthetic heart valves.
Porcine valves
In 1969, Kaiser et al.12 described a valve substitute consisting of an explanted glutaraldehyde-treated porcine aortic valve mounted on a rigid support frame. This rigid frame was eventually replaced by a rigid ring with flexible posts and the resulting valve became commercially available as the Hancock Porcine Xenograft (Medtronic, Irvine, CA, USA) valve in 1970. Today, this valve remains one of the most popular valve substitutes of this type. Another popular valve is the Carpentier-Edwards Bioprosthesis (Edwards Lifesciences, Irvine, CA, USA). This valve, introduced commercially by Edwards Laboratories in 1976, uses porcine valve tissue mounted on a totally flexible support frame. The use of intact, biologically formed valves in porcine prostheses eases the production process by alleviating the need to manufacture individual valve cusps. However, a large quantity of porcine valves has to be harvested in order to produce prosthetic valves of appropriate size and suitable quality.
Pericardial valves
Valves based on calf pericardium, developed by Ionescu et al.,13 overcome the large demands associated with porcine valves. The original Ionescu–Shiley pericardial xenograft valve was first marketed in 1976, but was discontinued in the mid-1980s owing to structural failure. The Carpentier–Edwards pericardial valve was introduced to clinical use in 1981 and approved for commercial use in the US in 1991. It provides superior haemodynamic performance with better durability than previous pericardial valves.14 The latest bioprosthetic valve approved by the US FDA, in 2007, is the Mitroflow valve by Sorin Group Canada Inc. (Burnaby, BC, Canada).
A recent major change in tissue valve design is the introduction of stentless bioprostheses. Tissue valves with no stent are believed to be better than stented valves because the aortic root is expected to distribute mechanical stresses into the aortic tissue instead of focusing them on the leaflet tissue near the stents of traditional bioprostheses. The first stentless heart valve, the Toronto SPV valve (St Jude Medical), was approved by the US FDA in 1997. Two other stentless aortic valve designs have since then been brought to the market: (i) the Medtronic Freestyle (Medtronic Inc.); and (ii) the Edwards Prima (Edwards Lifesciences) valves. Stentless bio-prostheses are currently only approved for aortic valve replacements in the US. Clinical trials with stentless mitral valves were first reported in 1992,15 but their approval has been delayed because of questionable valve durability and the complexity of the implantation technique.
The latest major breakthrough pertains to the development of percutaneous bioprosthetic heart valves. The first deployment of a porcine heart valve contained within a stent using a percutaneous approach in an animal heart was achieved by Andersen et al. in 1992.16 The first human implant was performed 9 years later by Bonhoeffer et al.17 In this procedure, a valve was implanted, in the pulmonary position, via surgically placed conduits. The first successful human percutaneous transcatheter implantation was reported in 2002 by Cribier et al.18 The valve deployed was an equine pericardium tissue heart valve, which was placed in the aortic position. The future of percutaneous heart valve replacement depends on advances in biomaterials, anticalcification treatment and the development of collapsible and compressible valve–stent devices for optimal valve delivery and deployment.
HEART VALVE FLUID MECHANICS
Despite the widespread use of artificial heart valve designs, neither mechanical nor bioprosthetic heart valves are free from complications. The overall complications associated with prosthetic heart valves can be divided into six main categories: structural valvular deterioration, non-structural dysfunction, valve thrombosis, embolism, bleeding and endocarditis.19 On the one hand, bioprosthetic heart valves are plagued with leaflet calcification and leaflet tearing. On the other, mechanical heart valves are associated with haemolysis, platelet activation and thromboembolic events arising from clot formation and their subsequent detachment.
These complications are believed to be associated with non-physiological blood flow patterns in the vicinity of heart valves. In fact, the potential of abnormal flow patterns to promote blood cell damage has long been recognized, because they may initiate thrombus formation by: (i) imposing forces on cell elements (regions of high shear stress cause tearing of the blood elements, thus leading to haemolysis and platelet activation); and (ii) changing the frequency of contact (recirculation and flow stagnation regions increase the contact time between blood elements, in particular activated platelets, thereby promoting thrombus formation). In addition, these abnormal flow patterns may induce leaflet calcification and tearing in tissue and polymeric valves by creating elevated regions of shear in the immediate vicinity of the leaflet surfaces.
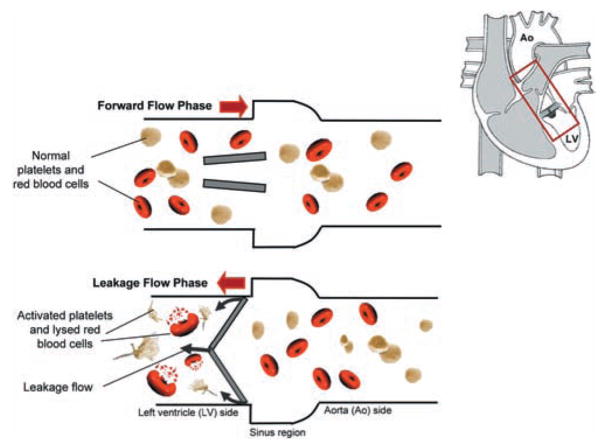
Figure 2 illustrates what is hypothesized to be the problem with artificial heart valves. Shown is a bileaflet mechanical heart valve in the aortic position during the leakage flow phase. As mentioned previously, bileaflet heart valves in the closed position are not perfectly sealed and leakage flow may occur. Figure 2 depicts red blood cells and platelets leaking back from the aorta into the left ventricle. While flowing through the valve prosthesis these cells are subjected to non-physiological flows, leading to rupture or activation, the first step towards initiation of the coagulation response. It is therefore clear that the EOA and the regurgitant volume alone do not sufficiently reflect the potential of artificial heart valves in inducing thrombus formation and thus the clinical performance of the valve. Hence, it is essential to assess the fluid mechanics of prosthetic heart valves to understand clinical valve successes or failures and improve the designs of these devices by minimizing the procoagulant potential and increasing valve durability.
Fig. 2.
Schematic of a bileaflet mechanical heart valve implanted in the aortic position during the leakage flow phase. Shown are the blood cells damaged from the high shear environment experienced within the leakage gaps (not to scale). Top panel: forward flow phase; bottom panel: leakage flow phase. Grey arrows, leakage flow; red arrows, bulk flow direction; Ao, aorta; LV, left ventricle.
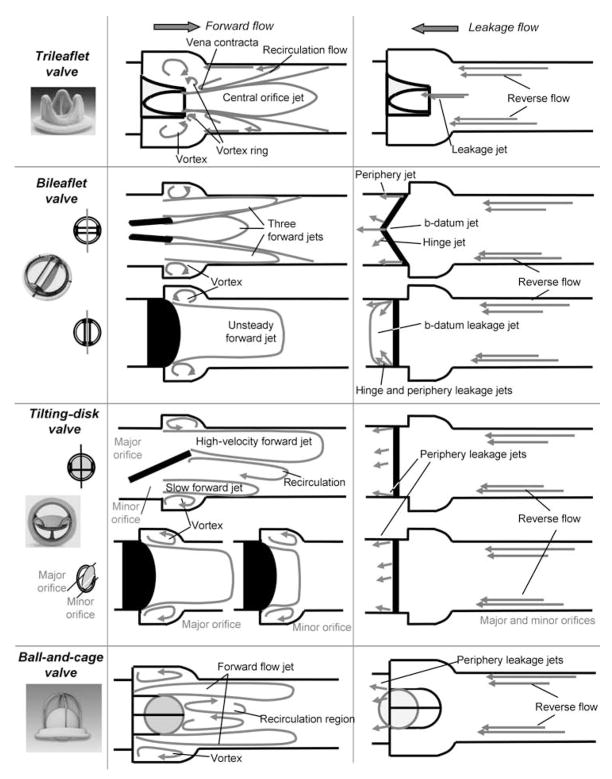
Therefore, the following paragraphs concentrate on the fluid mechanics of major prosthetic heart valves in the scope of establishing a relationship between fluid mechanics and valve success rate. The focus will be placed on the ball-and-cage valve, the tilting-disc valve, the bileaflet mechanical heart valve and the trileaflet valve. The flow fields downstream of these four valve designs during both the forward and leakage flow phases are shown in Fig. 3.
Fig. 3.
Flow fields downstream of selected valve designs during the forward flow phase (left) and the leakage flow phase (right).
Ball-and-cage valve
During the forward flow phase, the flow emerging from the valve forms a circumferential jet that separates from the ball, hits the wall of the flow chamber and then flows along the wall. At peak forward flow, a maximum velocity as high as 2.20 m/s was reported near the annulus in this forward flow jet under aortic conditions.20,21 This velocity decreases to 1.80 m/s 30 mm downstream of the valve. Immediately downstream of the apex of the cage, a wake develops and a region of low-velocity recirculating flow is present throughout the forward flow phase. A region of high-velocity gradient, and thus of high shear, exists at the edge of the forward flow jet and the recirculation region. A maximum turbulent shear stress up to 1850 dyn/cm2 was measured in this region.20,22 Turbulent shear stresses reach as high as 3500 dyn/cm2 in the annular region between the flow channel wall and the ball.20,22 Such high shear levels are clearly above the established thresholds to activate platelets.4–6 The activated platelets eventually get trapped in the recirculation region, where platelet-to-platelet contact is enhanced and thus where thrombus formation may occur. This is confirmed by clinical results that revealed the presence of thrombi at the apex of the cage.22
During the leakage flow phase, the ball moves back on the valve seat, but a small gap may form, thus permitting a mild regurgitation. Such regurgitant flow is believed, but not proven, to be beneficial by dislodging thrombi. However, regions of elevated shear may exist at the edges of the leakage jets, thus promoting platelet activation.
Tilting-disc valve
In the open position, the disc tilts to form a major orifice and a minor orifice for the blood to flow. A large forward flow jet emanates from the major orifice, whereas a smaller jet of lesser velocity magnitude emanates from the minor orifices. These two jets of different velocity magnitudes induce a recirculation in the wake of the disc. A recirculating flow pattern forms in the sinus region. Studies report that in the major orifice region, high turbulent shear stresses are confined to narrow regions at the edges of the major orifice jet.9,23 Maximum turbulent shear stresses measured at peak systole are in the order of 1500 dyn/cm2.9,23 High turbulent shear stresses are more dispersed in the minor orifice than those in the major orifice region.
During the closed phase, the tilting disc moves back and seats on the valve housing to occlude the valve orifice; however, a small gap may be present at the periphery of the disc, thus permitting a small amount of flow regurgitation. Moreover, tilting-disc valve designs include a retaining mechanism for the disc. This mechanism, in some designs, requires the presence of a hole in the centre of the tilting disk. In such a design, blood may flow through the orifice in the centre of the disc during valve closure. As highlighted for the ball-and-cage valve, the flow through such gaps may be hypothesized to be associated with regions of elevated shear on the edge of the leakage jets, thus promoting blood damage.
Bileaflet valve
As mentioned earlier, the open leaflets divide the area available for the flow into three regions: two lateral orifices and a central orifice. The major part of the forward flow emerges from the two lateral orifices. Measurements along the centreplane of the valve 8 mm distal to the valve annulus indicate that the velocity in the lateral orifices (2.2 m/s) is higher than in the central orifice (2 m/s).9 Two recirculation regions are seen in the sinus region of the aortic root. High turbulent shear stresses are present at locations of high-velocity gradients and at locations immediately distal to the valve leaflets. The flow becomes more disturbed as it travels further downstream of the valve. A close look at the vorticity contours (curl of the velocity field) indicates the formation of alternately rotating counter vortices in the wake of the leaflets at peak forward flow. This structure disappears during the deceleration phase and is replaced by a highly chaotic flow field.24 The peak turbulent shear stresses measured along the centreline plane at peak systole are in the order of 1500 dyn/cm2 downstream of the valve.9
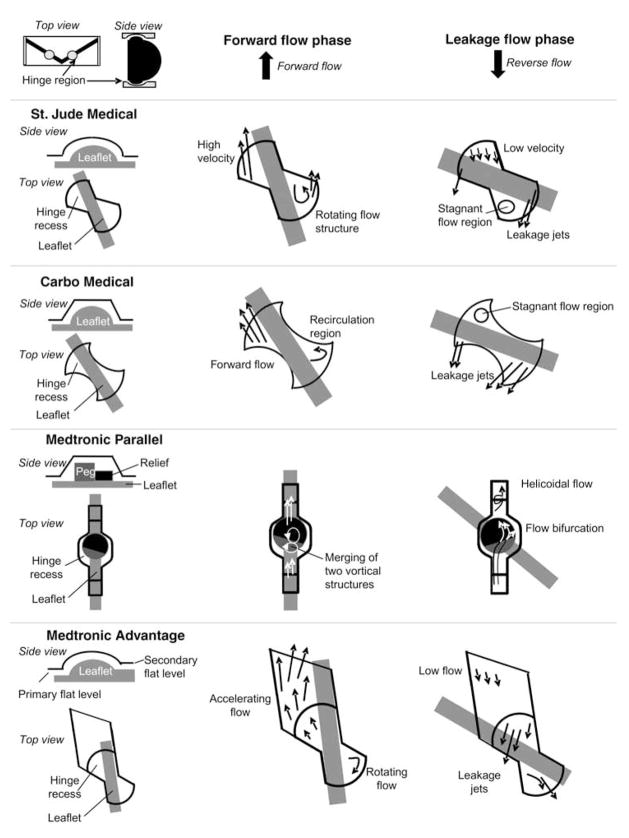
During the leakage flow phase, the leaflets rotate to occlude the valve orifice. However, the design of bileaflet mechanical heart valves includes some degree of leakage flow through the b-datum gap (the line where the two leaflets touch each other) and the periphery gaps, but occurs mainly in the gap present in the hinge region. The high pressure difference across the closed valve causes fast-moving hinge leakage flow that eventually emerges on the upstream end of the valve as narrow jets. The flow through the hinge region during the closed phase was originally designed to wash the hinges of the valve, thus preventing areas of flow stasis and inhibiting micro-thrombus formation. However, the magnitude of this retrograde flow has been shown to be detrimental to blood cells.25 Figure 4 shows the qualitative flow sketches during the forward and leakage flow phases within the hinge region of four bileaflet heart valves, namely SJM, CarboMedics, Medtronic Parallel and Medtronic Advantage, based on published data.26–35 Of these four designs, the Medtronic Parallel was discontinued owing to the observation of severe clot formations within the hinge region during clinical trials. All the hinge flow fields are characterized by the presence of recirculation regions during the forward flow phase. Such recirculating flow regions are the primary locations where clots begin to form by increasing cell-to-cell contact time. Therefore, it is important to note that the hinge design plays an essential role in thrombus formation. It is interesting to note that a highly convoluted flow, which could be associated with an elevated potential for thrombus formation, exists in the hinge region of the Medtronic Parallel hinge during the closed phase, whereas a more streamlined flow is present in the other hinges.
Fig. 4.
Complexity of the flow fields in four recessed hinge designs.
Trileaflet valve
Bioprosthetic heart valves are composed of three leaflets that open to form a central orifice for the blood to flow through, thus closely resembling the native trileaflet aortic valve. A jet-like flow emerges from the open leaflets during the forward flow phase. The jet is characterized by a vena contracta immediately downstream of the valve, followed by an expansion region. Velocities as high as 3.7 m/s have been reported downstream of a porcine heart valves at peak forward flow phase.9 Slower velocities, in the order of 1.75 and 1.7 m/s, were reported during the acceleration and deceleration phase, respectively.9 The forward flow jet is surrounded by counter-rotating recirculation regions. High turbulent shear stresses occur at the edge of the jet. Mean and maximum turbulent shear stresses of up to 2000 and 4500 dyn/cm2, respectively, have been measured at peak systole.9 During the closed phase, the leaflets coapt to prevent regurgitation; however, leakage flow may emanate from the centre of the valve if the leaflets do not close properly. Similar flow patterns have been reported33,36 throughout the cardiac cycle downstream of trileaflet polymeric heart valves, which are used in artificial hearts but not yet as heart valve substitutes.
Although the general flow characteristics of all bioprosthetic valves are the same, differences do exist on a valve-to-valve basis because of subtle differences in valve design, such as the height of the valve, the material (porcine, pericardial etc.) and stent characteristics (stented vs stentless valves). Of all the bioprosthetic valves, stentless valves have superior performance with the largest EOAs. Among the stented valves, pericardial stented valves are known to have larger EOAs than porcine bioprostheses.37
CURRENT FLUID MECHANICS RESEARCH AND FUTURE DIRECTION
As highlighted in the previous section, the performance of artificial heart valves is closely governed by the fluid mechanics within these valves, which, in turn, is strongly related to the geometry, material and mechanism of the valve design. The obvious engineering challenge is to design an optimal heart valve. Such a challenge is currently being pursued in three main directions. In the first direction, researchers have taken a step back from the concept of artificial devices and have taken the route of engineering a living tissue valve with as many characteristics of the native heart valve as possible. In the second direction, engineers are developing advanced computational fluid dynamics (CFD) tools to accurately predict the fluid mechanics in the vicinity of heart valves down to the resolution of individual blood cells. Finally, in the third direction, research is being conducted to pinpoint the exact coagulation mechanisms triggered by haemodynamics in heart valves using ex vivo and in vitro experiments, thus opening avenues to improve on existing designs based on direct coagulation measures.
Tissue-engineered heart valves
Recent advances in tissue engineering have spurred the interest in heart valve tissue engineering. The success of a tissue-engineered heart valve (TEHV) relies heavily on determination of the optimal scaffold material, cell source and in vitro preconditioning settings.
Current scaffold technologies used for TEHVs consist of biological and synthetic scaffolds, as reviewed extensively by Mol et al.,38 summarized in this paragraph. Biological scaffolds include decellularized allograft and xenograft valves, autologous collagen and autologous fibrin gel. The concept of a decellularized valve originated from the need for removing the immunogenic cellular components while retaining the essential extracellular matrix (ECM) proteins that can serve as an intrinsic template for cell attachment. The maintenance of mechanical properties depends on the decellularization technique and the degree of cross-linking. In general, non-fixed acellular allograft valve leaflets have been shown to promote remodelling of the prosthesis by inducing neovascularization and recellularization by the host and to possess sufficient mechanical integrity to withstand physiological conditions after implantation, even in the aortic position. Although acellular matrices as a scaffold material may be promising for future clinical use, important disadvantages include the infectious risk when using animal-derived materials, as well as immunological complications. In fact, the first clinical application using a decellularized xenograft composite aortic valve in children showed rapid failure of heart valves due to severe foreign-body reactions associated with a 75% mortality rate.39 To create a TEHV that is completely autologous, and thus free of the complications seen in the acellular grafts, the scaffold material must degrade in conjunction with the development of autologous tissue.
Two types of biodegradable biomaterials have been used for TEHVs: collagen and fibrin. Collagen as a scaffold material has been made into various forms: foam, gel, sheet or a sponge.40,41 Collagen is very difficult to obtain from the patient; most collagens are of animal origin. Owing to the slow degradation of collagen, scaffold materials will still be present at the time of implantation and will present risks of immune response or inflammation. Fibrin42,43 can be obtained from the patient’s blood and therefore is not expected to initiate an immune response from the patient. In addition, the degradation of fibrin can be controlled. As a scaffold material, fibrin has disadvantages, including low diffusion capacity and poor overall mechanical properties. Synthetic materials that have been tested for TEHV scaffold include polyglycolic acid (PGA), polylactic acid (PLA) and PLGA (PGA-PLA copolymer), which all belong to the aliphatic polyester family, and polyhydroxyalkanoates (PHAs).44–47 The major limitations of the aliphatic polyesters are their thickness, initial stiffness and non-pliability, making the fabrication of a trileaflet valve a difficult process. The PHAs are thermoplastic and can be easily moulded into any desired shape; however, they do have the drawback of having a slow degradation rate.
Cells that have been used for TEHVs include dermal fibroblasts and vascular-derived cells. Vascular cells have been shown to be superior to dermal fibroblasts and constructs seeded with venous cells were superior to those seeded with arterial cells in terms of collagen formation and mechanical stability. Although autologous, venous cells represent the most promising cell source for clinical use in the near future. Other cells, including genetically engineered allogenic vascular cells and stem cells, are also being explored to alleviate the potential shortage of venous cells.
Despite the tremendous progress that has been made in scaffold technology and cell sourcing during the past few decades, a functional, stable TEHV that is competitive with current replacement valves is hampered by the inability to isolate the optimal preconditioning environment. Preconditioning is required because the newly formed cell-scaffold composites are generally too weak to withstand the in vivo mechanical loading conditions. This is especially true for the aortic position. Indeed, various studies have shown that mechanical preconditioning can accelerate tissue maturation, resulting in higher mechanical strength, as well as tissue morphology, that is closer to the native counterparts.48,49 Currently, there are a number of systems patented for heart valves: the Pearson system,50 which simulates the in vivo conditions for heart valves; the Goldstein system,51 which imitates the dynamic flow environment of the aorta; the Dumont system,52 which mimics the circulation system; and the Hoerstrup pulse duplicator.49 Although these systems all have their own advantages in TEHV preconditioning, the determination of optimal conditions remains superficial and is hampered by our lack of knowledge of heart valve mechanobiology. A thorough understanding is needed of the relationship between the mechanical and structural characteristics of a native valve and the biological and mechanical stimuli required to mimic these characteristics in vitro.
Computational fluid dynamics-engineered heart valves
Advanced computational tools will eventually give the engineer the ability to design new valves and assess the performance of these new valve prototypes virtually, thus limiting the need to perform extensive, costly and time-consuming in vitro and animal tests. The increase in computer power, the advances in parallel processing and the development of sophisticated computational fluid dynamics algorithms have now made it feasible to simulate the flow within artificial heart valves using advanced computational fluid dynamics solvers. In 2007, the first fully validated, three-dimensional resolved, large-scale flow field within the bileaflet valve geometry was computed.24
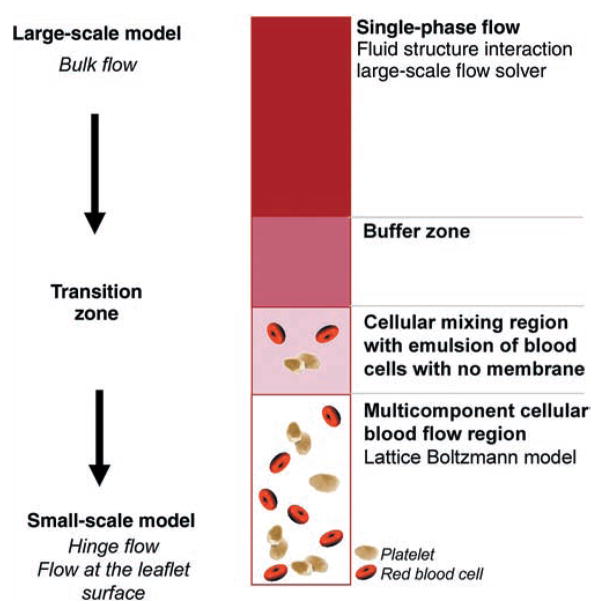
Research is currently moving to a multiscale approach where both the large-scale fluid mechanics (the bulk valvular flow field) and the small-scale fluid mechanics (such as the flow through the hinge region) can be resolved simultaneously. It is likely that within the next 5 years it will be possible to capture the entire range of fluid mechanics (from the blood cell level on the m scale to the valve level on the cm scale) and predict the mechanical loads on each individual blood cell that transits through the valve. A simulation that resolves all relevant length scales at the same time is critical because the small-scale structure of the flow is highly dependent on the multiphase properties of blood at the m length scale. This could be done by coupling a three-dimensional large-scale flow solver with a highly resolved small-scale solver deployed only on selected regions. Figure 5 shows a schematic of a proposed computational coupling scheme between a large-scale fluid-structure solver and a small-scale solver adjacent to the valve boundary. In this scheme, the large-scale solver resolves the coupled governing equations for mass and momentum balance for both the fluid phase and solid phase at a large scale (several hundred blood cells in length), whereas the small-scale solver uses a non-continuum formulation of blood to resolve the effects of discontinuities in fluid properties owing to the suspension of blood cells and platelets. A Lattice Boltzmann formulation53,54 used as the small-scale solver could provide a means to assess fluid forces on individual blood cells and obtain a new insight into the interaction of blood cells with the valve.
Fig. 5.
Schematic of a computational coupling scheme between a large-scale solver and a small-scale solver implemented at the hinge and leaflet surfaces.
Such advanced computational tools will eventually resolve all relevant length scales present in the blood flow in the heart. When developed and validated, these numerical tools will revolutionize current valve design and testing practices. These tools will be capable of yielding descriptions of the valve flow fields at a level of detail not currently accessible by experiments alone, leading to substantial time and cost savings during the research and development phase. Each new prototype could be tested numerically prior to any in vitro or animal testing, thus accelerating the process of valve optimization.
As mentioned earlier, previous studies have shown that high-flow shearing induces platelet activation and haemolysis, whereas regions of low flow promote thrombus formation. Therefore, it is of great importance to analyse the flow fields through the valve from the point of view of blood cells by performing a Lagrangian analysis. The flow environment experienced by blood cells as they are advected through the valve is what ultimately determines the rate of red blood cell lysis, platelet activation and deposition and the overall potential for thromboembolic complications. Thus, assessment of the likelihood that a particular artificial heart valve design may be prone to clinical complications requires knowledge of quantities such as exposure times to certain levels of shear stresses or residence times within recirculation regions. However, estimating these quantities cannot be done currently experimentally and only a numerical approach, using Lagrangian-tracking methods, may be used to assess the transport of platelets and red blood cells through artificial heart valves.
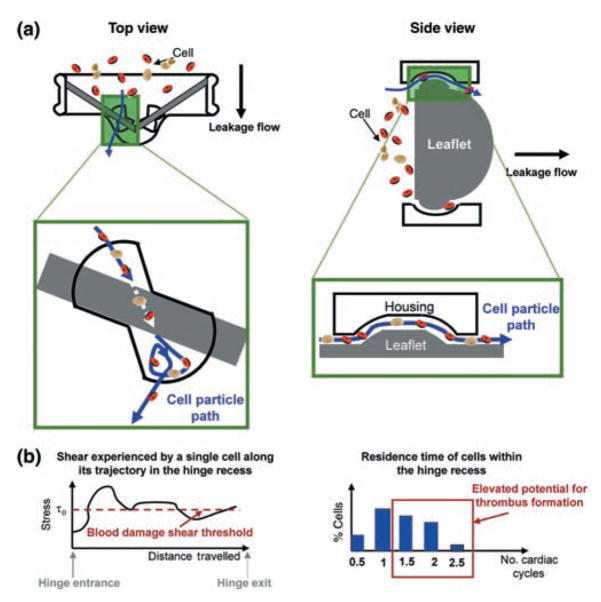
In these Lagrangian-tracking methods, trajectories of individual cells are computed via direct numerical integration, based on the velocity fields computed by a highly resolved computational solver. Such a numerical approach enables: (i) evaluation of the stress histories experienced by the cells transiting through critical regions, such as the hinge region, thus providing an insight into the potential for platelet activation and haemolysis; and (ii) estimation of the cell residence time, thus giving a surrogate measure of thrombus formation potential. Figure 6a shows a schematic of the cell trajectory through the hinge region. Once the trajectories are computed, thromboembolically relevant information, such as cell residence time and shear stress history, may be computed (Fig. 6b). This Lagrangian analysis could be used to assess the blood damage potential associated with alternative designs of the critical regions in a cost-effective and timely manner to eventually select a design where a maximum number of cells transiting the valve are not exposed to stresses above a blood damage shear threshold. Therefore, a Lagrangian approach, combined with a fully validated, accurate and efficient numerical solver, would eventually provide the necessary tool to identify the potential role of specific flow structures in inducing haemolysis, platelet activation and blood clot formation.
Fig. 6.
Schematic of (a) blood cell paths inside the hinge recess computed from coupled computational approaches and (b) stress environment experienced by a cell along its trajectory (left) and residence time of the cells within the hinge recess (right).
Coagulation experiments
The past 5 years have seen an increased number of blood experiments focused on quantifying shear-induced coagulation properties of heart valves. Novel ex vivo and in vitro methodologies have enabled the quantification of the thromboembolic potential of different heart valve and hinge designs.55–59 In these experiments, fresh citrated blood is used as the working fluid in physiological flow loops containing the test heart valve. Upon gradual recalcification, the coagulation properties of the blood are restored, with platelet activation and thrombin formation occurring in a valve-dependent manner. Platelet-specific assays, haemolysis assays and thrombin–antithrombin assays enable accurate quantification of the procoagulant potential of any cardiovascular device, including heart valves.60,61 Results have shown significant differences in the thromboembolic potential for various valves, with the Medtronic Parallel having the worst level (consistent with the recall of this valve during clinical trials associated with high levels of thrombus formation).57 Experiments also show that subtle differences in hinge geometries can impact the net thromoembolic potential of valves.55,57,59 Such biological experiments are crucial to fully understand the effect of non-physiological flow on blood damage and, once combined with computational methods, constitute the essential approach for valve design optimization.
CONCLUSION
In conclusion, a review of the fluid mechanics of artificial valves is presented with an emphasis on the engineering perspective. Heart valve fluid mechanics are design-specific phenomena that vary from valve to valve and are intimately related to the blood damage and coagulation potential. Thus, designing optimal artificial heart valves with reduced thromboembolic potential and better clinical outcomes requires a thorough understanding of the valvular flow fields. Such flow description can only be assessed with a highly engineering-intensive process, both tissue engineering as well as using state-of-the-art experimental and numerical methodologies. These methodologies are now within reach and we envision that, in the near future, superior valves will be designed with the use of multiscale computational approaches, fully validated with experimental measurements. This engineering approach in solving the valvular flow physics down to the blood cell scale will be used to assess the valve performance of new valve prototypes virtually, thereby leading to a considerable time and cost savings during the research and development phase.
References
- 1.Silverthorn DU. Human Physiology: An Integrated Approach. 4. Benjamin Cummings; San Francisco: 2006. [Google Scholar]
- 2.Yacoub N, Takkenberg J. Will heart valve tissue engineering change the world? Nat Clin Prac Cardiovas Med. 2005;2:60–1. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 3.Black MM, Drury N. Mechanical and other problems in artificial valves. In: Berry C, editor. Pathology of Devices, Vol. 84, Current Topics in Pathology. Springer-Verlag; Heidelberg: 1994. pp. 127–59. [DOI] [PubMed] [Google Scholar]
- 4.Wurzinger LJ, Opitz R, Wolf M, Schmidschonbein H. Shear induced platelet activation: A critical reappraisal. Biorheology. 1985;22:399–413. doi: 10.3233/bir-1985-22504. [DOI] [PubMed] [Google Scholar]
- 5.Ramstack JM, Zuckerman L, Mockros LF. Shear-induced activation of platelets. J Biomech. 1979;12:113–25. doi: 10.1016/0021-9290(79)90150-7. [DOI] [PubMed] [Google Scholar]
- 6.Hellums JD. 1993 Whitaker Lecture – Biorheology in thrombosis research. Ann Biomed Eng. 1994;22:445–55. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- 7.Lu PC, Lai HC, Liu A. Reevaluation and discussion on the threshold limit for hemolysis in a turbulent shear flow. J Biomech. 2001;34:1361–4. doi: 10.1016/s0021-9290(01)00084-7. [DOI] [PubMed] [Google Scholar]
- 8.Bokros JC, Gott VL, La Grange LD, Fadall AM, Vos KD, Ramos MD. Correlations between blood compatibility and heparin adsorptivity for an impermeable isotropic pyrolytic carbon. J Biomed Mater Res. 1969;3:497–528. doi: 10.1002/jbm.820030311. [DOI] [PubMed] [Google Scholar]
- 9.Chandran KB, Rittgers SE, Yoganathan AP. Biofluid Mechanics: The Human Circulation. CRC Press; Boca Raton: 2006. [Google Scholar]
- 10.Carpentier A, Lemaigre G, Robert L, Carpenti S, Dubost C, Gerbode F. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58:467–83. [PubMed] [Google Scholar]
- 11.Carpentier A. The concept of bioprosthesis. Thoraxchir Vask Chi. 1971;19:379–83. doi: 10.1055/s-0028-1099149. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser GA, Hancock WD, Lukban SB, Litwak RS. Clinical use of a new design stented xenograft heart valve prosthesis. Surg Forum. 1969;20:137–8. [PubMed] [Google Scholar]
- 13.Ionescu MI, Wooler GH, Smith DR, Grimshaw VA. Mitral valve replacement with aortic heterografts in humans. Thorax. 1967;22:305–13. doi: 10.1136/thx.22.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao GQ, Wu YX, Grunkemeier GL, Furnary AP, Starr A. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol. 2004;44:384–8. doi: 10.1016/j.jacc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Vrandecic M, Gontijo BF, Fantini FA, et al. Anatomically complete heterograft mitral valve substitute: Surgical technique and immediate results. J Heart Valve Dis. 1992;1:254–9. [PubMed] [Google Scholar]
- 16.Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial-heart valves: Description of a new expandable aortic-valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13:704–8. doi: 10.1093/oxfordjournals.eurheartj.a060238. [DOI] [PubMed] [Google Scholar]
- 17.Bonhoeffer P, Boudjemline Y, Qureshi SA, et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002;39:1664–9. doi: 10.1016/s0735-1097(02)01822-3. [DOI] [PubMed] [Google Scholar]
- 18.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation. 2002;106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 19.Grunkemeier GL, Anderson WN. Clinical evaluation and analysis of heart valve substitutes. J Heart Valve Dis. 1998;7:163–9. [PubMed] [Google Scholar]
- 20.Chandran KB, Khalighi B, Chen CJ. Experimental-study of physiological pulsatile flow past valve prostheses in a model of human aorta. 1. Caged ball valves. J Biomech. 1985;18:763–5. doi: 10.1016/0021-9290(85)90051-x. [DOI] [PubMed] [Google Scholar]
- 21.Figliola RS, Mueller TJ. Fluid stresses in vicinity of disk, ball, and tilting disk prosthetic heart-valves from in vitro measurements. J Biomech Eng Trans ASME. 1977;99:173–7. doi: 10.1115/1.3138265. [DOI] [PubMed] [Google Scholar]
- 22.Murphy DA, Levine FH, Buckley MJ, et al. Mechanical valves: A comparative-analysis of the Starr-Edwards and Bjork-Shiley prostheses. J Thorac Cardiovasc Surg. 1983;86:746–52. [PubMed] [Google Scholar]
- 23.Chandran KB, Cabell GN, Khalighi B, Chen CJ. Laser anemometry measurements of pulsatile flow past aortic-valve prostheses. J Biomechanics. 1983;16:865–73. doi: 10.1016/0021-9290(83)90011-8. [DOI] [PubMed] [Google Scholar]
- 24.Dasi LP, Ge L, Simon HA, Sotiropoulos F, Yoganathan AP. Vorticity dynamics of a bileaflet mechanical heart valve in an axisymmetric aorta. Physics Fluids. 2007:19. Article 067105. [Google Scholar]
- 25.Leo HL, Simon HA, Dasi LP, Yoganathan AP. Effect of hinge gap width on the microflow structures in 27-mm bileaflet mechanical heart valves. J Heart Valve Dis. 2006;15:800–8. [PubMed] [Google Scholar]
- 26.Shu MCS, Gross JM, O’Rourke KK, Yoganathan AP. An integrated macro/micro approach to evaluating pivot flow within the medtronic ADVANTAGE(™) bileaflet mechanical heart valve. J Heart Valve Dis. 2003;12:503–12. [PubMed] [Google Scholar]
- 27.Leo HL, He ZM, Ellis JT, Yoganathan AP. Microflow fields in the hinge region of the CarboMedics bileaflet mechanical heart valve design. J Thorac Cardiovasc Surg. 2002;124:561–74. doi: 10.1067/mtc.2002.125206. [DOI] [PubMed] [Google Scholar]
- 28.Ellis JT, Yoganathan AP. A comparison of the hinge and near-hinge flow fields of the St. Jude Medical Hemodynamic Plus and Regent bileaflet mechanical heart valves. J Thorac Cardiovasc Surg. 2000;119:83–93. doi: 10.1016/s0022-5223(00)70221-2. [DOI] [PubMed] [Google Scholar]
- 29.Ellis JT, Travis BR, Yoganathan AP. An in vitro study of the hinge and near-field forward flow dynamics of the St. Jude Medical(R) Regent(™) bileaflet mechanical heart valve. Ann Biomed Eng. 2000;28:524–32. doi: 10.1114/1.297. [DOI] [PubMed] [Google Scholar]
- 30.Healy TM, Fontaine AA, Ellis JT, Walton SP, Yoganathan AP. Visualization of the hinge flow in a 5: 1 scaled model of the medtronic parallel bileaflet heart valve prosthesis. Experiments Fluids. 1998;25:512–18. [Google Scholar]
- 31.Ellis JT, Healy TM, Fontaine AA, Saxena R, Yoganathan AP. Velocity measurements and flow patterns within the hinge region of a Medtronic Parallel(™) bileaflet mechanical valve with clear housing. J Heart Valve Dis. 1996;5:591–9. [PubMed] [Google Scholar]
- 32.Gross JM, Shu MCS, Dai FF, Ellis J, Yoganathan AP. Microstructural flow analysis within a bileaflet mechanical heart valve hinge. J Heart Valve Dis. 1996;5:581–90. [PubMed] [Google Scholar]
- 33.Leo HL, Simon H, Carberry J, Lee SC, Yoganathan A. A comparison of flow field structures of two tri-leaflet polymeric heart valves. Ann Biomed Eng. 2005;33:429–43. doi: 10.1007/s10439-005-2498-z. [DOI] [PubMed] [Google Scholar]
- 34.Simon HA, Dasi LP, Leo HL, Yoganathan AP. Spatio-temporal flow analysis in bileaflet heart valve hinge regions: Potential analysis for blood element damage. Ann Biomed Eng. 2007;35:1333–46. doi: 10.1007/s10439-007-9302-1. [DOI] [PubMed] [Google Scholar]
- 35.Simon HA, Leo HL, Carberry J, Yoganathan AP. Comparison of the hinge flow fields of two bileaflet mechanical heart valves under aortic and mitral conditions. Ann Biomed Eng. 2004;32:1607–17. doi: 10.1007/s10439-004-7814-5. [DOI] [PubMed] [Google Scholar]
- 36.Leo HL, Dasi LP, Carberry J, Simon HA, Yoganathan AP. Fluid dynamic assessment of three polymeric heart valves using particle image velocimetry. Ann Biomed Eng. 2006;34:936–52. doi: 10.1007/s10439-006-9117-5. [DOI] [PubMed] [Google Scholar]
- 37.Yoganathan AP, He ZM, Jones SC. Fluid mechanics of heart valves. Annu Rev Biomed Eng. 2004;6:331–62. doi: 10.1146/annurev.bioeng.6.040803.140111. [DOI] [PubMed] [Google Scholar]
- 38.Mol A, Bouten C, Baaiejns F, Zund G, Turina M, Hoerstrup S. Review article: Tissue engineering of semilunar heart valves: Current status and future development. J Heart Valve Dis. 2004;13:272–80. [PubMed] [Google Scholar]
- 39.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT™ in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–6. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 40.Rothenburger M, Volker W, Vischer J, et al. Tissue engineering of heart valves: Formation of a threedimensional tissue using porcine heart valve cells. ASAIO J. 2002;48:586–91. doi: 10.1097/00002480-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PM, Allen SP, Dreger SA, Yacoub MH. Human cardiac valve interstitial cells in collagen sponge: A biological three-dimensional matrix for tissue engineering. J Heart Valve Dis. 2002;11:298–306. [PubMed] [Google Scholar]
- 42.Ye Q, Zund G, Jockenhoevel S, et al. Scaffold precoating with human autologous extracellular matrix for improved cell attachment in cardiovascular tissue engineering. ASAIO J. 2000;46:730–3. doi: 10.1097/00002480-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Jockenhoevel S, Zund G, Hoerstrup S, et al. Fibrin gel: Advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19:424–30. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal C, Ray R. Biodegadable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–50. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Sodian R, Hoerstrup SP, Sperling JS, et al. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation. 2000;102(Suppl 3):III–22. 9. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- 46.Shinoka T, Ma P, Shum-Tim D, et al. Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation. 1996;94(Suppl):II-164–8. [PubMed] [Google Scholar]
- 47.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–50. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 48.Niklason L, Gao J, Abbott W, Hirschi K, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 49.Hoerstrup S, Sodian R, Daebtriz S, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102(Suppl III):III-44–9. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- 50.Pearson R, Bhandari R, Quirk R, Shakesheff K. Recent advances in tissue engineering: An invited review. J Long Term Eff Med Implants. 2002;12:1–33. [PubMed] [Google Scholar]
- 51.Goldstein S, Clarke D, Walsh S, Black K, O’Brien M. Transpecies heart valve transplant: Advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg. 1999;70:1962–9. doi: 10.1016/s0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- 52.Dumont K, Yperman J, Verbeken E, et al. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif Organs. 2002;26:710–14. doi: 10.1046/j.1525-1594.2002.06931_3.x. [DOI] [PubMed] [Google Scholar]
- 53.Ding EJ, Aidun CK. Cluster size distribution and scaling for spherical particles and red blood cells in pressure-driven flows at small Reynolds number. Phys Rev Lett. 2006:96. doi: 10.1103/PhysRevLett.96.204502. Article 204502. [DOI] [PubMed] [Google Scholar]
- 54.Aidun CK, Lu YN. Lattice Boltzmann simulation of solid particles suspended in fluid. J Statist Phys. 1995;81:49. [Google Scholar]
- 55.Fallon AM, Dasi LP, Marzec UM, Hanson SR, Yoganathan AP. Procoagulant properties of flow fields in stenotic and expansive orifices. Ann Biomed Eng. 2008;36:1–13. doi: 10.1007/s10439-007-9398-3. [DOI] [PubMed] [Google Scholar]
- 56.Fallon A, Marzec U, Hanson SR, Yoganathan AP. Thrombin formation in vitro in response to shear-induced activation of platelets. Thromb Res. 2007;121:397–406. doi: 10.1016/j.thromres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Fallon AM. PhD thesis. Georgia Institute of Technology; Atlanta, GA, USA: 2005. The development of a novel in vitro flow system to evaluate platelet activation and thromboembolic potential induced by bileaflet mechanical heart valve leakage jets. [Google Scholar]
- 58.Fallon AM, Shah N, Marzec UM, Warnock JN, Yoganathan AP, Hanson SR. Flow and thrombosis at orifices simulating mechanical heart valve leakage regions. J Biomech Engin Trans ASME. 2006;128:30–9. doi: 10.1115/1.2133768. [DOI] [PubMed] [Google Scholar]
- 59.Travis BR, Marzec UM, Leo HL, et al. Bileaflet aortic valve prosthesis pivot geometry influences platelet secretion and anionic phospholipid exposure. Ann Biomed Eng. 2001;29:657–64. doi: 10.1114/1.1385808. [DOI] [PubMed] [Google Scholar]
- 60.Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Steady flow in an aneurysm model: Correlation between fluid dynamics and blood platelet deposition. J Biomech Engin Trans ASME. 1996;118:280–6. doi: 10.1115/1.2796008. [DOI] [PubMed] [Google Scholar]
- 61.Bluestein D. Research approaches for studying flow-induced thromboembolic complications in blood. Expert Rev Med Devices. 2004;1:65–80. doi: 10.1586/17434440.1.1.65. [DOI] [PubMed] [Google Scholar]