Abstract
Objective
The incidence of heart attack and stroke undergo diurnal variation. Molecular clocks have been described in the heart and the vasculature; however it is largely unknown how the suprachiasmatic nucleus (SCN) entrains these peripheral oscillators.
Methods and Results
Norepinephrine and epinephrine, added to aortic smooth muscle cells (ASMCs) in vitro, altered Per1, E4bp4, and dbp expression and altered the observed oscillations in clock gene expression. However, oscillations of Per1, E4bp4, dbp, and Per2 were preserved ex vivo in the aorta, heart, and liver harvested from dopamine β-hydroxylase knockout mice (Dbh−/−) that cannot synthesize either norepinephrine or epinephrine. Furthermore, clock gene oscillations in heart, liver, and white adipose tissue phase shifted identically in Dbh−/− mice and in Dbh+/− controls in response to daytime restriction of feeding. Oscillation of clock genes was similarly preserved ex vivo in tissues from Dbh+/− and Dbh−/− chronically treated with both propranolol and terazosin, thus excluding compensation by dopamine in Dbh−/− mice.
Conclusions
Although adrenergic signaling can influence circadian timing in vitro, peripheral circadian rhythmicity is retained despite its ablation in vivo.
Keywords: circadian, catecholamines, norepinephrine, adrenergic, sympathetic
Circadian timing has evolved as an adaptation to regular and predictable changes in the environment associated with the alternation of day and night. Endogenously programmed rhythmicity permits temporal organization of physiological processes and behavior in relation to a cyclic environment.1 Heart rate and blood pressure exhibit time-dependent oscillations and the incidence of acute myocardial infarction, sudden death, and stroke all vary according to the time of day.2 It has been unclear whether these observations relate to the molecular clock or reflect a time-dependent alteration in exposure to environmental stress, assumption of the upright posture, and exercise, which themselves result in sympathoadrenal activation and an elevation of blood pressure. 3,4 The discovery of a circadian clock within the cardiovascular system5 added a layer of complexity to such considerations. Peripheral clocks may serve to amplify, dampen, or phase shift a peripheral rhythm and are likely to be synchronized by the master clock6 in the suprachiasmatic nucleus (SCN) by hormones,5,7 which may, in turn, be activated by environmental stimuli.
Components of the mammalian molecular clock include 3 Period proteins (Per1, Per2, and Per3), 2 Cryptochromes (Cry1 and Cry2), and 2 PAS helix-loop-helix proteins, Clock and Bmal1.8,9 Clock and Bmal1 heterodimerize to form an active transcription complex that leads to increases in Per and Cry. Complexes of Per and Cry proteins translocate to the nucleus to inhibit Clock:Bmal1 mediated transcription. Clock:Bmal1 heterodimers also stimulate the transcription of many other clock controlled genes which, in turn, influence functions external to the timing mechanism and mediate the output of the clock.10 Food intake, hormonal synthesis and release,11 endogenous regulation of thrombolysis,12 body temperature maintenance, and aspects of metabolism13 are among the rhythmic physiological processes influenced by the clock.
Molecular clocks are reset each day by external cues; the SCN is synchronized primarily through photic signals, whereas peripheral oscillators may be adjusted by neural, humoral, and food-induced signals. Peripheral clocks are capable of autonomous12,14 and centrally coordinated function. Aside from diurnal variation in cardiovascular and sympathoadrenal function, the SCN is linked to peripheral tissues via sympathetic nerves.15 In addition, exogenous norepinephrine and epinephrine (NE and E) have been shown to increase Per1 expression in the liver.16 Thus, catecholamines are potential signals from the master clock that might entrain the periphery, including the cardiovascular system. Here we show that although activation of discrete adrenergic receptors alters oscillatory gene expression induced in vascular smooth muscle cells in vitro, disruption of catecholamine biosynthesis by deletion of dopamine β-hydroxylase (DBH) or adrenergic receptor antagonism does not detectably phase alter peripheral oscillators in vivo.
Methods
Cell Culture
Human aortic smooth muscle cells (ASMCs; Cambrex) and mouse ASMCs harvested from C57BL/6J mice were synchronized with 50% fetal calf serum. Norepinephrine (NE), epinephrine (E), phenylephrine, isoproterenol, procaterol, CL316243, propranolol, ICI118551, and SR59230A were obtained from Sigma. HEAT was obtained from Tocris.
Taqman Polymerase Chain Reaction (PCR)
RNA isolated using RNeasy kits (Qiagen) was reverse transcribed, and subjected to qPCR using SyBr green detection (Applied Biosystems). Primer sequences are detailed in the supplemental methods section. Melting (dissociation) analysis was performed to ensure consistent target sequences were amplified. Relative quantitation was achieved using a standard curve for each transcript examined in each tissue.
Mouse Tissue Analysis
All mouse experiments were previously approved by the Institutional Animal Care and Use Committee. Dbh+/− and Dbh−/− males used were hybrids of C57BL/6J and 129/SvCPJ.17 Dbh+/− females were mated with Dbh−/− males and treated with 100 µg/mL each of phenylephrine and isoproterenol from embryonic day (E)8.5 to E16.5 and 2 mg/mL of L-threo-3,4-dihydroxyphenylserine(L-DOPS, Sumitomo Pharmaceuticals) from E16.5 to birth in the maternal drinking water to enhance fetal survival of the Dbh−/− mice. NE and E are not essential for survival postnatally, so litters were not treated after birth. Sex-matched littermate Dbh+/− mice were used as controls because tissue content of NE and E is normal in these mice, and no phenotypic differences have been observed in comparison with wild-type mice.18 Mice were acclimatized to a 12 hour:12 hour light:dark(LD) regimen for 2 weeks before being placed in total darkness (DD) for 24 hours before the first tissue harvest at 7 am (CT24). Aortae, heart, liver, white and brown adipose harvested were flash frozen in liquid nitrogen (LN2).
Restricted Feeding Experiments
Dbh−/− mice and littermate Dbh+/− controls were acclimatized to LD for 2 weeks before restricted feeding during the light period (7 am to 7 pm) commenced. After the 5th day mice were placed in DD for 24 hours before tissue harvests at CT24 and CT36. Food was withheld on the day of tissue collection.
Minipump Implantation
Wild-type C57BL/6J, Dbh−/−, and littermate Dbh+/− controls were anesthetized with an i.p. injection of pentobarbital 72 mg/kg. Two-week Osmotic minipumps were surgically implanted and 50 mg/mL propranolol, and 25 mg/mL terazosin was administered for 2 weeks at a flowrate of 0.5 µL/h. Mice were acclimatized to LD and then placed in DD on the 13th day, for 24 hours before the first tissue harvest. Terazosin administered at 12.5 µg/h was sufficient for complete inhibition of the acute blood pressure response to 10 µg/kg phenylephrine. Propranolol administered at 25 µg/h was sufficient for complete inhibition of contextual memory in a fear conditioning paradigm (supplemental Figure I, available online at http://atvb.ahajournals.org).
Results
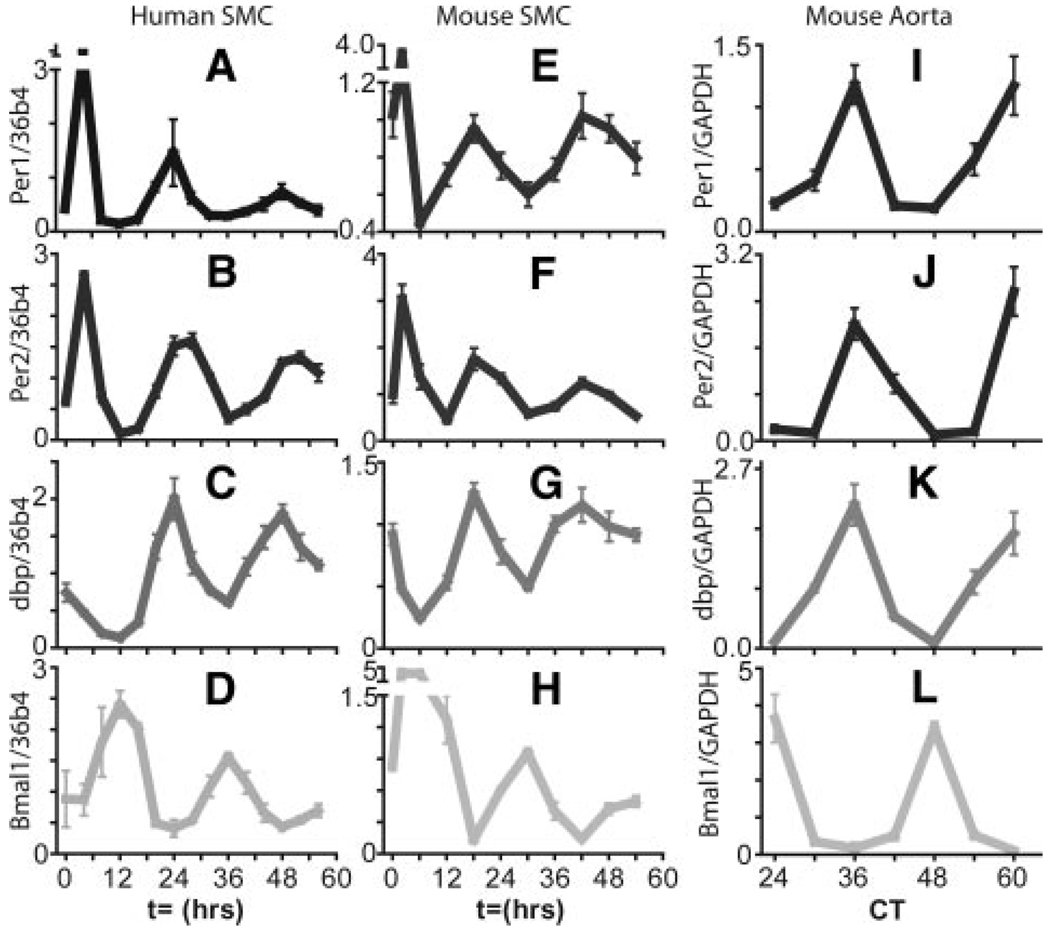
The mRNA expression of clock genes Per1, Per2, dbp, and Bmal1 all show a circadian pattern of expression in aortic smooth muscle cells (ASMCs) after treatment with 50% serum (Figure 1A through 1H). The expression of clock genes in the aorta of C57BL/6J mice show an identical relative pattern of expression with accumulation of Bmal1 mRNA antiphase to that seen for Per1, Per2, and dbp (Figure 1I through 1L).
Figure 1.
Accumulation of circadian clock gene transcripts in smooth muscle cells and mouse aorta. Human (A through D) or mouse (E through H) smooth muscle cells were synchronized with serum. Per1, Per2, Bmal1, and dbp expression was monitored by qPCR. I through L, Aortae harvested from wild-type C57BL6/J mice in constant darkness.
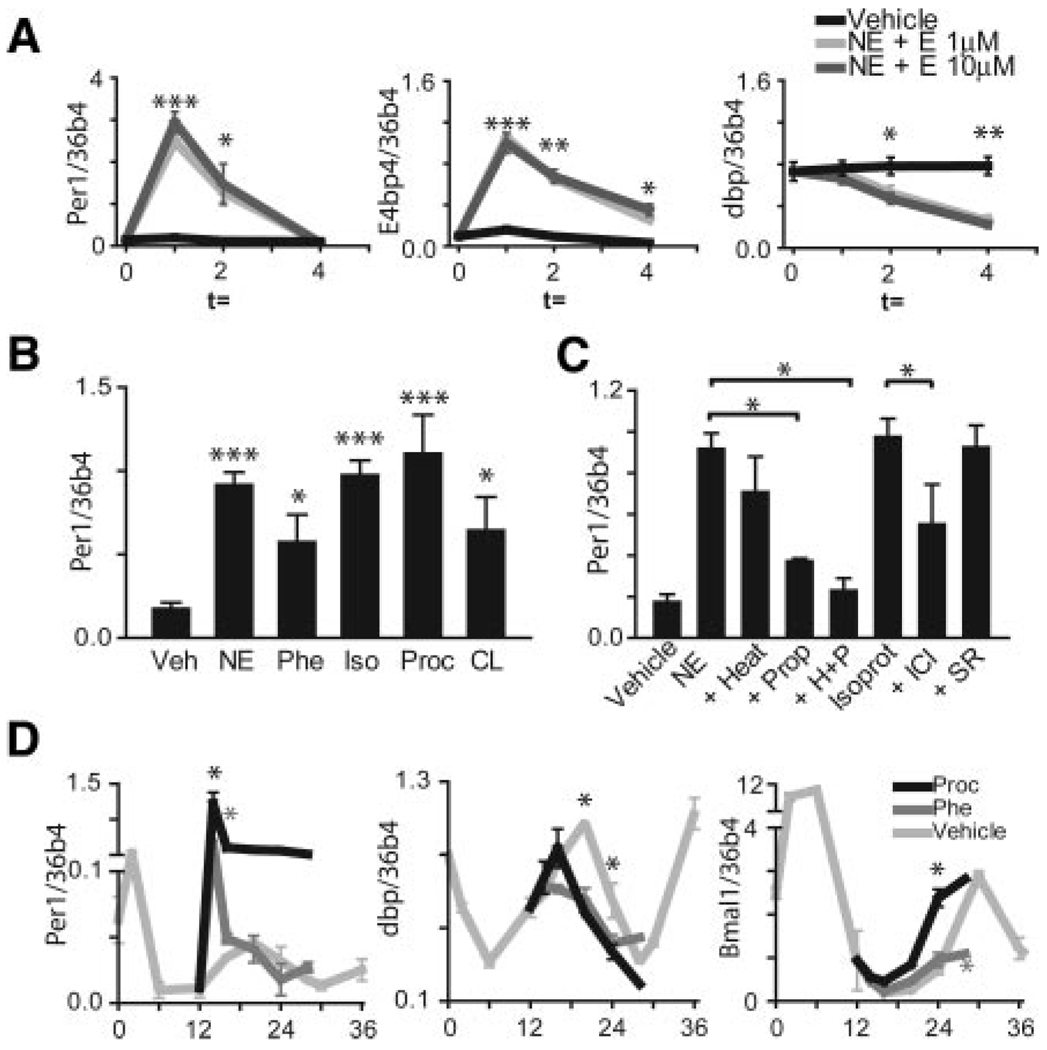
When added to murine ASMCs, NE and E at 1 µmol/L and 10 µmol/L increased Per1 expression at t = 1 hour, and significantly decreased dbp expression at t = 4 hours (Figure 2A). Increases in E4bp4 expression were also observed at both 1 µmol/L and 10 µmol/L NE and E. Pharmacological probes of α and β adrenergic receptor (AR) function were examined for their effects on expression of Per1, E4bp4, and dbp in unsynchronized mouse ASMCs (Figure 2B and supplemental Figure II). NE 1 µmol/L (or E 1 µmol/L-data not shown) alone can elicit a maximal response. Maximal responses were observed with the βAR agonist, isoproterenol (Iso), whereas a ≈60% maximal response was observed with the α1AR agonist, phenylephrine (Phe). We used specific agonists and antagonists of the β1, β2, and β3ARs (β1-xamoterol (Xam; data not shown), β2-procaterol(Proc), and β3-CL316243[CL]) to determine which βAR was mediating the response seen with isoproterenol. Significant increases in Per1 and E4bp4 mRNA levels were observed with procaterol, with a slight increase seen in response to CL316243.
Figure 2.
NE and E phase advance rhythms in mouse aortic smooth muscle cells. Unsynchronized mASMCs were treated with (A) NE and E, (B and C) NE, Phenylephrine, Isoproterenol, Procaterol, and CL316243. HEAT, propranolol, HEAT plus Propranolol, ICI118551, and SR59230A were preincubated for 30 minutes. D, Procaterol and phenylephrine were added to mASMCs at t = 12 hours after serum shock.
The NE response was partially inhibited by the α1AR antagonist HEAT and the βAR antagonist, propranolol (Prop; Figure 2C). HEAT plus propranolol combined inhibited agonist-induced changes in clock gene expression completely. Isoproterenol responses were then challenged with specific antagonists of β2 and β3ARs, ICI118551, and SR59230A, respectively. β2 antagonism caused the larger reduction in isoproterenol-mediated increases in Per1, dbp, and E4bp4. Regulation of clock gene expression by NE and E is mediated predominantly via β2ARs with a variable and lesser contribution from β1 and β3ARs.
Oscillating mouse ASMCs were stimulated with procaterol 1 µmol/L or phenylephrine 1 µmol/L at t = 12 hours after serum shock (Figure 2D). Either α1 or β2 stimulation induced a rapid increase in expression of Per1. Procaterol advanced the peak in dbp and Bmal1 expression. Phenylephrine blunted the increase in dbp expression to a greater extent, thus advancing the peak in dbp expression. Phenylephrine blunted the peak in Bmal1 mRNA accumulation at t = 30 hours after serum shock. In summary, catecholamines, acting via β2 and α1 ARs, can impact the oscillating expression of clock components and clock-dependent output genes in mouse and human (supplemental Figure III) SMC in vitro.
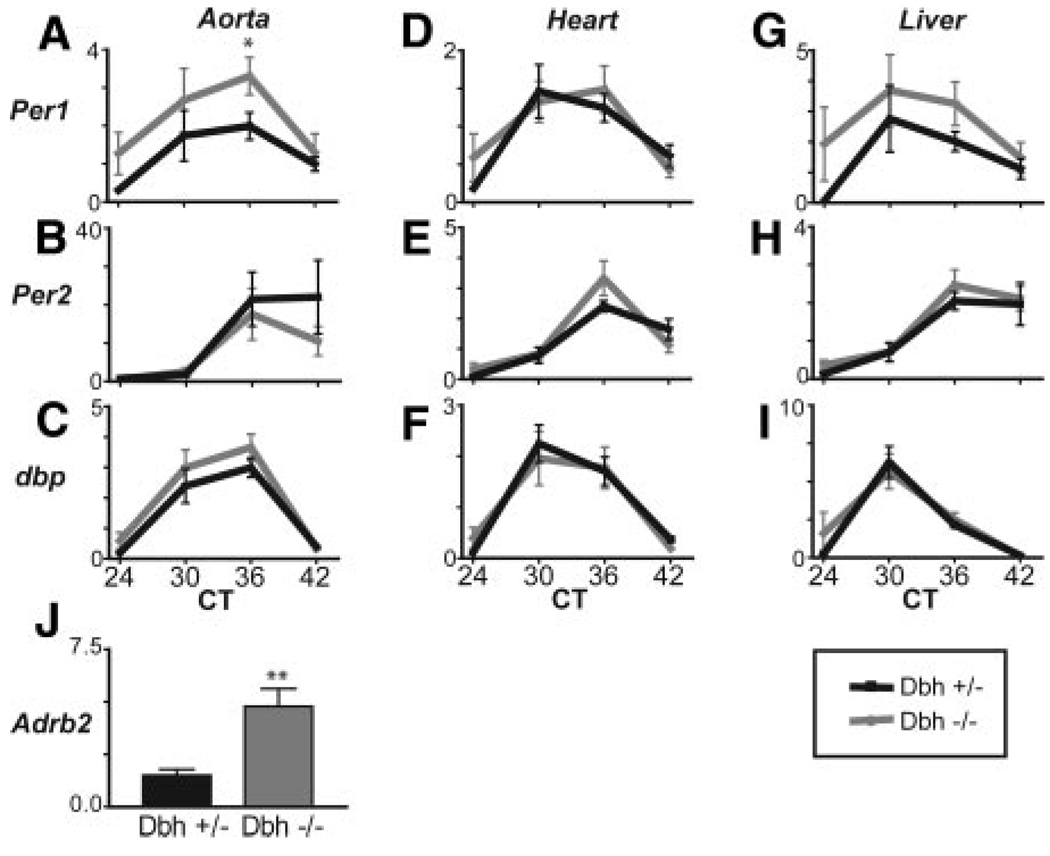
Dbh+/− mice have normal levels of NE and E and therefore serve as suitable controls for Dbh−/− mice, which lack these catecholamines systemically, but retain dopamine.17,18 Littermate control heterozygous and homozygous Dbh mice were entrained to LD followed by 24 hours of DD. Peripheral tissues from control and littermate knockout mice were harvested at 6-hour intervals. Dbh+/− mice showed rhythmic accumulation of mRNA for Per1, Per2, dbp (Figure 3A through 3I), and E4bp4 (data not shown) in all tissues examined. Rhythms in Per1, Per2, dbp, and E4bp4 in the aorta, heart, and liver were indistinguishable between Dbh−/− and control Dbh+/− mice. Expression of the β2 AR was elevated in Dbh−/− compared with the Dbh+/− mice (Figure 3J), presumably reflecting its upregulation in response to depletion of its endogenous ligands.
Figure 3.
Rhythms in peripheral tissues are maintained in Dbh−/− mice. A through I, Dbh+/− (black) and Dbh−/− (gray) mice entrained on a 12-hour light-dark regimen (Lights on at ZT0) were switched to constant darkness for 24 hours before tissue harvest (n = 6). Per1, Per2, dbp, E4bp4, and Adrb2 mRNA was monitored by qPCR.
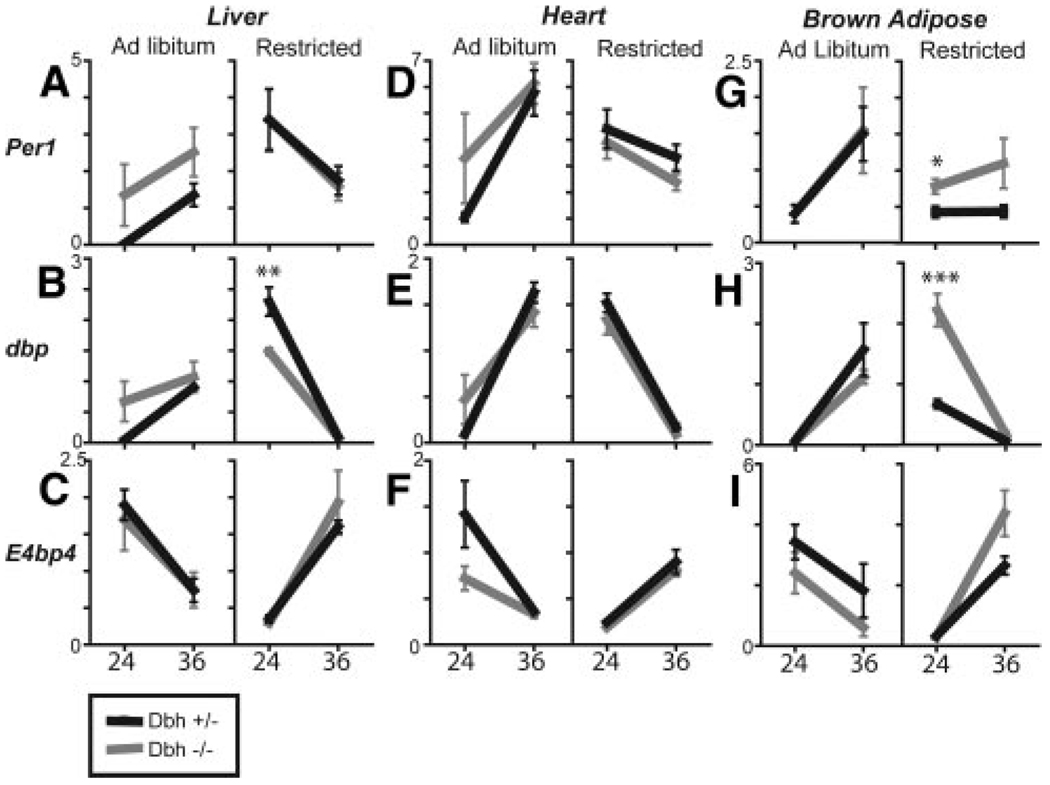
Dbh+/− and littermate Dbh−/− mice were placed on a restricted feeding regimen to daylight hours from 7 am to 7 pm for 6 days. Rhythms in clock genes peaked in the opposite phase to ad libitum conditions in food-restricted Dbh+/− mice (Figure 4). Data were obtained at CT24 and CT36, representing the peak and nadir of expression of most of the genes examined under ad libitum conditions. In the liver, Per1 and dbp peaked at CT24, whereas E4bp4 and Bmal1 now peaked at CT36 (Figure 4C and supplemental Figure IV). Very low amplitude oscillations of Per2 were evident in the liver of food-restricted Dbh+/− and Dbh−/− mice (supplemental Figure IV). Rhythms in Dbh−/− mice appeared to phase align with Dbh+/− mice in most of the clock genes examined. Thus, it appears that mice that lack NE and E retain the ability to reentrain their peripheral rhythms according to food availability. Significant differences between Dbh+/− and littermate Dbh−/− clock gene expression were apparent in some of the tissues examined; however, all of the tissues appear to have reentrained under restricted feeding conditions. In brown adipose tissue, rhythms in Per1 and Per2 did not appear to reentrain as well as in other tissues (Figure 4G through 4I and supplemental Figure IV). However, the overall rhythm in dbp, E4bp4, and Bmal1 appears to have phase altered similar to the other tissues examined.
Figure 4.
Rhythms in peripheral tissues of food-restricted mice are similar in Dbh+/− and Dbh−/−. A through I, Dbh+/− and Dbh−/− littermate mice were food-restricted for 5 days during daytime hours (7 am to 7 pm) or ad libitum and switched to dark-dark for 24 hours before tissue harvest at CT24 (7 am) and CT36 (7 pm) from Dbh−/− (gray) and Dbh+/− (black; n = 6) littermate controls.
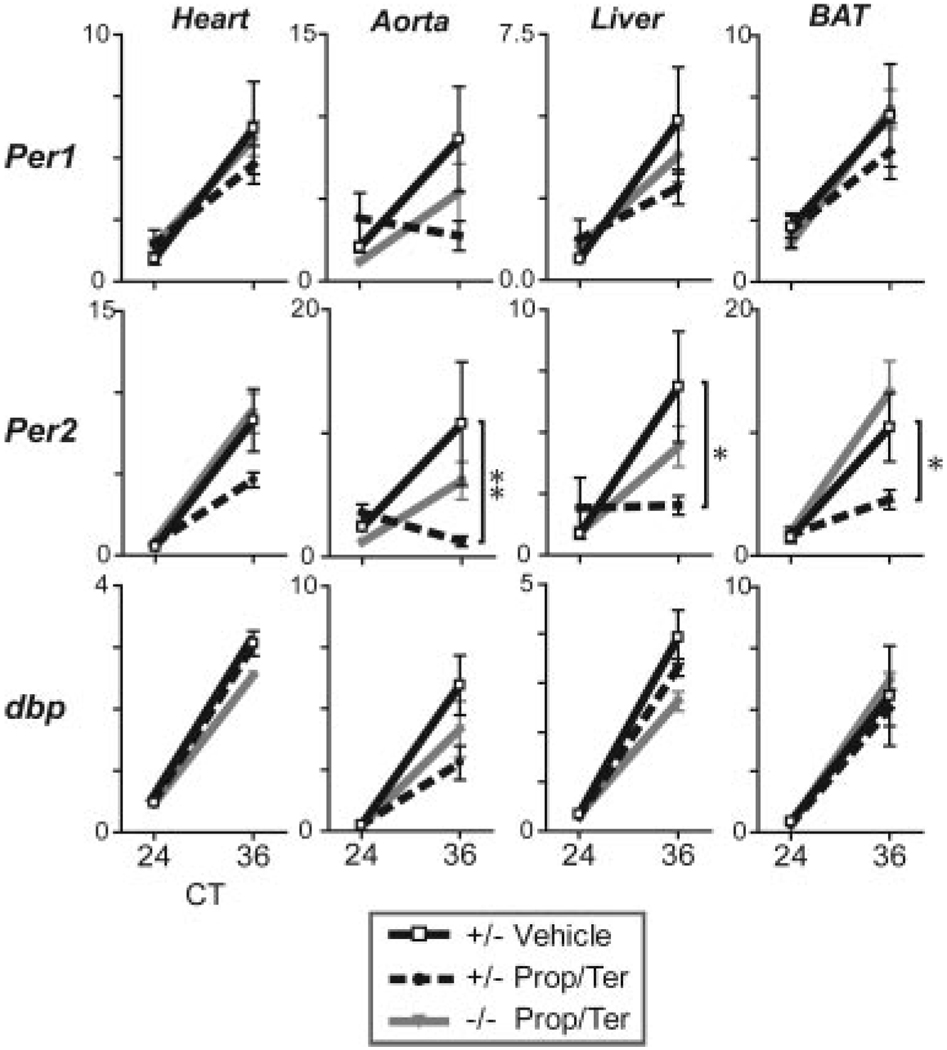
Dbh+/− and littermate Dbh−/− mice were implanted with propranolol/terazosin osmotic minipumps or vehicle pumps and maintained on a light dark schedule for 12 days before tissue harvest under constant darkness. Even with complete inhibition of both α and β adrenergic receptors, rhythmic accumulation of mRNA for Per1, dbp, E4bp4, and Bmal1 was observed in the heart, aorta, liver, and brown adipose of Dbh+/− and Dbh−/− (Figure 5 and supplemental Figure V). Per2 rhythms were observed in antagonist treated Dbh−/−, similar to vehicle controls, but a significantly damped rhythm in Per2 expression was observed in the aorta, liver, and brown adipose of Dbh+/− treated with both antagonists.
Figure 5.
Antagonism at α and βAR affects Per2 in Dbh+/− but does not disrupt peripheral rhythmicity in Dbh+/− and Dbh−/−. Two week minipumps with 50 mg/mL propranolol and 25 mg/mL terazosin were implanted. Tissues were harvested in constant darkness. Per1, Per2, and dbp expression levels were monitored by qPCR.
Discussion
The discovery that most, if not all, peripheral tissues harbor molecular clocks has raised interest in the means by which the SCN communicates with the periphery and the effects of potential modulators on phasic biorhythms in physiological function. Circulating hormones are prime candidates for such a function, and we have previously described how activation of a nuclear receptor can phase delay periodic gene expression in the vasculature.5 Both NE and E are subject to diurnal variation in both plasma and urine.19 A diurnal variation in both sympathetic tone and vascular reactivity to AR antagonists has also been established.20 Here, we show that these catecholamines, acting via β2 and α1ARs, have the capacity to alter expression of clock components and clock-dependent output genes in mouse and human ASMCs in vitro. Dbh−/− mice retain central clock function, as reflected by behavioral rhythms,21 but lack a diurnal oscillation in blood pressure, reflective of depletion of both NE and E22 and their impact on vascular tissue in the periphery. Here, we show that despite the modulatory capacity of catecholamines on vascular oscillatory gene expression in vitro, Dbh disruption does not influence molecular rhythms in peripheral tissues in vivo. Phase alterations influenced by daytime food restriction are similarly unaffected in Dbh−/−. Chronic treatment with α and β adrenergic antagonists similarly does not induce detectable phase shifting in vivo. These studies illustrate the capacity of catecholamines to modulate the vascular clock in vitro, but suggest that they are not important modulators of peripheral clock function under physiological conditions in vivo.
Catecholamines are potent vasoactive hormones which oscillate in train with endogenous rhythms in blood pressure and airways function. Thus, catecholamines seemed attractive candidates to investigate as potential modulators of the vascular clock that might signal in response to a central cue, such as environmental stress. We initially determined the influence of catecholamines and AR subtype-specific agonists on the oscillatory expression of a series of clock genes and clock-dependent output genes in ASMCs in vitro. Per1 is such a gene and participates in phase resetting.23 Per1 expression increases in the SCN in response to light pulses in mice previously kept in constant darkness for several days: this results in a phase shift of the central clock. Analogously, treatment with AR agonists elevates Per1 expression in both human and mouse cells (the present study) or in liver.16 Induction of Per1 by phenylephrine and isoproterenol in liver slices in vitro is dampened by inhibitors of MAPK and PKA, respectively. Signaling pathways downstream of the α1 AR include Gq-mediated PLC activation and Gq/Gs activation of MAPK. β2-mediated signaling via Gs leads to activation of adenylate cyclase, elevation of cAMP and subsequent PKA activation. Overall, our results are compatible with previous literature showing that CRE elements in the Per1 promoter are responsive to synergistic activation of cAMP and MAPK pathways.24 Thus, catecholamines, acting via β2 and α1ARs modulate clock gene and clock dependent output gene expression in ASMC in vitro.
We used Dbh−/− mice to address the importance of endogenous modulators of the clock at concentrations attained in vivo. Despite complete depletion of NE and E, oscillation of Per1, Per2, dbp, E4bp4, and Bmal1 is retained in aorta, heart, and livers of Dbh−/− mice. Consistent with these observations local sympathectomy does not affect the daily variation in clock-gene mRNA expression in the liver.25 The phase of peripheral clocks can be completely uncoupled from the SCN by restricted feeding.26 Thus, feeding time, although not affecting the phase of the SCN pacemaker, is a dominant modulator of peripheral circadian oscillators.27 We have assessed the impact of catecholamine depletion on phase shifting peripheral rhythms by daytime food restriction in the Dbh−/− model. Food was restricted for 6 days to permit phase reversal of cardiovascular rhythms, as previous literature had shown that the mouse heart requires between 3 to 6 days of restricted feeding to reverse its phase.26 Restriction of feeding to daytime periods phase shifts rhythms in peripheral tissues however, oscillations in clock genes in the SCN remain in phase with light and activity, similar to ad libitum feeding. Despite successful phase alteration by food restriction, Dbh disruption had no discernable impact in all tissues studied, save for subtle effects in brown adipose tissue.
Dbh disruption leads to complete depletion of NE and E, but an accumulation of dopamine is observed in many tissues, albeit at lower concentrations than that normally observed for NE.18 Dopamine, at least in vitro, is a much less potent agonist of both α and β adrenergic receptors than NE and E.28 However, we observed upregulated levels of β2AR in the aorta. Thus, we wished to exclude compensation for depletion of E and NE by dopamine in the Dbh−/− mice. Both Dbh−/− and Dbh+/− mice were chronically treated with antagonists of α and βAR. However, rhythmic accumulation of clock genes was observed in the heart, liver, and brown adipose of both treated and untreated Dbh−/− mice, excluding adrenergic compensation by dopamine. Thus, in multiple tissues in vivo, peripheral clock rhythmicity is unaffected by depletion of NE and E, disruption of α and βAR signaling, or a combination of both as seen in the antagonist treated Dbh−/−. It must be considered that the accumulated dopamine in many tissues may increase dopaminergic signaling in the periphery of Dbh−/− mice. In the current study of Dbh−/− we cannot rule out the possibility that enhanced dopaminergic signaling may compensate in the chronic absence of adrenergic signaling. However, rhythmicity is similarly maintained in wild-type mice where adrenergic signaling was disrupted with antagonists.
What might explain the apparent discrepancy between the ability of catecholamines to modulate clock function in ASMCs in vitro and the apparent failure of Dbh disruption to influence peripheral clock function in vivo? Firstly, catecholamines are only one set of multiple potential modulators of vascular clock function in vivo, among them retinoic acids,5 epidermal growth factor,29 angiotensin II,30 and glucocorticoids. 7 Rhythms in peripheral circadian clock genes examined under steady state conditions in adrenalectomized or sham operated mice are similarly unaffected.31 However, adrenalectomized mice show a more rapid phase adjustment in response to altered feeding time, reflective of the absence of glucocorticoid signaling.32
A second possibility relates to precision. The detection of phase alterations in the in vitro experiments was configured on the ability to sample over short 1-to 4-hour intervals after subjecting tissues to serum shock and catecholamine exposure. The in vivo experiments, by contrast, relied on samples obtained less frequently—at 6-hour intervals. Although it is possible that such technical distinctions obscured the ability to detect an impact of Dbh deletion on oscillatory gene expression under either physiological or food-restricted conditions, significant alterations in phase should be detectable in clock genes and clock-dependant output genes such as dbp. Finally, it is possible that although the catecholamine concentrations used in vitro can modulate the clock, depletion of those concentrations that are evident in vivo do not result in a detectable impact on clock function. This may be because the endogenous concentrations of catecholamines attained in vivo never do or because they must act in concert with other mediators to modulate clock function.
Although we focused on depletion experiments to examine the relevance of endogenous catecholamines in vivo, there is little reason to believe that manipulated elevation of endogenous epinephrine and norepinephrine would have led to different conclusions. Thus, the circadian expression of Per1, Per2, and Bmal1 measured at 3-hour intervals in the liver, heart, and kidney are unaffected by immobilization stress,33 which elevates substantially endogenous catecholamines.34 Distinct from the impact of catecholamines on the vascular clock, the central clock appears to influence sympathoadrenal function. Cry-deficient mice lack diurnal changes in blood pressure and the vascular responses to α1AR agonists, but show remarkably enhanced baroreflex sensitivity,35 and disruption of core clock genes ablates the diurnal variation in catecholamines, blood pressure and the sympathoadrenal activation response to stress.34
In summary, catecholamines can modulate clock genes and clock-dependent output genes, acting via β2 and α1ARs in either human or mouse ASMCs in vitro. However, depletion of endogenous levels of NE and E in vivo, by disruption of Dbh, does not influence peripheral clock function, in that diurnal variation in clock gene transcript accumulation is retained. Phase shifting in response to food restriction also occurs unaltered by inactivation of Dbh. Chronic antagonism of α and βAR in Dbh+/− and Dbh−/− similarly did not disrupt rhythms in essential clock genes, save for Per2, ruling out potential compensation by dopamine. Despite the influence of the central molecular clock on sympathoadrenal function and the direct effects of catecholamines on vascular cells in vitro, endogenous concentrations of NE and E do not appear to modulate peripheral clock function in vivo.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported in part by grants from the National Institutes of Health to Drs FitzGerald (HL-62250 and HL-54500) and Thomas (MH063352), and grants from the American Heart Association to Dr Reilly (325661U), Dr Cheng (0730264N), and Dr Rudic (0225478U). Dr FitzGerald is the McNeil Professor of Translational Medicine and Therapeutics.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Tofler GH. Circadian variation and cardiovascular disease. N Engl J Med. 1991;325:1038–1039. doi: 10.1056/NEJM199110033251410. [DOI] [PubMed] [Google Scholar]
- 3.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 4.Turton MB, Deegan T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta. 1974;55:389–397. doi: 10.1016/0009-8981(74)90014-x. [DOI] [PubMed] [Google Scholar]
- 5.McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 7.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 8.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 11.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Schoenhard JA, Muldowney JA, 3rd, Emens JS, Lewy AJ, Vaughan DE. Plasminogen activator inhibitor-1 has a circadian rhythm in blind individuals. Thromb Haemost. 2007;98:479–481. [PubMed] [Google Scholar]
- 13.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, FitzGerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 16.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 19.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Hossmann V, FitzGerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res. 1980;14:125–129. doi: 10.1093/cvr/14.3.125. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 22.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- 23.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 24.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pevet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005;22:2531–2540. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- 26.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WP, Ouyang M, Thomas SA. Potency of catecholamines and other L-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology. 2004;47:438–449. doi: 10.1016/j.neuropharm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, Ishida N. Genome-wide Expression Analysis Reveals 100 Adrenal Gland-dependent Circadian Genes in the Mouse Liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 32.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 34.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.