Abstract
More than 94 million Americans have tried marijuana, and it remains the most widely used illicit drug in the nation. Investigations of the cognitive effects of marijuana report alterations in brain function during tasks requiring executive control, including inhibition and decision-making. Endogenous cannabinoids regulate a variety of emotional responses, including anxiety, mood control, and aggression; nevertheless, little is known about smokers’ responses to affective stimuli. The anterior cingulate and amygdala play key roles in the inhibition of impulsive behavior and affective regulation, and studies using PET and fMRI have demonstrated changes within these regions in marijuana smokers. Given alterations in mood and perception often observed in smokers, we hypothesized altered fMRI patterns of response in 15 chronic heavy marijuana smokers relative to 15 non-marijuana smoking control subjects during the viewing of masked happy and fearful faces. Despite no between-group differences on clinical or demographic measures, smokers demonstrated a relative decrease in both anterior cingulate and amygdalar activity during masked affective stimuli compared to controls, who showed relative increases in activation within these regions during the viewing of masked faces. Findings indicate that chronic heavy marijuana smokers demonstrate altered activation of frontal and limbic systems while viewing masked faces, consistent with autoradiographic studies reporting high CB-1 receptor density in these regions. These data suggest differences in affective processing in chronic smokers, even when stimuli are presented below the level of conscious processing, and underscore the likelihood that marijuana smokers process emotional information differently from those who do not smoke, which may result in negative consequences.
Keywords: Marijuana, fMRI, Masked Affect, Cingulate, Amygdala
1. Introduction
More than 94 million Americans have tried marijuana at least once, and it remains the most widely used illicit drug in the nation (Johnston et al., 2005). When smoked, the main active ingredient in marijuana, Δ9 -tetrahydrocannabinol (THC) binds to CB-1 cannabinoid receptors in the brain, resulting in a variety of subjective experiences including mood changes, heightened sensitivity to external stimuli, relaxation, and altered perception of time and space (Gobbi et al., 2005; Hollister, 1986). Furthermore, endogenous cannabinoids have been shown to regulate a variety of emotional responses, including anxiety, mood control, and aggression (Martin et al., 2002). CB-1 receptors, the predominant cannabinoid receptor type within the central nervous system (CNS), have been shown to mediate the effects of both endogenous and exogenous cannabinoids, including effects on mood (Chaperon and Thiebot, 1999). CB-1 receptors are abundant within the cerebellum, basal ganglia, cingulate cortex, amygdala, and hippocampus (Witkin et al., 2005), and autoradiographic studies have demonstrated high densities of CB-1 receptors in the frontal cortex, reported to be nearly twice as high as those found in the posterior occipital cortex (Herkenham et al., 1990), and of higher density in these areas in humans relative to rat or monkey brain (Pertwee, 1997). Given the association between CB-1 receptors and mood, the current study is aimed at identifying the relationship between affective processing and activation within specific brain regions in chronic heavy marijuana smokers.
The anterior cingulate, an area with a high concentration of CB-1 receptors, has been shown to play a key role in affective regulation and the inhibition of impulsive behavior (Devinsky et al., 1995). Results from investigations using PET and fMRI techniques have reported metabolic alterations within frontal regions of marijuana smokers, both at rest and during tasks which require executive function, inhibition, and decision making (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005). Previous studies have demonstrated significant increases in cingulate activation that were associated with the level of both injected (Mathew et al., 1998; 2002) and smoked (O'Leary et al., 2000; 2002) marijuana. Studies have also reported high CB-1 receptor density within the human limbic system, including the amygdala, a critical brain region involved with affective processing (Joseph, 1996; Killgore and Yurgelun-Todd, 2004). Results from animal studies have suggested an interaction between cannabinoids and stress in the activation of the amygdala, which may provide a context for understanding the affective changes observed following marijuana use (Patel et al., 2005). Specifically, increased c-Fos expression has been shown within the central amygdala (CeA) in mice following a combination of restraint (stress) and CB-1 agonist administration. This finding underscores the importance of considering this region when attempting to understand the relationship between the emotional and affective changes related to marijuana use and the potential interactive role of the endocannabinoid system.
The acute response to marijuana generally includes feelings of euphoria and relaxation (Hollister, 1986), and alterations of motor control, sensory function, sense of time and cognitive performance have been documented (Nahas, 1993). Studies of laboratory- controlled smoking of marijuana have reported dose-related effects on measures of heart rate, subjective feeling of being “high”, reports of being hungry, and on cognitive performance of tasks including digit span, divided attention, and recall (Chait et al., 1988; Chait et al., 1985; Haney et al., 1999a, b). Early studies of marijuana smokers reported that use of the drug also increased feelings of happiness, friendship, interpersonal warmth and empathy (Galanter et al., 1974; Tart, 1971), suggesting to the users a facilitation of interpersonal relationships. Interestingly, the marijuana-intoxicated subjects who were observed by raters blind to the subjects’ drug state reported that subjects’ use of marijuana actually decreased both social interaction (Galanter et al., 1974) and empathic communications (Janowsky et al., 1979). Despite this, marijuana smokers have reported an enhanced effect on sensory-perceptual abilities (Tinklenberg and Darley, 1976) and improved insight into others (Green et al., 2003) after using the drug. In one of the few studies designed to examine the direct influence of marijuana on the ability to perceive emotions, Clopton and colleagues (1979) administered the Affect Sensitivity Test (AS) both before and after subjects smoked either a marijuana cigarette (containing 6 mg of THC) or a placebo cigarette. Designed to measure the ability to perceive emotions in others, results from the scale indicated a decrease in test scores in the active but not the placebo subjects, suggesting that intoxication resulted in difficulty with emotional perception. These results provide the first evidence that affective processing is affected by marijuana use, and may in fact be altered in individuals who smoke marijuana.
Subsequent investigations have examined a range of clinical measures related to marijuana use, however, these are not direct assessments of affective perception and processing. Hart and colleagues compared the effects of smoked marijuana (3.1% THC) and oral THC (20 mg) administered to the same chronic marijuana smoking individuals over an 18 day period in a double-blind fashion, with four days of placebo administration separating each of the active drug conditions (Hart et al., 2002). Psychomotor performance, food intake and subjective effects of the drug were measured at several points for each condition for a period of three days. Results demonstrated that relative to the placebo baseline, both orally ingested THC and smoked marijuana produced similar subjective ratings on a visual analog scale (VAS) which included levels of feeling “high” or “mellow”. While no specific measures of mood or affective perception were acquired, subjects reported more negative subjective effects, including ratings of “irritable” or “miserable” during the days following the smoking but not oral THC administration. The authors concluded that results were consistent with previous comparison studies of oral THC vs. smoked marijuana (Haney et al., 1999a, b; Wachtel et al., 2002) and that the behavioral profile associated with smoked marijuana is similar to the effects of oral THC, with some subtle differences. These findings are important when considering the results of individuals who smoke marijuana, which is the most common route of consumption.
In a study designed to examine the impact of marijuana use on mood and cognitive performance within the context of work and everyday life, Wadsworth and colleagues (2006) administered several neurocognitive measures and ratings of mood to a group of marijuana smokers and age and IQ matched non-smoking control subjects employed full time at the beginning and end of a typical work day in adults, both on Monday and Friday of the week. Measures of mood were derived from a computer administration of a bipolar visual analog scale (i.e. drowsy – alert) and included items categorized into alertness, hedonic tone and anxiety. Relative to the control subjects, the marijuana smokers demonstrated reduced alertness, as well as an effect of day, time and order of the mood testing. In general, marijuana smokers’ generally lower alertness levels were most evident prior to work at the start of the week, and tended to worsen with increased frequency of use. It is of note that despite the finding of reduced alertness in the smokers, which was assessed by self-report, no changes in anxiety ratings were detected between the groups. The finding of reduced alertness, while not a direct measure of affective processing, raises the question of how these individuals might differ from non –marijuana smokers on tasks related to affective discrimination or processing, given the importance of being able to visually attend to stimuli in order to process information appropriately.
In one of the few studies designed to examine subjective behavioral effects and impulsivity associated with marijuana use, McDonald et al. (2003) examined occasional users of marijuana after taking one of two acute doses (7.5,15 mg) of oral THC or a placebo. Subjects were administered clinical rating scales and tasks designed to measure impulsivity. The authors reported that THC administration increased subjective ratings of euphoria, dysphoria, somatic effects and sedation; decreases in intellectual efficiency and energy were also noted. Significantly increased scores on anxiety, fatigue, anger and confusion, as measured by the Profile of Mood States (POMS) (McNair et al., 1971) were also demonstrated following THC administration, which were noted to be dose dependent. Behavioral results from this study indicate that THC administration affected two of the four measures of impulsivity, increasing impulsive responses to the Stop task and altering patterns of response on a time estimation task, however, no significant effect was noted on either the Go/No Go task or the Delay Discount task. The authors interpret the findings to suggest that multiple processes underlie impulsivity, only some of which are affected by marijuana. In addition, this study provides clear evidence of alterations in mood following the administration of THC, as measured by the POMS, although no measure was specific to affective processing. Despite the fact that specific areas of cognitive function appeared to be altered following the THC administration, several remained unaffected, underscoring the importance for examining affective processing.
As noted, although previous investigations have reported altered cortical function in marijuana smokers during the completion of primarily cognitive-based tasks using fMRI techniques, and many have focused on the physiologic changes secondary to either acute oral or smoked marijuana, none thus far have focused on the effects of chronic marijuana use on affective processing. Given the behavioral alterations often demonstrated by marijuana smokers, and their difficulty in appropriately judging emotional and affective cues, it is likely that processing differences are present in this population. Further, given the pattern of high CB-1 receptor density within frontal and limbic regions, functions subserved by these regions, including affective processing, may very well be affected by chronic, heavy exposure to marijuana.
In order to address this issue, we acquired BOLD fMRI data from both chronic heavy marijuana smokers and non-marijuana smoking control subjects while undergoing a masked facial affect paradigm to examine whether chronic, heavy marijuana smokers would demonstrate a different pattern of activation during the completion of this task. One clear advantage of using a masked paradigm is that since stimuli are presented very rapidly, in fact, below the level of conscious awareness, it avoids confounding interpretation by the presence of other cognitive processes during the scan. We hypothesized that given the increased behavioral impulsivity often noted in chronic marijuana smokers and previous findings of altered anterior cingulate activation, chronic heavy marijuana smokers would likely demonstrate different frontal patterns of activity relative to non-marijuana smoking control subjects in response to affective stimuli, even if presented below the level of conscious processing. We also predicted differential amygdalar response to the masked affective stimuli, given the CB1 receptors located within the amygdala-prefrontal circuit (Laviolette and Grace, 2006), which have been shown to modulate emotional associative learning. Given previous fMRI findings of altered cingulate activation in chronic, heavy marijuana smokers during tasks requiring inhibitory function, we hypothesized that relative to the non-marijuana smoking control subjects, the chronic marijuana smokers would demonstrate reduced cingulate activity during the masked affective tasks and altered amygdalar activity, which may be correlated with measures of marijuana use.
2. Methods
2.1. Subjects
Fifteen adult chronic heavy marijuana smokers who had smoked at least 3,000 joints in their lifetime, and who smoked at least four of the last seven days and tested positive for urinary cannabinoids and fifteen non-marijuana smoking control subjects who were age, sex and education matched were included in the study (see Table 1). Subjects were recruited from the greater Boston area, with participants from both downtown and suburban locations included. Recruitment sites included local colleges and universities, sports clubs and athletic centers, supermarkets, community centers and other public locations. All subjects received the Structured Clinical Interview for DSM-IV (SCID-P) (First et al., 1995) to ensure that no Axis I pathology was present (except for marijuana abuse, a requirement for the marijuana smoking group) and had no history of head trauma or medical condition. No subjects met diagnostic criteria for current or previous alcohol abuse or dependence; subjects were excluded if they reported more than 5 lifetime episodes of using any category of illicit drugs (including sedative-hypnotics, stimulants, cocaine, opioids, hallucinogens, and MDMA) except for marijuana in the case of the smokers. Subjects were required to provide a urine sample to be tested for marijuana, ethanol, amphetamines, opioids, phencyclidine, barbiturates, benzodiazepines, and cocaine (TRIAGE) just prior to imaging. This procedure was required for three reasons: 1) to ensure that subjects did not test positive for other substances of abuse, and 2) to determine whether subjects had used marijuana recently enough to have a positive urine screen, and 3) to encourage subjects, as requested, to abstain from marijuana from the previous evening until arriving at the laboratory, to ensure subjects were not acutely intoxicated at the time of the visit. Subjects were repeatedly reminded that they would be tested for marijuana use upon their arrival at the lab, and were led to believe that we would be able to determine if they had smoked within the previous 12 hours. A portion of the sample was sent to an outside laboratory for quantification of urinary cannabinoid concentration via gas chromatography mass spectrometry (GCMS). In addition, all subjects completed the Addiction Severity Index (ASI), Positive and Negative Affect Scale (PANAS) (Watson et al., 1988), Beck Depression Inventory (BDI) (Beck, 1987), and the Profile of Mood States (POMS) (McNair et al., 1971) prior to their scanning sessions to evaluate their clinical state at time of testing. Prior to their participation in any study related activity, study procedures were explained and all subjects were required to read and sign an informed consent form, which described in detail the scanning procedures and which had been approved by the McLean Hospital Institutional Review Board.
Table 1.
Demographics
| Normal Controls | MJ Smokers | |||
|---|---|---|---|---|
| Variable (x̄) | (N=15) | (N=15) | P value | |
| Age | 26 (±9.0) | 25 (±8.8) | 0.81 | |
| Education | 14.8 (±2.67) | 13.9 (±2.11) | 0.32 | |
| Sex | ||||
| Male | 14 | 14 | ||
| Female | 1 | 1 | ||
| Handedness | ||||
| Right | 13 | 13 | ||
| Left | 2 | 2 | ||
| MJ Age of Onset (years) | 14.9 (±2.50) | |||
| MJ Avg. smokes per week (joints) | 25.6 (±27.8) | |||
| MJ Avg. Urinary Concentration (ng/ml) | 505.8 (±734.7) | |||
| Alcohol Use (days/month) | 4.0 (±4.5) | 9.6 (±5.4) | 0.05 | |
| Alcohol Use to intoxication (days/month) | 2.3 (±3.0) | 4.8 (±4.1) | 0.07 | |
2.2. Image Acquisition and Analyses
Scanning was performed on a Siemens whole body 3T system using a quadrature head coil; 35–41 contiguous coronal slices were acquired from each subject to ensure whole brain coverage. Slices were 5 mm thick, with a 0 mm skip, and images were collected every 3 seconds (TR=3000) using a single shot, gradient pulse echo sequence (TE=30 ms, flip angle =90, 50 images per slice). Each subject completed two masked facial affect tasks conducted as separate scanning runs (anger, happy). The presentation order of tasks was counterbalanced, and subjects were unaware of the backward masking nature of the paradigm. In order to ensure that subjects remained engaged, focused and attentive to the task, subjects were told that they would see a series of briefly presented photographs of faces and be required to make a gender discrimination for each face by pressing a small hand held key pad.
The fMRI stimuli were comprised of faces obtained from the picture set from the Neuropsychiatry Section of the University of Pennsylvania and consisted of black and white photographs of males and females posing each of three different emotional states (happy, anger, neutral). Emotional and neutral faces were used as the target stimuli and neutral faces from each subject were used as masking stimuli. Scanning epochs consisted of a total of 150 seconds with an additional 6 seconds initially for calibration during which time no data was acquired; thus, the total time required for each condition (masked happy or masked anger) was two minutes, thirty-six seconds. The task was comprised of five alternating blocks which consisted of a total of ten trials. Each individual trial consisted of an emotional or neutral target face presented for 30 milliseconds, followed immediately by a neutral masking face of the same poser for 170 milliseconds. Trials were separated by a 1 second interstimulus interval. Blocks 1, 3 and 5 consisted of neutral targets and neutral masks while blocks 2 and 4 were comprised of emotional targets and neutral masks. No commingling of emotional stimuli type occurred within a scanning epoch- only one emotional target type per scan was presented. Stimuli were presented this way to facilitate contrast analyses between the emotional masked stimuli and the neutral condition of neutral targets followed by neutral masks. The paradigms were presented using Psyscope 1.2.5 software generated from a Macintosh G5 computer and were rear projected onto a screen placed behind the top of the bore, visible through the mirror on the head coil. Immediately upon completion of the functional scan, subjects were presented with a post-test that included all facial expression stimuli and were asked to indicate for each expression whether it had been seen during the study. Subjects were also asked to describe what they had seen of the presented faces, and all reported the faces seen as having neutral expressions.
FMRI images were analyzed using a widely available software package SPM5 (Wellcome Department of Imaging Neuroscience, University College, London, UK) running in Matlab (MathWorks, Natick, MA, USA). Initially, blood oxygen level dependent (BOLD) fMRI data were corrected for motion in SPM5 using an intra-run realignment algorithm that uses the first image as a reference. A criterion of 2 mm of head motion in any direction was used as an exclusionary criterion. The realigned images were then normalized to an EPI template in Montreal Neurological Institute (MNI) stereotactic space. Normalized images were re-sampled into 2 mm cubic voxels and then spatially smoothed using an isotropic Gaussian kernel with 8 mm full width at half maximum (FWHM). Global scaling was not used, high-pass temporal filtering with a cut-off of 128s was applied, and serial autocorrelations were modeled with an AR(1) model in SPM5. Statistical parametric images were calculated individually for each subject and each task, using a general linear model (Friston et al., 1995a; Friston et al., 1995b). These images were subsequently entered into second level model, subjected to a voxel-wise contrast and t-test to assess statistical significance. Using the two-sample t-test, we made direct comparisons between the chronic, heavy marijuana smokers and the non-marijuana smoking controls. Contrast analyses were conducted for each region of interest and for each task condition, which consisted of the subtraction of one group map from the other; for example, anterior cingulate activity of marijuana smokers during the viewing of angry faces was subtracted from anterior cingulate activity of the healthy control subjects viewing angry faces to determine which areas showed increased activity in controls relative to smokers. The probability threshold was set at 0.005 uncorrected and a minimum cluster extent (k) of 20 contiguous voxels. Region of interest (ROI) masks were created using the Wake Forest University Pickatlas utility (Maldjian et al., 2003; 2004). These regions included the cingulate gyrus and the amygdala. The statistical threshold for the ROIs was set at 0.05, and k was set at 20 voxels. In addition, whole brain analyses were also completed. Finally, in order to determine the relationship between marijuana use and discrete patterns of activity during the masked affect task, regression analyses were completed between the BOLD signal data and both the total number of smokes per week and urinary cannabinoid level for the smokers.
3. Results
3.1. Demographic and Clinical Measures
Demographic and clinical variables for all subjects are included in Table 1 and Table 2. The subject groups did not differ with regard to any demographic or clinical variable, and no subject tested positive on the urine toxicology screen for illicit substances, with the exception of marijuana in the case of the smokers. Marijuana smokers had an average age of onset of 14.9 years, smoked an average of 25.6 joints per week and had a mean urinary cannabinoid concentration, normalized to their creatinine level, of 505.8 ng/ml on the day of scanning. One subject within each of the study groups endorsed using tobacco in an occasional fashion. Subjects did not differ on their past use of illicit substances according to their ASI scores, no subject in either group met diagnostic criteria for past abuse or dependence for any illicit substance (other than marijuana in the case of the marijuana smokers), and no subject in either group reported use of any illicit substance greater than 5 times in their lives. However, the chronic, heavy marijuana smokers reported using alcohol more days per month (9.6 days) than the non-marijuana smoking control subjects (4.0 days). This difference was statistically significant (p < 0.05), yet there were no significant differences between the groups for the number of days intoxicated in the past month (chronic heavy marijuana smokers = 4.8 days/month; non-marijuana smoking control subjects = 2.3 days/month), and no subject in either group met diagnostic criteria for alcohol abuse or dependence. With regard to clinical state, as seen in Table 2, no significant between-group differences were detected for the Beck Depression Inventory (BDI), Positive and Negative Affect Scale (PANAS), or Profile of Mood State (POMS), suggesting that the samples were equally matched on clinical state at the time of testing. No subject in either study group demonstrated clinically elevated scores on the PANAS, POMS, or the BDI. On the Beck Depression Inventory (BDI), all subjects within the non-marijuana smoking control subjects scored within the minimal range, as did all but one of the chronic, heavy marijuana smoking subjects, who had a total score which fell at the low end of the mild range. Within each of the subject groups, no subjects demonstrated clinically elevated scores on any of the POMS subscales or the PANAS positive or negative items, and all subjects within the non-marijuana smoking control subjects and all but one of the chronic, heavy marijuana smokers scored within the minimal (lowest possible) symptom range on the BDI. It is of note that while one chronic marijuana smoking subject had a total BDI score which was in the low end of the mild symptom range, this appeared to be related to a change in sleep and fatigue level secondary to a new living situation. Overall, scores on the PANAS, POMS and BDI for both groups suggested that the study samples were affectively stable at the time of testing. Given that one female subject was included in each sample, we completed analyses with and without these subjects. As the data were unchanged with the female subjects excluded, we report data with the entire sample intact.
Table 2.
Clinical Information
| Normal Controls | MJ Smokers | ||
|---|---|---|---|
| Variable (x̄), range | (N=15) | (N=15) | P Value |
| Beck Depression Index (Total) | 1.07 | 1.93 | 0.27 |
| (0–12) | (0–16) | ||
| Positive and Negative Affect Scale (PANAS) | |||
| Positive Item Score | 36.2 | 34.1 | 0.23 |
| (30–46) | (23–42) | ||
| Negative Item Score | 11.9 | 13.3 | 0.27 |
| (10–17) | (10–21) | ||
| Profile of Mood States (POMS) | |||
| Vigor | 21.95 | 20.92 | 0.48 |
| (18–29) | (15–29) | ||
| Anger | 3.66 | 6.14 | 0.16 |
| (0–13) | (0–12) | ||
| Confusion | 6.66 | 7.57 | 0.38 |
| (3–13) | (4–12) | ||
| Tension | 6.29 | 7.71 | 0.32 |
| (3–18) | (3–11) | ||
| Fatigue | 3.62 | 4.36 | 0.54 |
| (0–12) | (0–9) | ||
| Depression | 2.79 | 4.93 | 0.12 |
| (0–9) | (0–10) | ||
| Total | 45.51 | 51.87 | 0.33 |
| (28–84) | (29–67) | ||
3.2. Imaging Results
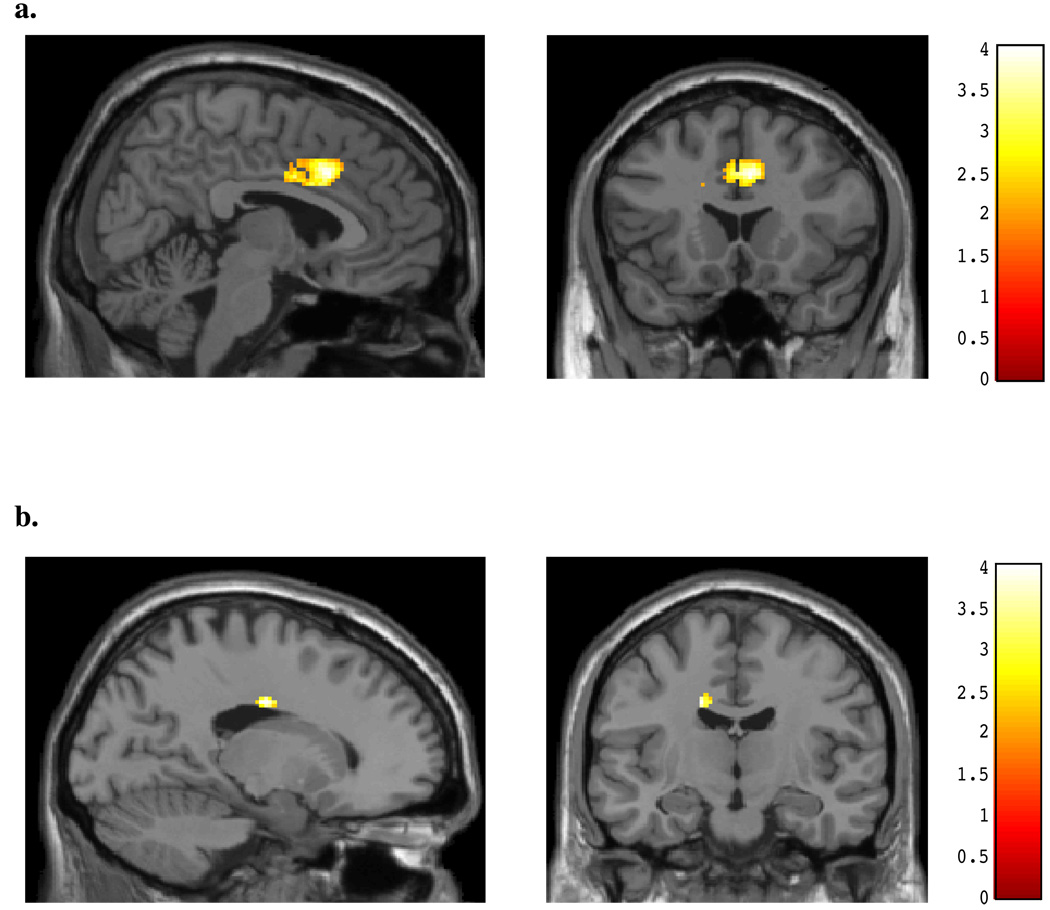
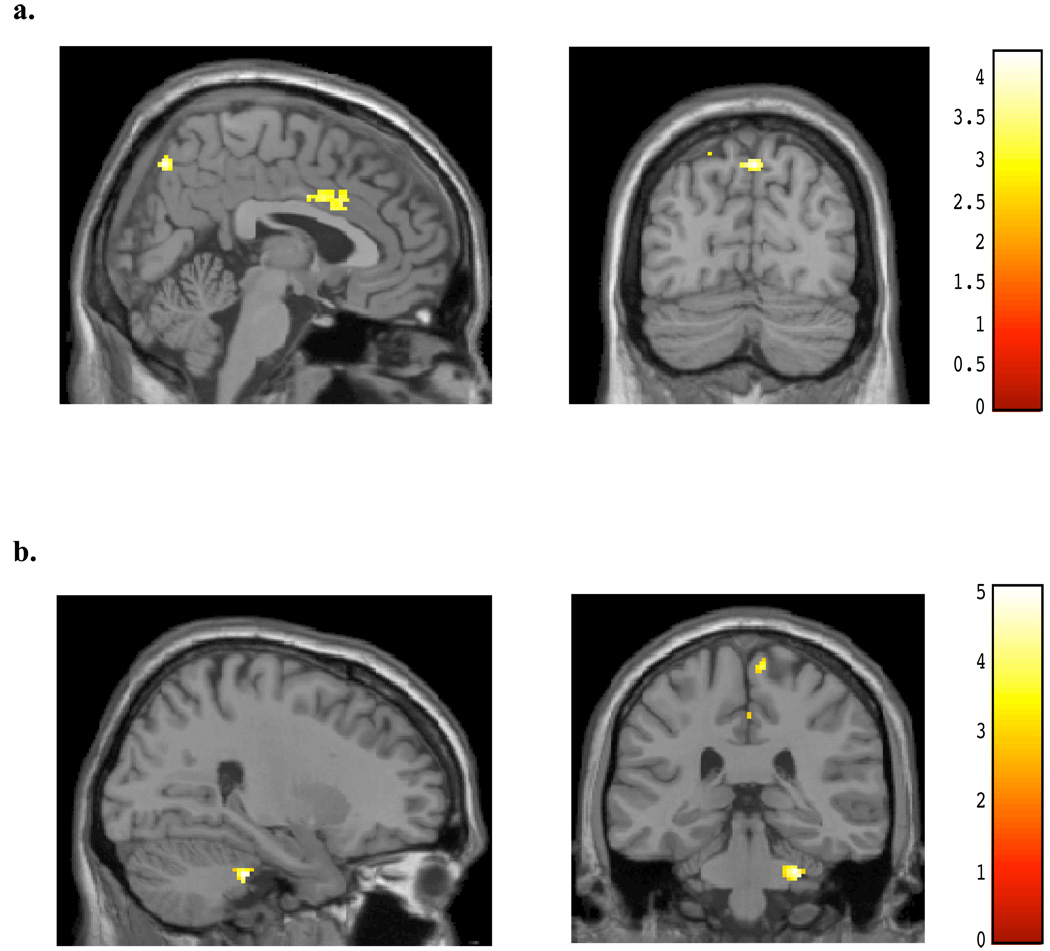
During the viewing of angry faces, non-marijuana smoking control subjects demonstrated significant differences relative to the chronic, heavy marijuana smokers (see Table 3). Greater activation was evident within an area of the midanterior cingulate relative to the marijuana smokers (peak x, y, z = 6, 18, 36; t = 3.58, p#x0003C; 0.05; k = 753; see Figure 1a). In contrast, the chronic, heavy marijuana smokers demonstrated much lower and more posterior cingulate activity than the non-marijuana smoking control subjects during the viewing of angry faces (peak x, y, z = −16, −12, 34; t = 3.23, p< 0.05; k = 41; see Figure 1b, Table 3). During the same condition, the control subjects exhibited activity within the left amygdala relative to the marijuana smokers (peak x, y, z = −20, −c4, −14; t = 2.32, p< 0.05; k = 35; see Figure 2a), who in contrast, showed no area of increased activation relative to the control subjects within the amygdala (see Figure 2b).
Table 3.
BOLD Activity by Region for Masked Angry Condition
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Talairach Results | x | y | z | k | t | z | |
|
NC > MJ Cingulate |
|||||||
| Right Frontal Lobe, Cingulate Gyrus, Brodmann area 32 | 6 | 18 | 36 | 753 | 3.58 | 3.21 | |
| Left Limbic Lobe, Cingulate Gyrus, Brodmann area 31, Parietal Lobe, Sub-Gyral, Frontal Lobe | −14 | −30 | 42 | 47 | 2.28 | 2.17 | |
| Amygdala | Left Limbic Lobe, Amygdala, Parahippocampal Gyrus | −20 | −4 | −14 | 35 | 2.32 | 2.20 |
|
MJ > NC Cingulate |
|||||||
| Left Limbic Lobe, Cingulate Gyrus | −16 | −12 | 34 | 41 | 3.23 | 2.94 | |
| Right Limbic Lobe, Cingulate Gyrus | 12 | −26 | 30 | 22 | 2.23 | 2.12 | |
Figure 1.
SPM contrasts: Cingulate Activation during Masked Angry Faces for (A) Normal controls > Marijuana smokers, and (B) Marijuana smokers > Normal Controls.
Figure 2.
SPM contrasts: Amygdalar Activation during Masked Angry Faces for (A) Normal controls > Marijuana smokers, and (B) Marijuana smokers > Normal Controls.
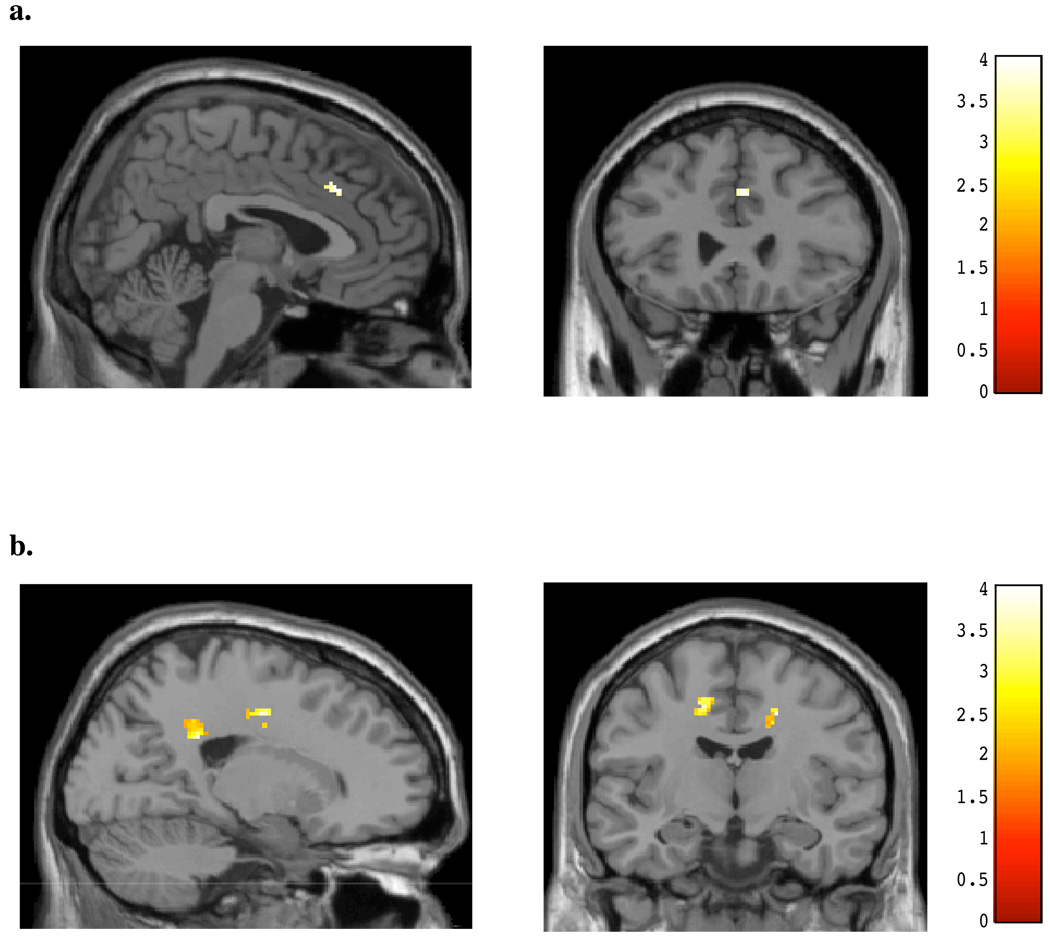
Interestingly, during the viewing of masked happy faces, a similar pattern of activation as to that demonstrated for masked angry faces was detected for both subject groups (see Table 4). Non-marijuana smoking control subjects demonstrated a small focal area of increased activity within the cingulate gyrus relative to the chronic, heavy marijuana smokers (peak x, y, z = 4, 26, 36; t = 2.12, p< 0.05; k = 30; see Figure 3a), while marijuana smokers showed greater activation within mid (x, y, z = 20, −10, 42; t = 3.62, p< 0.05; k =43) and posterior cingulate (x, y, z, = 20,−40, 30; t = 3.46, p< 0.05; k = 44) regions relative to the control subjects (see Figure 3b). Within the amygdala, non-marijuana smoking control subjects demonstrated small areas of increased activity bilaterally relative to the chronic, heavy marijuana smokers (x, y, z = 26, 2, −20; t = 2.64, p< 0.05; k =24; see Figure 4a), while marijuana smokers again showed no increased activity relative to the control subjects in this region (see Figure 4b).
Table 4.
BOLD Activity by Region for Masked Happy Condition
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Talairach Results | x | y | z | k | t | z | |
|
NC > MJ Cingulate |
|||||||
| Right Frontal Lobe, Cingulate Gyrus, Medial Frontal Gyrus, Brodmann area 32 | 4 | 26 | 36 | 30 | 2.12 | 2.02 | |
| Amygdala | Left Limbic Lobe, Amygdala, Uncus, Brodmann area 34 | −18 | −2 | −20 | 58 | 2.51 | 2.36 |
| Right Limbic Lobe, Uncus | 26 | 2 | −20 | 24 | 2.64 | 2.47 | |
|
MJ > NC Cingulate |
|||||||
| Right Limbic Lobe, Cingulate Gyrus, Frontal Lobe, Sub-Gyral | 20 | −10 | 42 | 43 | 3.62 | 3.24 | |
| Right Limbic Lobe, Cingulate Gyrus, Brodmann area 31, Frontal Lobe, Sub-Gyral, Parietal Lobe, Precuneus | 20 | −40 | 30 | 44 | 3.46 | 3.12 | |
Figure 3.
SPM contrasts: Cingulate Activation during Masked Happy Faces for (A) Normal controls > Marijuana smokers, and (B) Marijuana smokers > Normal Controls.
Figure 4.
SPM contrasts: Amygdalar Activation during Masked Happy Faces for (A) Normal controls > Marijuana smokers, and (B) Marijuana smokers > Normal Controls.
3.3. Correlations Between Clinical Variables and Activation
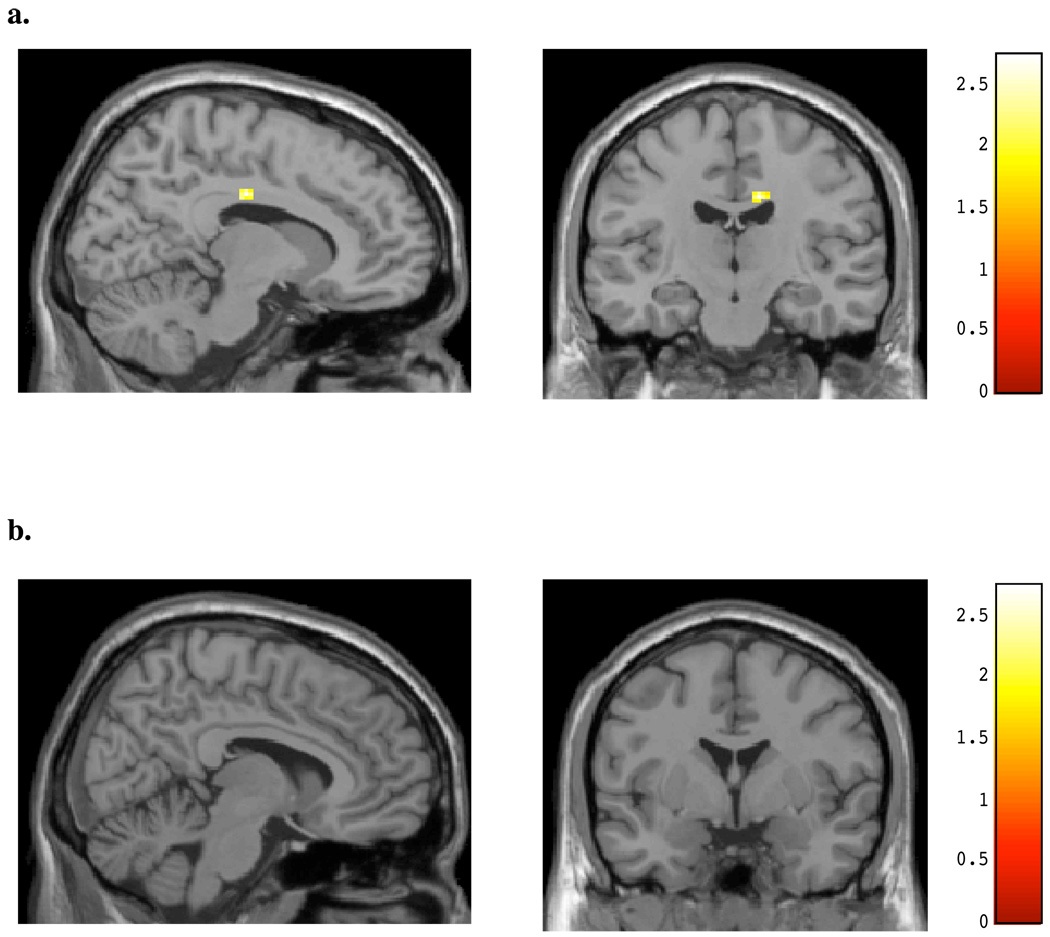
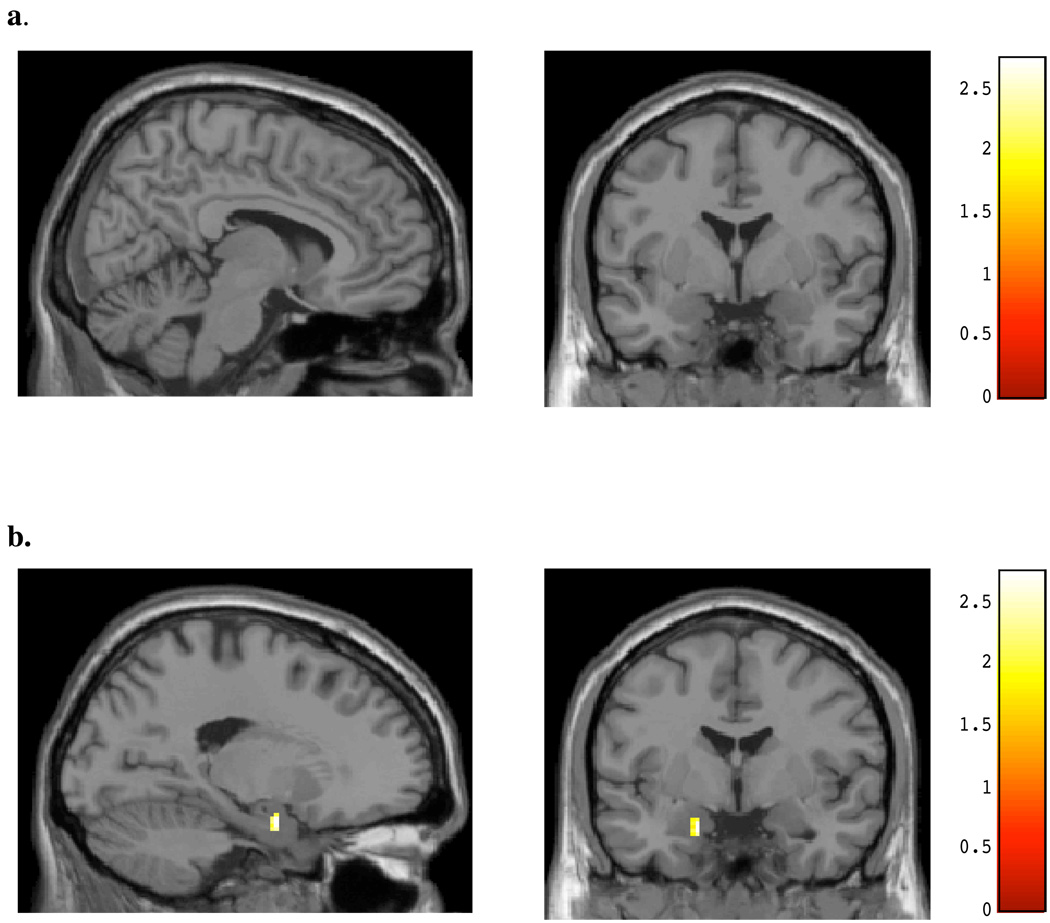
The relationship between marijuana use and BOLD signal changes was examined within the chronic, heavy marijuana smokers for both affective conditions. As seen in Figure 5a and Table 5, BOLD activity within the same posterior cingulate region noted as different between the marijuana smokers and controls during the viewing of masked angry faces was highly positively correlated with number of smokes (x, y, z = 12, −16, 34; t = 2.91, p< 0.05; k =25) per week; activity within the amygdala (Figure 5b) was not. For masked happy faces, no significant association was detected between cingulate activity and number of smokes per week (see Figure 6a), however, a significant positive correlation was noted for activity within the left amygdala and smokes per week (x, y, z = −18, −2, −22; t = 2.88, p< 0.05; k = 21; see Figure 6b, Table 5). This amygdalar activity was nearly identical to the region activated in healthy controls during the viewing of masked happy faces. A significant positive association was also detected for activity within the midcingulate during the viewing of masked happy faces and marijuana level, as determined by urinary cannabinoid level (x, y, z = 16, 10, 30; t = 3.70, p< 0.05; k = 37; see Figure 7, Table 5).
Figure 5.
SPM Maps: Positive regression for Number of Smokes and BOLD Activity during Masked Angry Faces for (A) cingulate region and (B) amygdalar region.
Table 5.
Regression of BOLD Activity by Region with MJ variables
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| MJ Variable | Talairach Results | x | y | z | k | t | z |
|
Number of Smokes Per Week | |||||||
|
Masked Anger Cingulate |
|||||||
| Right Limbic Lobe, Cingulate Gyrus | 12 | −16 | 34 | 25 | 2.91 | 2.51 | |
|
Masked Happy Amygdala |
|||||||
| Left Limbic Lobe, Amygdala, Uncus, Brodmann area 34, Brodmann area 28 | −18 | −2 | −22 | 21 | 2.88 | 2.49 | |
| Urinary Cannabinoid Level | |||||||
|
Masked Happy Amygdala |
|||||||
| Right Limbic Lobe, Cingulate Gyrus | 16 | 10 | 30 | 37 | 3.70 | 2.96 | |
| Left Limbic Lobe, Cingulate Gyrus | −16 | 0 | 46 | 36 | 3.07 | 2.59 | |
| Left Frontal, Sub-Gyral | −20 | −26 | 44 | 20 | 3.02 | 2.55 | |
Figure 6.
SPM Maps: Positive regression for Number of Smokes and BOLD Activity during Masked Happy Faces for (A) cingulate region and (B) amygdalar region.
Figure 7.
SPM Maps: Positive regression for Urinary Cannabinoid Level and BOLD Activity during Masked Happy Faces in the Cingulate region.
As exploratory analyses, we also completed whole brain analyses as contrasts for both task conditions which are presented in Figure 8 and Figure 9 and Table 6 and Table 7. During the viewing of masked angry faces, non-marijuana smoking control subjects demonstrated significant differences relative to the chronic, heavy marijuana smokers (see Table 6) in the cingulate gyrus (peak x, y, z = −2, 20, 30; t = 3.82, p< 0.05; k = 125) as well as regions in the left and right parietal lobe (see Figure 8a). In contrast, the chronic, heavy marijuana smokers demonstrated activity within primarily temporal, parietal and frontal regions, though these were less focal than those demonstrated by the non-marijuana smoking controls (see Figure 8b, Table 6). During the viewing of masked happy faces, non-marijuana smoking control subjects demonstrated significant differences relative to the chronic, heavy marijuana smokers (see Figure 9a, Table 7) in the superior temporal gyrus and sublobar space, while the chronic, heavy marijuana smokers demonstrated significant differences within the posterior lobe (see Figure 9b, Table 7).
Figure 8.
SPM contrasts: Whole Brain activation during Masked Angry Faces for (A) Normal Controls > Marijuana Smokers, and (B) Marijuana Smokers > Normal Controls.
Figure 9.
SPM contrasts: Whole Brain activation during Masked Happy for (A) Normal Controls > Marijuana Smokers, and (B) Marijuana Smokers > Normal Controls.
Table 6.
Whole Brain BOLD Activity for Masked Anger Condition
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Talairach Results | x | y | z | k | t | z | |
| NC > MJ | L. Limbic Lobe, Cingulate Gyrus, Brodmann area 32 | −2 | 20 | 30 | 125 | 3.82 | 3.34 |
| Inter-Hemispheric, Precuneus, R. Parietal Lobe, Brodmann area 7 | 4 | −74 | 50 | 105 | 4.29 | 3.66 | |
| L. Parietal Lobe, Superior Parietal Lobule, Brodmann area 7 | −24 | −64 | 60 | 28 | 3.51 | 3.12 | |
| MJ > NC | R. Posterior Lobe, Cerebellar Tonsil | 26 | −32 | −34 | 55 | 5.08 | 4.14 |
| R. Temporal Lobe, Middle Temporal Gyrus | 46 | −66 | 20 | 26 | 4.41 | 3.73 | |
| R. Frontal Lobe, Inferior Frontal Gyrus, Brodmann area 9 | 58 | 12 | 32 | 52 | 4.27 | 3.65 | |
| L. Temporal Lobe, Sub-Gyral | −44 | −20 | −18 | 40 | 4.12 | 3.55 | |
| R. Parietal Lobe, Precuneus | 2 | −36 | 46 | 35 | 3.76 | 3.30 | |
| R. Frontal Lobe, Paracentral Lobule, Brodmann area 5 | 8 | −44 | 58 | 31 | 3.47 | 3.09 | |
| R. Frontal Lobe, Superior Frontal Gyrus | 20 | 62 | 16 | 21 | 3.45 | 3.08 | |
Table 7.
Whole Brain BOLD Activity for Masked Happy Condition
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Talairach Results | x | y | z | k | t | z | |
| NC > MJ | L. Temporal Lobe, Superior Temporal Gyrus, Brodmann area 38 | −24 | 8 | −24 | 28 | 3.91 | 3.43 |
| L Sub-lobar, Extra-Nuclear | −32 | 6 | 2 | 20 | 3.52 | 3.15 | |
| MJ > NC | R. Posterior Lobe, Cerebellar Tonsil | 30 | −38 | −40 | 96 | 4.38 | 3.76 |
In order to address the question of whether or not marijuana use predicted brain activation above and beyond alcohol use, we completed both whole brain and ROI regression analyses for number of alcoholic drinks over the past 30 days and BOLD activation for each condition. Whole brain analyses did not reveal any significant relationship between alcohol use and BOLD activity for either the masked angry or masked happy condition, and results from the amygdalar ROI analyses also revealed no significant relationship between alcohol use and BOLD activation for either condition. Results from the cingulate gyrus (CG) ROI analyses revealed a small area of association between alcohol use in the last 30 days and BOLD activity during masked angry faces, which was in a more anterior and superiorly placed region within the cingulate than the pattern detected for the relationship between MJ use and BOLD activity, which is illustrated in Figure 5a. For the masked happy condition, an association between number of drinks and BOLD activity was detected for mid and posterior cingulate, however, no area of the cingulate gyrus was associated with MJ use and BOLD activity during masked happy faces, as illustrated in Figure 6a, suggesting independent patterns of BOLD activity for both alcohol and marijuana use.
4. Discussion
As hypothesized, chronic, heavy marijuana smokers demonstrated significant differences in both the magnitude and pattern of BOLD response within the cingulate and amygdala during the presentation of masked angry and masked happy faces relative to non-marijuana smoking control subjects. This was true, despite the fact that no significant between-group differences were noted for any clinical or demographic variable, with the exception of alcohol use, and all subjects appeared to be stable with regard to their mood. Specifically, ROI analyses revealed that the chronic, heavy marijuana smokers demonstrated relatively lower anterior cingulate and amygdalar activity during the presentation of masked angry stimuli sets relative to the non-marijuana smoking control subjects, who showed relatively higher activation within these regions during the task. In contrast, the chronic, heavy marijuana smokers demonstrated a larger, more diffuse pattern of activation during the presentation of masked happy faces within the cingulate as compared to the non-marijuana smoking control subjects, with no discernible increase in amygdalar activation. These differential patterns and magnitude of activation for the chronic, heavy marijuana smokers relative to the non-marijuana smoking control subjects underscore the likelihood that these subjects process emotional stimuli differently from those who do not smoke.
Regression analyses, which allowed us to examine the relationship between marijuana use and BOLD signal changes, also yielded interesting findings. Within the chronic, heavy marijuana smokers, the total number of smoking episodes per week was positively associated with cingulate activity during the viewing of masked angry faces. This region was nearly identical to the area of greatest activation seen when marijuana smokers were contrasted with non-marijuana smoking control subjects during the viewing of masked angry faces, highlighting the importance of the association between marijuana use and activation change. Within the marijuana smokers, total number of smoking episodes per week was also positively associated with amygdalar activity during the viewing of masked happy faces, with coordinates virtually overlapping those seen for the non-marijuana smoking controls during the viewing of masked happy faces. Finally, overall urinary cannabinoid level was positively related to cingulate activity during the viewing of masked happy faces, in approximately the same region as demonstrated for the chronic, heavy marijuana smokers compared to the non-marijuana smoking controls during the viewing of masked happy faces. The positive associations between marijuana use and BOLD activation within both cingulate and amygdalar regions depending on the stimuli may represent an alteration of fronto-limbic circuitry response within the marijuana smoking subjects. Given the relative activation differences between the marijuana smokers and non-smoking control subjects, it would appear that within the marijuana smoking group, increased marijuana use tends to normalize cingulate response to masked angry stimuli while it enhances the positive response to masked happy stimuli. This interpretation is consistent with previous self-reports of marijuana smokers feeling “mellow” and happy when smoking marijuana.
While no between-group differences emerged for any of the mood-related clinical variables, suggesting that the two groups were well matched in terms of their clinical state at the time of scanning, the question of whether heavier marijuana use might lead to increased emotionality was also explored. Correlations which examined the relationship between marijuana use, and included both frequency of use and total urinary THC concentration, and the clinical ratings of positive and negative symptoms (including all measures from the POMS, PANAS and BDI) did not reveal any statistically significant association, suggesting that marijuana use was not related to any of their clinical scores. Similarly, as a between-group difference was detected for number of alcoholic drinks in the last 30 days, with marijuana smokers having higher days of alcohol use relative to non-marijuana smoking controls, we also completed analyses to examine the potential relationship between alcohol use and marijuana use. The correlations for number of alcoholic drinks and number of smokes were not significant for parametric (r = .321; p = .243) or nonparametric (Kendall’s tau_b = .216; p = .313; Spearman’s rho = .281; p = .310) analyses, suggesting no relationship between alcohol and marijuana use. The findings reported here highlight the importance of examining both the frequency of use and overall level of marijuana present, and suggest a direct relationship between smoking marijuana and BOLD signal changes within specific brain regions for both masked affective tasks, which appear to be independent of other factors, including alcohol use. Finally, the findings are particularly noteworthy, as none of the chronic, heavy marijuana subjects were acutely intoxicated or “high” at the time of the scan and had been abstinent for a minimum of 12–16 hours, and are therefore not likely to reflect acute effects of the drug.
The activation patterns noted between the groups during this masked affective task underscore the likelihood that individuals who have smoked marijuana process emotional information in a different way from those who do not smoke, which may result in negative consequences. These data suggest differences in affective processing in chronic smokers even when the stimuli are presented below the level of conscious awareness. The cingulate has been hypothesized to play a critical role in the evaluation of the motivational significance of emotional stimuli (Devinsky et al., 1995) and the amygdala has been reported to be specifically involved in the detection and early processing of stimuli not available to conscious awareness (Morris et al., 1999; Whalen et al., 1998). Therefore, the finding of increased anterior cingulate and amygdalar response in the non-marijuana smoking control subjects but not the chronic, heavy marijuana smokers during a masked affective paradigm is perhaps not unexpected. The relationship between both amount of use and overall cannabinoid concentration level and the BOLD signal changes suggest an effect of marijuana use in these regions which may impact the ability for individuals who are chronic, heavy marijuana smokers to process affective stimuli, even if not consciously doing so. This finding may explain why, as a group, the chronic, heavy marijuana smoking group demonstrates altered patterns of activation in these regions, as the amount of marijuana smoked per week may affect cingulate and amygdalar activity, evident during these two tasks. Further, in addition to lower overall activation in the marijuana smokers relative to the control subjects in the anterior cingulate, the activation detected for the marijuana smokers was more posteriorly located, perhaps reflective of a compensatory action of this region. In previous studies of cingulate function in marijuana smokers, findings have suggested that these individuals utilize different cortical processes from control subjects in order to complete tasks (Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005), which may represent an inherent alteration of the cingulate itself.
The whole brain data, while exploratory, were not constrained by a prioi hypothesized regional analyses, yet still demonstrated a robust difference between the groups for each of the task conditions. During the viewing of masked angry faces, the non-marijuana smoking control subjects demonstrated significant activation within nearly the same region of the cingulate demonstrated in the ROI analyses, suggesting that this region is important for the processing of masked angry stimuli. The chronic, heavy marijuana smokers did not exhibit the same pattern of activity as the non-marijuana smokers, and in fact appeared to activate posterior and temporal regions for both masked affective tasks in the whole brain analyses, perhaps underscoring the notion of a compensatory process at work in these subjects. It is of note, however, that despite the regional differences seen for both groups during the tasks, each group activated significant numbers of voxels, which, while distributed differently, argues against a global reduction in activation during the tasks for either group.
Results from this study are consistent with those of Phan and colleagues who utilized fMRI and a variation of the Emotional Face Processing Task in occasional marijuana smokers after the acute administration of oral THC or placebo (Phan et al., 2008). Briefly, this task requires subjects to match a target, consisting of either an emotional face (angry, fearful or happy) or a shape, with one of two choices, and has been shown to produce robust amygdalar activity during pharmacologic challenges (Hariri et al., 2002; Paulus et al., 2005). During the placebo condition, amygdalar activity was greater for threatening (angry, fearful) faces than non-threatening (happy) faces. In contrast, administration of THC significantly attenuated amygdalar activity in response to both types of threatening faces (angry or fearful). Interestingly, the extent of amygdalar attenuation was positively related to extent of increase of “feeling drug” reported by subjects on the Drug Effects Questionnaire. Given that subjects did not report any change in their reported levels of anxiety (neither an increase nor a decrease) following the THC administration, yet the drug attenuated threat-related amygdalar activity, the authors suggest that activation patterns detected by fMRI may be a more relevant indicator of the centralized effects of THC on conscious threat perception than behavioral measures, or that the self-report scales used, which were visual analog scales, may not be sensitive to the emotion or fear specific changes elicited by THC for the stimuli used. In either case, administration of THC altered amygdalar reactivity in response to overt social signals of threat in casual marijuana users, raising the likelihood that similar alterations in neural networks associated with affective processing would be detected using non-overt or masked stimuli in chronic, heavy marijuana smokers.
A study by Limonero and colleagues examined the association between self-reported perceived emotional intelligence (PEI) using an abbreviated version of the Trait Meta Mood Scale (TMMS), a measure designed to assess an individual's ability to perceive, understand and manage emotion in university students who frequently smoked marijuana compared to non-smoking students (Limonero et al., 2006). The authors report that the marijuana smoking students scored significantly lower on emotional repair, a measure of the ability to regulate and modulate one’s emotions or feelings relative to the non-smokers, and that this measure was inversely correlated with marijuana use. No differences were detected between the groups on the ability to think about one’s feelings (termed “attention”) or in the ability to discriminate among moods or emotions (termed “clarity”). These findings provide further evidence that marijuana smoking individuals may have difficulty regulating or controlling their emotions, and may not process affective or emotional stimuli in the same way as those who do not smoke.
Findings from the current investigation also complement previous animal studies which have demonstrated alterations in behavior in genetically altered animals and those which administer both low (Cherek et al., 1980) and high doses (Alves et al., 1973) of THC. Animal studies have reported that the administration of cannabinoids can induce both anxiogenic and anxiolytic response, depending on both the dose administered and the familiarity of the animal to its environment (Rodriguez de Fonseca et al., 1996). Specifically, low doses appear to produce anxiolytic-like responses while higher doses result in more anxiogenic-like behaviors, including increased muricidal behavior (Bac et al., 1998). In one recent study of CB-1 knockout mice, increased aggressive behavior was noted following a resident-intruder procedure, where an animal is exposed to a novel, or intruding animal for four minutes, relative to wild-type animals (Martin et al., 2002), underscoring the relationship between CB-1 receptors and behaviors related to anxiety. Hill and colleagues (2006) examined the effects of administering both a very low (5 µg/kg) and very high (100 µg/kg) dose of the cannabinoid CB1 agonist HU-210 in rats, and reported increased anxiety-like behavior and higher plasma corticosterone levels in animals treated with chronic high but not low dose HU-210. These results suggest that higher cannabinoid doses may result in increased emotionality and sensitization of the stress axis. Finally, Patel and colleagues’ (2005) finding of increased CeA c-Fos expression following the combination of stress and the administration of a CB-1 agonist in mice lends support to the current study findings. The authors conclude that CB-1 receptor activation effectively reduces the threshold at which salient sensory stimuli activate the basolateral amygdala (BLA) –CeA pathway (Katona et al., 2001). This mechanism may account for the finding that subthreshold environmental stress or even normally neutral stimuli can acquire emotional or affective salience during marijuana intoxication (Patel et al., 2005). This may help to explain why individuals who smoke marijuana regularly do not appear to process affective stimuli in the same way as those who do not smoke, even when the stimuli are presented at a level which does not result in conscious processing. Findings from these animal studies, which have demonstrated both anxiolytic and anxiogenic responses to cannabinoid agonists depending on the doses administered are somewhat different from human studies of marijuana use, which often report the more positive subjective experiences related to marijuana use, including increased feelings of relaxation and euphoria (Hollister et al., 1986). These seemingly discrepant findings may in fact be related to overall dose administered over time, environmental factors, and socialization, as well as the inherent difference between human subjects’ self-report of mood and affective state as compared to more pure laboratory-based observations, which are possible with animal subjects. Regardless, results from animal studies which have included cannabinoid agonists provide evidence of a relationship between CB-1 receptor activation and affective or emotional state, which may facilitate interpretation of our human study results.
It is tempting to assume that based on animal studies, which show an activation of a specific amygdalar pathway following the combination of stress and a CB-1 agonist, chronic, heavy marijuana smokers would show increased amygdalar response to affective stimuli. While we observed an increase in amygdalar activity in the contrast analyses which examined the non-smoking control subjects relative to the marijuana smokers during the tasks (NC > MJ), we did not see this response in the contrast analyses which examined the chronic, heavy marijuana smoking group relative to the non-smoking control subjects (MJ > NC). This may be a result of the stimuli being masked, which does not allow the conscious processing of the affective state displayed on the faces, although the non-marijuana smoking control subjects did demonstrate an amygdalar response. The lack of relative increased activity in amygdalar regions in the chronic, heavy marijuana smokers relative to the non-smoking control subjects may suggest a disruption early in the network responsible for detection and early processing of stimuli, which is then further reflected in the altered cingulate response to the masked faces. The positive association noted between number of smokes per week and amygdalar activity during the viewing of masked happy stimuli despite the relative lack of activity noted in the region when compared to non-smoking healthy controls suggests that the use of marijuana may lead to a system-wide alteration in inhibitory/excitatory circuitry which engages both cingulate and amygdalar regions. It is also possible that increased marijuana use may result in reduced inhibitory function for positive stimuli and increased inhibitory function for negative stimuli therefore modulating response within fronto-limbic regions. This may help to explain why marijuana smokers often report increased feelings of relaxation and affiliatory behavior after smoking (Green et al., 2003).
Results from this study are also consistent with previous fMRI studies neuroimaging studies which demonstrated increased activity of the anterior cingulate and amygdala during the presentation of masked affective faces in healthy control subjects. In an fMRI study of healthy control subjects, Whalen and colleagues (1998) utilized a backward masking paradigm which included masked happy and fearful faces. The authors reported increased activation within the amygdala during masked fearful faces and decreased activity within the amygdala during the viewing of masked happy facial affect. Killgore and Yurgelun-Todd (2004) examined healthy control subjects during a backward masking paradigm which included masked happy and sad faces. Results from that study suggest increased activity within bilateral anterior cingulate gyrus and the amygdala in response to masked happy faces, and increased left anterior cingulate gyral activity in response to masked sad faces. The authors conclude that both the anterior cingulate and amygdala are key regions of the neural network responsible for detecting and discriminating affective information presented below the level of conscious awareness.
Studies of individuals with affective disorders that have used masked paradigms also report activity within anterior cingulate and amygdalar regions. Sheline et al. (2001) used a backwad masking paradigm to examine depressed subjects prior to and following a course of antidepressant treatment and compared their activation levels to healthy control subjects. At baseline, prior to treatment, depressed subjects demonstrated exaggerated left amygdalar activity relative to control subjects for masked happy and fearful faces, which was greatest for masked fearful faces. Following antidepressant treatment, bilateral activation within the amygdala was reduced or “normalized” relative to baseline levels for both facial affect types. The authors conclude that even when presented below conscious awareness, depressed subjects demonstrate a hyperarousal of the amygdala in response to masked facial affect. Results from these investigations underscore the importance of the anterior cingulate and amygdala in affective processing, even when the stimuli is not consciously perceived by the individual. These data are also consistent with other investigations, suggesting that the anterior cingulate serves an indirect function by integrating emotional and attentional processing, and that the amygdala is specifically involved in detection and early processing of stimuli not available to conscious awareness (Morris et al., 1999). Individuals who smoke marijuana, even though not currently intoxicated, may experience a disruption or alteration of these networks which in turn affects the processing of emotional stimuli.
Alterations within the brain following THC administration are not surprising, given the wide distribution of CB-1 receptors throughout the brain, highly concentrated in areas which have been shown to mediate emotional and affective behavior. Postmortem studies have, in fact, provided evidence for a relationship between CB-1 receptor activity and affective processing. Increases in CB-1 receptor levels have been reported within the prefrontal cortex of suicide victims (Hungund et al., 2004) who met diagnostic criteria for both depression and alcoholism relative to those who met only for alcohol dependence (Vinod et al., 2005), indicating a specific change relative to the affective disorder. In another investigation of CB-1 receptors, Zavitsanou et al. (2004) examined radioligand binding of [3H]SR141716A, an antagonist that specifically targets CB1 receptors of the endogenous cannabinoid system, using quantitative radiographic techniques on postmortem tissue of schizophrenic patients and healthy controls. The authors report a significant increase of CB-1 binding in the schizophrenic brains relative to those of the control subjects, suggesting changes in the endogenous cannabinoid system in the ACC may be involved in the pathology of schizophrenia (Zavitsanou et al., 2004).
Several limitations should be considered with regard to interpreting the study findings. The data sample acquired in this study was moderate in size (15 per study group) which limits the generalizability of the study findings. In addition, fMRI data in this study was acquired for whole brain, and these findings were reported as exploratory analyses, however, the main results reported here are focused on specific cortical regions, hypothesized a priori to be associated with the challenge paradigms. The regions chosen, the cingulate gyrus and amygdala, are known to be sensitive to susceptibility artifact. This type of artifact makes it difficult to interpret null or negative results, as the absence of a significant difference between study conditions may either reflect the true state or be due to signal loss. Given the fact that we observed significant differences in both cingulate and amygdalar responses to both masked affective stimuli types between groups, the question of signal distortion possibly resulting in null effects is not likely. Further, regions of interest used in the current study were based on the use of an atlas within SPM, rather than hand-drawn individualized ROIs. While this method affords consistency of localization for the regions of interest for analyses both within and between subject groups, results must be interpreted as regional, rather than absolutely anatomically precise.
One additional limitation for the current study findings is related to the nature of the study participants. In general, all participants were well educated, and in the case of the chronic marijuana smoking subjects, did not meet diagnostic criteria for marijuana dependence. This was somewhat surprising, given the smokers’ high levels and frequency of use. Marijuana smokers willingly and openly reported their level of use, (which was highly correlated with their urinary cannabinoid levels, adding credibility to their reporting), yet did not report any negative impact of their smoking on their social, physical, educational or occupational function, nor did they report experiencing symptoms associated with withdrawal while undergoing testing. In order to better understand the characteristics of the marijuana smoking sample, we examined scores for each of the seven elements which comprise marijuana dependence. We therefore calculated the percentage of the sample who endorsed (with a rating of 2 or subthreshold, or 3, threshold) the following criterion: tolerance (increased amount needed or decreased effect with same amount of marijuana= 33.3%; withdrawal = 0%; marijuana taken in larger amounts or for longer period than planned = 39.9%; desire to control or stop use of marijuana = 0 %; time required to obtain drug or recover from effects = 26%; given up activities = 6%; continued use of marijuana despite knowledge of worsening physical or psychological issue = 0%. Criteria for dependence is met with a score of “3” or “threshold” for a minimum of three items, and while no individual subject within our sample endorsed any three items, and therefore did not meet criteria for dependence, subjects did acknowledge some degree of tolerance, greater use than planned and time required to acquire the drug. The study findings, therefore, may be limited in generalizability to individuals who are chronic, heavy marijuana smokers but who do not endorse the more negative effects of marijuana use (i.e. psychological issues, inability to stop or cut down on use, withdrawal effects), and to those who do not meet for dependence, despite frequent, heavy use.
Further, while our study cohort did not include subjects with any comorbid psychiatric disorders or a current or past history of other substance dependence or abuse, our marijuana smoking subjects did report more frequent use of alcohol than the non-marijuana smoking control subjects. Regression analyses of the association between total number of drinks over the last 30 days and brain activation within the marijuana smokers, however, suggest that while there is some detectable cingulate gyrus activation associated with alcohol use in the last month for the marijuana smokers, this is in different regions of the cingulate cortex than those reported to be associated with marijuana use alone. Therefore, it appears that the relationship between marijuana use and BOLD activation during both masked affective tasks is different from the one detected for alcohol use and BOLD activity. Future studies will need to address the relationship between alcohol and marijuana use in order to determine the specific effects of each substance on brain activation patterns.
Differences in personality or social style between the subject groups must also be considered. Subjects from the current investigation were well characterized with regard to their clinical and demographic status, and were investigated using a backward masking task, preventing subjects from consciously “overthinking” about the stimuli, however, formal personality testing was not completed. Although we did not evaluate subjects on the basis of their overall level of social interaction or other social functions, it is possible that marijuana use exerts an effect on social style, thus, some marijuana smokers may be more social than others, or less so. As our sample is comprised of fairly young adults, all of whom are clinically stable, are functioning at a fairly high level in either their academic or employment settings and who primarily belong to extended social groups, we do not believe that our findings are related to differences in social interactive style between the groups. The potential confound of marijuana use on social behavior should be investigated in future studies which include measures of personality and interactive behaviors. It is also unlikely that our results are due to acute marijuana intoxication. All study subjects reported being abstinent from marijuana use for a minimum of 12–16 hours prior to their scanning session, and fully expected that the investigators would be able to tell if they had used the drug since the previous evening upon their arrival at the lab, underscoring the likelihood that subjects were not intoxicated at the time of testing. Similarly, it is unlikely that the observed changes in the chronic marijuana smokers are the result of withdrawal effects from the drug. No subject endorsed any withdrawal symptoms at the time of testing, however, given the previous findings of withdrawal-related symptoms emerging between 24 and 48 hours after abstinence from marijuana (Haney et al., 1999; Kouri and Pope, 2000; Budney et al., 2001, 2002, 2004), this is not surprising, as subjects were seen well within a 24 hour period from their last use. Of course, despite subjects’ own report of no overt withdrawal symptoms, we cannot rule out the possibility that some of the observed differences are not related to short term withdrawal that occurs at a biologic and not psychological level, and thus, remains undetectable to subjects themselves. One strength of the current study, however, is the naturalistic design, which allowed for the examination of marijuana smokers who were not asked to complete an extended period of abstinence. Study findings are therefore more likely to be reflective of chronic marijuana smokers in everyday life and not those who are constrained by laboratory based situations. Future investigations should include more sensitive, quantifiable measures related to withdrawal from marijuana, especially given that most of the withdrawal syndrome in marijuana smokers does not begin until at least 24 hours after last use. An important next step in this research is the examination of a sample of chronic marijuana smokers who complete a lengthy period of abstinence in order to assess whether the findings reported in the current investigation remain once the smokers are free of THC.
To our knowledge, this is the first fMRI study of regional brain differences in response to the masked affective stimuli in chronic, heavy marijuana smokers and non-marijuana smoking control subjects. Results from the current investigation suggest that individuals who are chronic, heavy marijuana smokers do not appear to process affective stimuli in the same way as those who do not smoke. This is true despite the fact that the stimuli used in the current investigation were administered below the level of conscious awareness, suggesting a disruption early in the neural circuit responsible for affective processing. Given the behavioral alterations and difficulty in inhibiting inappropriate responses often seen in marijuana smokers, this finding may have implications for decision making, which may result in negative consequences. The ability to accurately perceive emotion and identify the affective states of others is critical for effective communication. Further research is needed to determine the long-term effect of marijuana on affective processing and the potential for altering these neural changes through moderation or abstinence from the drug.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves CN, Goyos AC, Carlin EA. Aggressiveness induced by marijuana and other psychotropic drugs in REM sleep-deprived rats. Pharmacol Biochem Behav. 1973;1:183–189. [Google Scholar]
- Bac P, Pages N, Herrenknecht C, Paris M. Measurement of the three phases of muricidal behavior induced by delta9-tetrahydrocannabinol in isolated, fasting rats. Physiol Behav. 1998;63:815–820. doi: 10.1016/s0031-9384(97)00543-x. [DOI] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RA, Hughes JR. Onset, magnitude and duration of abstinene effects following heavy marijuana use. Drug Alcohol Depend. 2002;66S23 [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandry R. Review of the validity and significance of the cannabis withdrawal syndrome. Am J Psychaitry. 2004;11:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacol Biochem Behav. 1988;29:553–557. doi: 10.1016/0091-3057(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. 'Hangover' effects the morning after marijuana smoking. Drug Alcohol Depend. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-x. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Thompson T, Kelly T. Chronic delta 9-tetrahydrocannabinol administration and schedule-induced aggression. Pharmacol Biochem Behav. 1980;12:305–309. doi: 10.1016/0091-3057(80)90374-3. [DOI] [PubMed] [Google Scholar]
- Clopton PL, Janowsky DS, Clopton JM, Judd LL, Huey L. Marijuana and the perception of affect. Psychopharmacology (Berl) 1979;61:203–206. doi: 10.1007/BF00426737. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-Axis DIsorders - Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Galanter M, Stillman R, Wyatt RJ, Vaughan TB, Weingartner H, Nurnberg FL. Marihuana and social behavior; a controlled study. Arch Gen Psychiatry. 1974;30:518–521. doi: 10.1001/archpsyc.1974.01760100082013. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999a;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999b;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Little M, Johnson M, Melvin L, DeCosta B, Rice K. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, Mann JJ, Arango V. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Clopton PL, Leichner PP, Abrams AA, Judd LL, Pechnick R. Interpersonal effects of marijuana. A model for the study of interpersonal psychopharmacology. Arch Gen Psychiatry. 1979;36:781–785. doi: 10.1001/archpsyc.1979.01780070059006. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2004. Rockville, MD: National Institutes of Health; 2005. p. 72. [Google Scholar]
- Joseph R. Neuropsychiatry, Neuropsychology, Clinical Neuroscience. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharm. 2000;4:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limonero JT, Tomas-Sabado J, Fernandez-Castro J. Perceived emotional intelligence and its relation to tobacco and cannabis use among university students. Psicothema. 2006;18 Suppl:95–100. [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain Res. 1998;797:183–189. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, Provenzale J. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res. 2002;116:173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood States. San Diego: Educational & Industrial Testing Services; 1971. [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating "unseen" fear. Proc Natl Acad Sci USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas G. General Toxicity of cannabis. In: Nahas G, Latour C, editors. Cannabis: Physiopathology, Epidemiology, Detection. Boca Raton, FL: CRC Press; 1993. pp. 5–17. [Google Scholar]
- O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, Watkins GL, Hurtig RR, Andreasen NC, Hichwa RD. Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport. 2000;11:3835–3841. doi: 10.1097/00001756-200011270-00047. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Tart CT. On Being Stoned: A Psychological Study of Marijuana Intoxication. Palo Alto, CA: Science and Behavior Books; 1971. [Google Scholar]
- Tinklenberg J, Darley C. A model of marihuana's cognitive effects. New York: Raven Press; 1976. [Google Scholar]
- Vinod KY, Arango V, Xie S, Kassir SA, Mann JJ, Cooper TB, Hungund BL. Elevated levels of endocannabinoids and CB1 receptor-mediated G-protein signaling in the prefrontal cortex of alcoholic suicide victims. Biol Psychiatry. 2005;57:480–486. doi: 10.1016/j.biopsych.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP. Cannabis use, cognitive performance and mood in a sample of workers. J Psychopharmacol. 2006;20:14–23. doi: 10.1177/0269881105056644. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]