Abstract
Background
Tetralogy of Fallot (TOF) typically results in clinical cyanosis or volume overload of the left ventricle (LV) depending upon the direction and magnitude of shunting across the ventricular septal defect (VSD). This study examines the effects of surgical TOF repair on LV mechanics and compares these changes between patients with VSD shunts that are predominantly right-to-left (R-L; “blue TOF”) and those with VSD shunts that are predominantly left-to-right (L-R; “pink TOF”).
Methods and Results
11 patients (6 R-L and 5 L-R) age 4.3-18.4 months, (median 7.1 months) were studied. LV end-diastolic area (EDA) was calculated from transesophageal echocardiograms obtained during initiation and weaning of cardiopulmonary bypass. LV end-diastolic pressure (EDP) was measured by micromanometer. Compliance was assessed by EDP-EDA curves. Contractility was assessed from preload recruitable stroke work (PRSW), using the stroke work vs. LV EDA relation. VSD shunt direction was determined by pre-operative Doppler echocardiography. Changes in LV function at the conclusion of cardiopulmonary bypass included decreased stroke area (6.6±0.9 to 4.1±0.4 cm2/m2, p=0.012) and ejection fraction (55±2% to 41±3%, p<0.001). LV EDA at a common pressure in 8 patients decreased (10.4±1.4 to 7.6±1.2 cm2/m2, p=0.003), suggesting a decrease in ventricular compliance. Additionally, the end-diastolic P-A curves shifted to the left in all patients. PRSW decreased (34.8±2.4 to 21.8±2.6 mmHg, p=0.007) demonstrating a decrease in ventricular contractility. When separated by preoperative shunt direction, LV EDA increased in R-L patients by 0.9±0.5cm2/m2 postoperatively, but decreased in L-R by 4.3±0.8 cm2/m2 (p<0.001). Area ejection fraction decreased in all patients independent of shunting or change in LV EDA.
Conclusions
LV diastolic and systolic function are depressed after TOF repair. Mechanical effects of the VSD patch and myocardial depressant effects of ischemia and reperfusion during surgery probably contribute to the observed changes in LV mechanics. Different effects of surgical repair on LV preload in “pink” and “blue” TOF also contribute to the spectrum of clinical results observed after surgery.
Keywords: Tetralogy of Fallot, Pediatrics, Mechanics (Ventricular), Surgery, Cardiopulmonary bypass
Introduction
Tetralogy of Fallot (TOF) is a cyanotic congenital heart lesion with a prevalence of 0.26-0.80 per 1000 live births, accounting for approximately 10 percent of all cases of congenital heart disease1. The surgical repair of TOF involves closure of the ventricular septal defect (VSD) and relief of the right ventricular (RV) outflow tract obstruction. Originally, surgical repair of TOF was performed in older children; however, current clinical management involves complete repair in infancy, usually before the age of one-year. These surgical corrections result in several acute physiological changes, including a decrease in RV hypertension and the elimination of ventricular shunting, resulting in a change in the volume load of both ventricles.
While the postoperative changes in function and mechanics of the RV and interventricular septum have been studied extensively2-6, the effects upon the left ventricle (LV) have not been fully evaluated in the modern surgical era.
We aimed to describe the acute changes in LV mechanics and function after surgical TOF repair, and to determine if the direction of preoperative VSD shunting affects these changes.
Methods
Intraoperative data were collected prospectively from 1997-2004. Patients were enrolled prior to surgery for complete repair of Tetralogy of Fallot. Method of repair was determined by the surgeon at the time of surgery based upon available clinical information. The Columbia University Institutional Review Board approved the protocol and informed consent was obtained from the patient's legal guardian.
Clinical Data
Patient demographics including age, weight and body surface area (BSA) at the time of surgery, prior interventions, and associated anatomic anomalies were noted. Intraoperative variables recorded included type of surgical repair, total cardiopulmonary bypass time, aortic cross-clamp time and the presence or absence of a residual atrial communication. Preoperative transthoracic echocardiograms were analyzed for right ventricular outflow tract anatomy and a pulmonary annulus diameter was measured in the parasternal long-axis. The absolute diameter was converted to a z-score based upon BSA. The predominant direction of shunting through the ventricular septal defect preoperatively was determined by qualitative assessment of color Doppler images by a clinical echocardiographer independent from the study. For further analysis, patients were divided into two groups based upon the predominant direction of blood flow through the ventricular septal defect, right-to-left (R-L) or left-to-right (L-R).
Intraoperative Data Acquisition
All patients were anesthetized, heparinized and cannulated for cardiopulmonary bypass per clinical protocol. A 5-Fr MPC500 micromanometer-tipped catheter (Millar Instruments, Houston, TX) was inserted through a purse-string suture in the proximal ascending aorta and advanced into the left ventricle. All waveform data, including ventricular pressure, systemic arterial pressure and surface electrocardiogram, were digitized at 200 Hz and imported via a 16-channel analog-to-digital converter (ADInstruments Inc., Milford, MA) to a portable computer (Apple Computers, Cupertino, CA). Transesophageal short-axis echocardiograms at the level of maximal LV area were obtained using a Sonos 5500 ultrasound system (Hewlett-Packard Co., Palo Alto, CA) and recorded on videotape.
Pressure data and echocardiograms were recorded in steady state periods (periods of stable ventricular preload without changes in vasoactive medications) immediately before and after cardiopulmonary bypass, and during ventricular emptying and filling upon initiation and withdrawal of bypass. To facilitate correlation of beats, pressure and electrocardiogram data were recorded with the echo images, and artifacts were added to the pressure channel. This allowed for accurate beat-to-beat comparison of the echocardiographic images with the pressure tracings7.
Data Analysis
Echocardiograms were planimetered offline on a VingMed CFM800 (GE Healthcare, Chalfon St. Giles, United Kingdom). During the steady state period, cyclic variation in the mean arterial pressure was used to identify beats at end-expiration, which were then used for analysis. During preload alteration with initiation and withdrawal of cardiopulmonary bypass, all beats were reviewed and beats were excluded from analysis for atrial or ventricular ectopy, poor echo quality or artifacts in the pressure signal. End-diastolic area (EDA) and end-systolic area (ESA) were measured by planimetry of the LV endomyocardial borders in accordance with American Society of Echocardiography standards. The eccentricity index was used as an index of geometry and was defined as the ratio of the septal-freewall diameter to the anterior-posterior diameter in short-axis images of the LV with a value of 1 indicating circular cross-sectional geometry8. LV mass was approximated by Am, defined as the EDA subtracted from the area created by the epicardial tracing in end diastole. Am vs. EDA was plotted and modeled as previously reported to correct for changes in end-diastolic volume9. The corrected postoperative Am was used for comparison with the preoperative Am.
Utilizing artifacts on the ventricular pressure tracing, corresponding beats were identified on the echocardiogram in order to correlate EDA and end-diastolic pressure (EDP) in the same cardiac cycle. Pressure was digitally differentiated. EDP was identified as the pressure preceding the rapid upstroke, determined from the rate of increase of ventricular pressure (dP/dt). EDP was plotted against EDA for the creation of compliance curves and calculation of diastolic indices. This process generally yielded a data set of 10 to 15 beats for analysis at the initiation and withdrawal of bypass.
Pre-bypass and post-bypass ventricular and arterial pressure were analyzed with custom routines developed in Matlab (The Mathworks Inc., Natick, MA), including identification of EDP and mean ejection pressure (MEP).
Functional Indices
All measurements of area were normalized to patient BSA. Stroke area (SA) was used as a surrogate for stroke volume and defined as SA=EDA-ESA. Ejection fraction (EFa) was calculated from the equation EFa= (SA/EDA)×100%. Preload recruitable stroke work (PRSW), a load-independent measure of contractility, was defined as the slope of the linear regression between stroke work (SW=SA×(MEP-EDP)) and EDA. Cardiac index was calculated by the formula CI=SA×(heart rate). Diastolic function was assessed by creating end-diastolic pressure-area curves by fitting data to the equation EDP=αeβ(EDA), where α and β are curve fitting constants. For all curves, a correlation coefficient (r) was calculated to evaluate the goodness of fit.
Statistical Methods
Data are expressed as the group mean ± standard error of the mean. The paired t-test was used to analyze pre-post data within group, and the student's t-test was used to compare differences between shunting groups. Repeated measures Analysis of Variance (ANOVA) was used to compare pre-post data between the shunting groups. All data were analyzed using SAS system software (SAS Institute, Cary, NC). Significance was defined as a p-value less than 0.05. P-values were not adjusted for the multiple tests. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
11 patients were studied at the time of full repair of TOF at a median age of 7.1 months (range: 4.3-18.4) with a mean weight of 8.1 kg (range: 6.9-10.0) and mean BSA of 0.39 m2 (range: 0.34-0.48). Previous interventions were performed in three patients consisting of two modified Blalock-Taussig shunts and one central shunt, all performed for cyanosis in the newborn period. These three patients did not differ to their unshunted counterparts in all preoperative characteristics (i.e. hemoglobin concentration, LV dimensions, systolic function) except for age. These patients were 6.7, 15.1 and 18.4 months of age at the time of repair, somewhat older than the rest of the patients studied.
A pulmonary valve annulus sparing procedure was done in 8 patients and a transannular patch was used in 2 patients. Of the 8 patients with an annulus sparing procedure, 5 had extensive infundibular resections without the need for an infundibular patch, and 3 did not require extensive infundibular resection. The remaining patient required a right ventricle to pulmonary artery homograft for an anomalous course of the left circumflex artery crossing the right ventricular outflow tract. Mean cardiopulmonary bypass time was 80±40 minutes with a mean aortic cross-clamp time of 42±16 minutes.
No patient had a residual atrial communication postoperatively. VSD patch leaks were identified in 4 patients; all were trivial shunts by color Doppler echocardiogram and restrictive to pressure based upon Doppler waveform analysis. Seven of the 8 patients undergoing valve sparing procedures had residual pulmonary stenosis with a mean gradient of 30mmHg (range: 13mmHg-46mmHg) on post-operative transthoracic echocardiogram. All patients were in normal sinus rhythm before and after cardiopulmonary bypass and all exhibited a right ventricular conduction delay or a right bundle branch block pattern on postoperative 12-lead surface electrocardiogram.
Preoperative echocardiograms showed predominantly right-to-left (R-L) shunting through the ventricular septal defect in 6 patients and left-to-right (L-R) shunting in 5 patients. The mean preoperative hemoglobin for the R-L shunting group was 15.8±3.2 g/dl, which was significantly higher than the mean hemoglobin in the L-R shunting group of 11.8±0.4 g/dl (p=0.025). Only one patient in the R-L shunting group had a hemoglobin value in the normal range for age. Furthermore, the mean pulmonary valve annulus diameter z-score was significantly smaller in patients in the R-L shunting group as compared to the L-R shunting group (-2.7±0.4 vs. -0.5±0.3, p=0.001). Only one patient in the R-L shunting group had a z-score greater than -2 (-1.76), and no patient in the L-R shunting group had a z-score smaller than -1.2. There were no other significant differences in baseline clinical characteristics between the two groups. One patient in each group required a transannular patch as part of the surgical repair.
Postoperative Changes
Steady state parameters were compared from immediately before and after cardiopulmonary bypass (see Table 1). Systolic function decreased immediately post-bypass as exhibited by a decrease in mean LV stroke area (6.6±0.9 cm2/m2 to 4.1±0.4 cm2/m2, p=0.012) and mean ejection fraction (56±2% to 41±3%, p<0.001). Despite this decrease in function there was no significant decrease in calculated cardiac index (p=0.28) as mean heart rate increased from 132±5 bpm to 158±5 bpm (p=0.004). Additionally, there was an increase in mean corrected Am from 10.6±1.2 cm2/m2 pre-bypass to 12.5±1.3 cm2/m2 post (p=0.014).
Table 1.
Mean pre- and post-cardiopulmonary bypass (CPB) values for all patients. (PRSW=Preload Recruitable Stroke Work, LV SA=Left Ventricular Stroke Area, LV SW=Left Ventricular Stroke Work, LV EDA=Left Ventricular End-Diastolic Area, Am=EDA corrected Myocardial Area)
| Pre-CPB | Post-CPB | p-value | |
|---|---|---|---|
| Heart Rate (bpm) |
132±5 | 158±5 | 0.004 |
| PRSW (mmHg) |
34.8±2.4 | 21.8±2.6 | 0.007 |
| LV SA (cm2/m2) |
6.6±0.9 | 4.1±0.4 | 0.012 |
| LV SW (cm2 × mmHg/m2) |
279±54 | 171±21 | 0.032 |
| Ejection Fraction (%) |
56±2 | 41±3 | <0.001 |
| Cardiac Index (cm2/min/m2) |
890±133 | 683±49 | 0.15 |
| LV EDA at constant pressure (cm2/m2) |
10.4±1.4 | 7.6±1.2 | 0.003 |
| Am (cm2/m2) |
10.6±1.2 | 12.5±1.3 | 0.014 |
Complete data during preload alteration via initiation and weaning from cardiopulmonary bypass were available in eight patients. Three patients were excluded from this analysis due to technically poor echocardiograms. Similar to the steady state data, LV systolic function as measured by PRSW decreased in all patients from a mean of 34.8±2.4 mmHg pre-bypass to a mean of 21.8±2.6 mmHg post-bypass (p=0.007). To assess diastolic function, individual compliance curves were created for each patient. All curves had a correlation coefficient value (r-value) between 0.90-0.99. In each individual patient, the curve shifted to the left post-bypass indicating a post-bypass decrease in ventricular compliance. Representative curves are presented in Figure 1. Furthermore, all eight patients exhibited a decrease in LV EDA when compared at a constant pressure nearest to 5 mmHg pre- and post-bypass. The mean pre-bypass EDA at a constant pressure was 10.4±1.4 cm2/m2 which decreased to a mean of 7.6±1.2 cm2/m2 after surgical repair (p=0.003).
Figure 1.
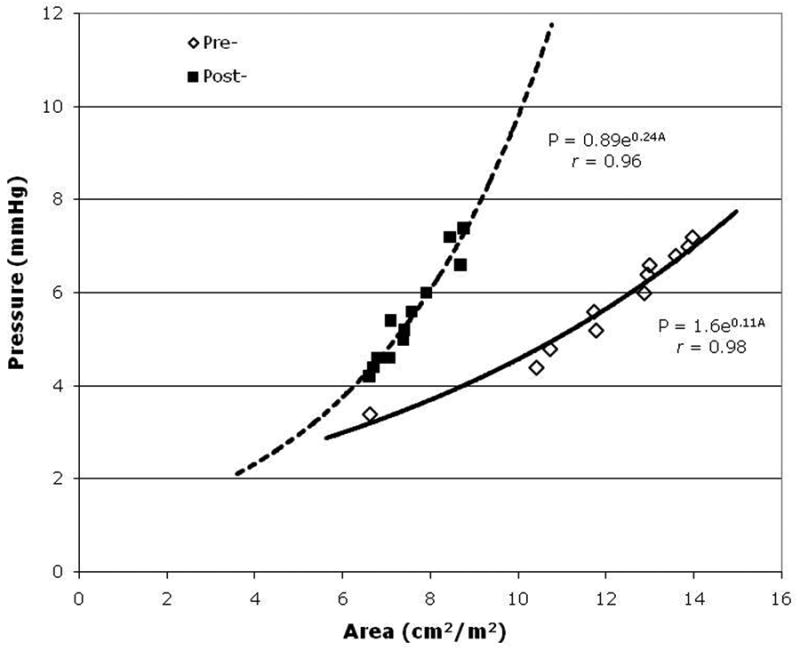
Representative pressure-area (P-A) curve generated from data collected pre-(open diamonds) and post- (black squares) cardiopulmonary bypass.
Effect of Preoperative VSD Shunt Direction
Preoperative measurements were compared between the two groups. Most measures were equal in both groups (see Table 2), with the exception of the end-diastolic eccentricity index which was lower in patients with R-L shunting (0.61±0.04 vs. 0.85±0.05 p=0.01). There was no significant difference in the relative changes pre- to postoperatively between the two groups and the difference between the groups persisted post-bypass (0.51±0.04 vs. 0.78±0.04, p=0.03). Further comparisons between groups revealed a difference in the response of LV EDA and SA after repair. Steady state EDA increased by a mean of 0.9±0.5 cm2/m2 in the R-L shunting group, whereas it decreased by a mean of 4.3±0.8 cm2/m2 in the L-R shunting group (p<0.001, see Figure 2). This resulted in nearly equal postoperative LV EDA mean values of 10.1±1.6 cm2/m2 and 11.1±2.2 cm2/m2 respectively (p=0.7). While SA decreased in all patients, the decrease was significantly larger in patients with L-R shunting physiology (-4.6±1.3 cm2/m2 vs. -0.8±0.3 cm2/m2, p=0.013, see Figure 3). Similarly there was a larger decrease in calculated cardiac index in the left-to-right shunting patients (-460±224 cm2/min/m2) as compared to the right-to-left shunting group (-5±83 cm2/min/m2) although this difference was not statistically significant (p=0.075).
Table 2.
Mean pre- and post-cardiopulmonary bypass (CPB) values for patients separated by ventricular septal defect shunt direction. There was no significant difference between the right-to-left (R-L) and left-to-right (L-R) shunting groups in these parameters at baseline. P-values are for the difference in the pre- to post- change between the two groups. (PRSW=Preload Recruitable Stroke Work, LV SA=Left Ventricular Stroke Area, LV SW=Left Ventricular Stroke Work, LV EFa=Left Ventricular Ejection Fraction, LVEDA=Left Ventricular End-Diastolic Area, Am=EDA corrected Myocardial Area).
| R-L (n=6) |
L-R (n=5) |
||
|---|---|---|---|
| PRSW (mmHg) |
Pre-CPB | 36.0±3.6 | 32.9±3.6 |
| Post-CPB | 21.8±3.7 | 21.9±5.0 | |
| Change, p=0.68 | -14.2±4.5 | -11.0±6.1 | |
| LV SA (cm2/m2) |
Pre-CPB | 5.0±0.8 | 8.6±1.5 |
| Post-CPB | 4.2±0.6 | 4.0±0.3 | |
| Change, p=0.01 | -0.8±0.3 | -4.6±1.3 | |
| LV SW (cm2 × mmHg/m2) |
Pre-CPB | 215±24 | 360±111 |
| Post-CPB | 144±23 | 206±34 | |
| Change, p=0.34 | -70±8 | -154±95 | |
| EFa (%) |
Pre-CPB | 54±3 | 56±2 |
| Post-CPB | 42±4 | 40±6 | |
| Change, p=0.55 | -13±3 | -16±5 | |
| LVEDA at constant pressure (cm2/m2) |
Pre-CPB | 8.6±1.4 | 12.2±2.3 |
| Post-CPB | 6.6±0.9 | 8.7±2.2 | |
| Change, p=0.26 | -2.1±0.6 | -3.5±1.0 | |
| Am (cm2/m2) |
Pre-CPB | 10.9±1.5 | 10.0±2.1 |
| Post-CPB | 13.5±1.8 | 11.0±1.9 | |
| Change, p=0.28 | 2.6±1.0 | 1.1±0.5 |
Figure 2.
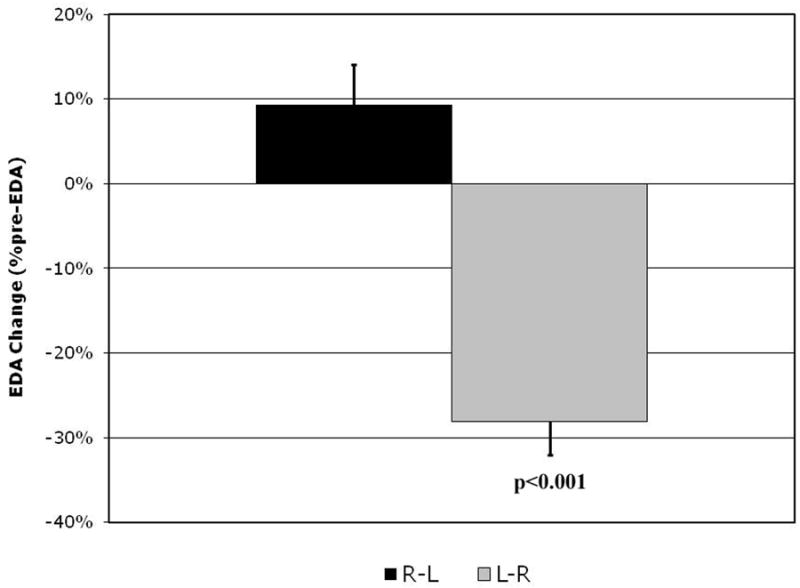
Change in postoperative left ventricular end diastolic area (EDA) expressed as a percent change from preoperative in patients with right-to-left (R-L) shunting (black column) vs. left-to-right (L-R) shunting (gray column).
Figure 3.
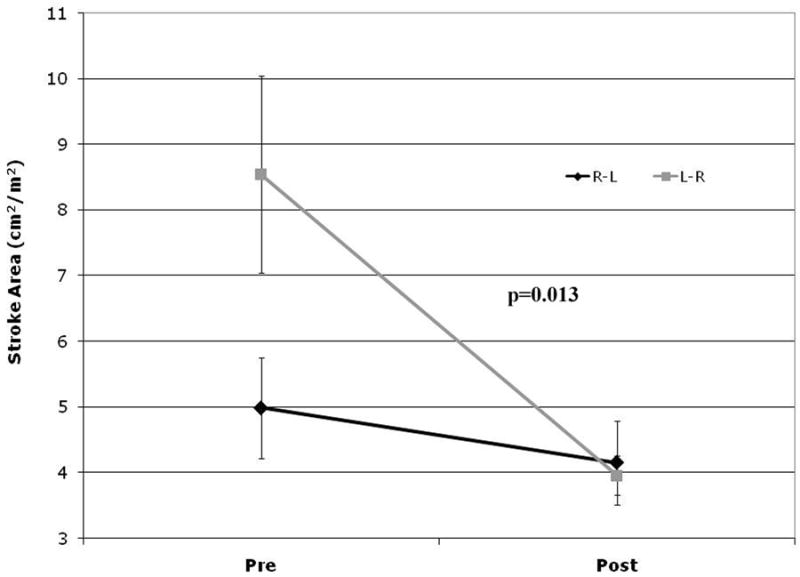
Graph representing pre- and postoperative stroke area in patients with right-to-left (R-L) shunting (black line) vs. left-to-right (L-R) shunting (gray line).
Discussion
Patients at one end of the spectrum of Tetralogy of Fallot have minimal right ventricular outflow obstruction, or so called “pink TOF”. These patients usually are acyanotic and are physiologically similar to patients with a simple VSD. At the other extreme are cyanotic patients with severe RV outflow obstruction, occasionally with complete pulmonary atresia with the degree of cyanosis reflecting the degree of right-to-left shunting across the VSD. The treatment strategies for patients along this spectrum of disease may differ (i.e. systemic to pulmonary shunt placement as a neonate vs. full repair at an older age), however all current methods require cardiac surgery with cardiopulmonary bypass.
Our study utilizes pressure-area measurements to demonstrate distinct changes in systolic and diastolic function of the LV after repair of TOF in the modern surgical era. LV systolic function decreased in all patients as measured by stroke area, stroke work, ejection fraction and preload-recruitable stroke work. Ventricular compliance also decreased, as demonstrated by a decrease in LV end-diastolic area at a constant end-diastolic pressure. This change in compliance was associated with a postoperative increase in myocardial mass in all patients.
Changes in LV geometry and loading after surgery were affected by the direction of preoperative VSD shunting. LV EDA increased in patients with predominant R-L shunting (“blue TOF”). Conversely, LV EDA decreased in patients with predominant L-R shunting (“pink TOF”). These divergent changes trended toward “normalization” of LV volumes, with convergence of postoperative LV EDA (Table 2). Ventricular eccentricity also differed between these two groups preoperatively, and, despite the convergence of LV EDA, this difference persisted postoperatively. This supports the view that elimination of anatomic lesions during surgery does not acutely normalize ventricular geometry10. Rather, reversal of chronic abnormalities of ventricular shape requires a period of weeks postoperatively, suggesting active remodeling. Follow-up studies of this phenomenon are indicated.
Most of the changes in systolic and diastolic LV function we observed were independent of the direction of preoperative VSD shunting. These include decreased LV compliance and increased LV mass. This combination suggests myocardial edema due to hemodilution and/or ischemic injury7, 9. Decreased compliance was also associated with decreases in systolic function. This may indicate a need for better myocardial protection strategies. However, the degree of systolic and diastolic LV dysfunction did not correlate with the duration of cardiopulmonary bypass or aortic cross-clamp (data not shown). The impact of the ventricular septal patch upon LV and septal function is unknown. Future studies examining the mechanical effects of septal patches upon the septum and ventricular systolic and diastolic function are indicated
Most previous studies of LV function in TOF repair involved older children, and a previous era of surgical and cardiopulmonary bypass techniques11-15. The differences between the conditions under which previously published data were collected and current practice is important, because it is likely that the ventricular mechanics of TOF and the effects of the surgical repair are affected by patient age 2, 11, 16.
Regarding unique surgical responses of TOF, Levin et al. noted abnormalities of isovolumic systole which increased with the severity of TOF13. Lower peak LV pressures and a lower LV end-diastolic volume have also been noted in cyanotic TOF11, 12. In 1972, Jarmakani et al. reported decreased ejection fraction pre- and postoperatively 11. In addition, LV wall mass increased postoperatively, suggesting that myocardial edema contributed to decreased systolic function. Studies of RV restrictive physiology also imply limitations of intraoperative myocardial protection6.
Decreased postoperative LV ejection fraction was also reported by Lange et al. in 1982 12. In Jarmakani's study LV end-diastolic volume increased after an elective left-to-right shunt, but not after complete repair11. This suggests that while increased LV preload tends to improve LV mechanics, other factors can cause a net decrease in systolic function postoperatively. Our data support this hypothesis (Figure 4).
Figure 4.
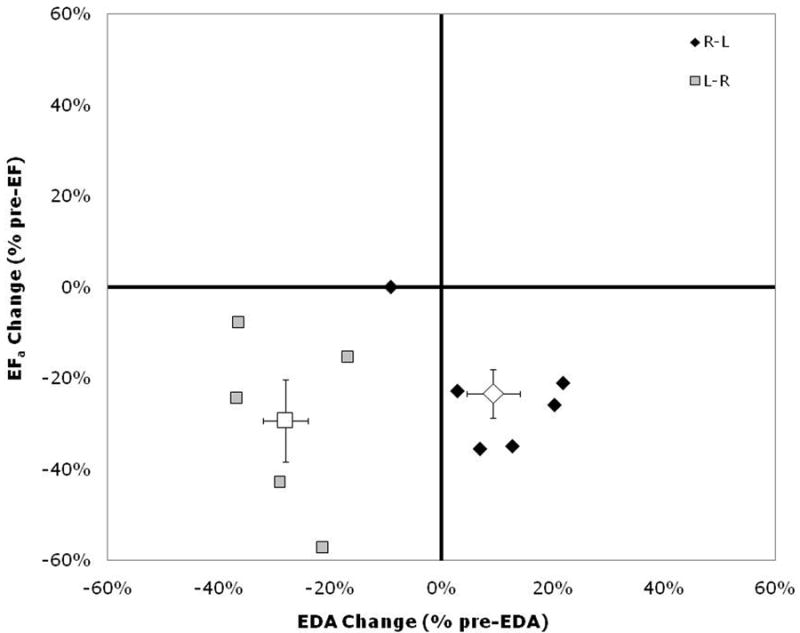
Changes in postoperative left ventricular end-diastolic area (EDA) and ejection fraction (EFa) expressed as a percentage of preoperative values. Right-to-left shunting (R-L) patients (black diamonds) fall in the lower-right quadrant, while the left-to-right shunting (L-R) patients (gray squares) fall in the lower-left quadrant. Group means are shown by the open symbols. Error bars indicate group standard error. A simultaneous decrease in EFa and EDA is consistent with a primary effect of reduced preload, but a decrease in EFa while EDA increases implies decreased contractile function or a large increase in afterload.
RV ejection fraction is also subnormal before and after surgery17. Shunting or repair normalizes RV end-diastolic volume similar to the normalization of LV EDA in our study17.
Cullen et al. associated RV restrictive physiology with prolonged postoperative recovery and clinical evidence of low cardiac output, despite normal LV systolic function18. Others have confirmed that RV restriction increases morbidity postoperatively6, 18. It is speculative whether the RV or LV is the primary cause of low output states after TOF repair. But when infundibulectomy is not extensive, LV dysfunction may dominate in some patients. Postoperative management of primary LV dysfunction and RV restrictive physiology differ in important respects. Accordingly, studies of the relative importance of RV and LV dysfunction in this population are needed.
Our data are the first to describe an effect of the direction of preoperative ventricular septal defect shunting upon pre- and postoperative LV mechanics in TOF. Others have previously reported decreased LV volume in severe RV outflow tract obstruction and exaggerated right-to-left shunting11. Jarmakani et al. showed normalization of LV volumes in all patients postoperatively, regardless of the magnitude or direction of shunting preoperatively11.
Our group has previously reported functional effects of repair of congenital heart lesions7, 10, 19. A relation similar to Figure 4 was derived between EDA and ejection fraction after repair of atrial or ventricular septal defects10. All patient data fit a linear correlation model. Atrial septal defect (ASD) patients fell into the upper-right quadrant of the graph, while VSD patients fell into the lower-left quadrant. This reflects increased volume loading of the LV after ASD repair and decreased volume loading after VSD repair. The clinical implication is that volume loading is appropriate for treatment of low output states after VSD closure, while inotropic agents may be preferable after ASD closure.
Figure 4 localizes L-R shunting “pink” TOF patients to the same quadrant as VSD patients studied previously10. These patients experience decreased LV preload as well as decreased LV systolic function. LV function would be expected to improve substantially if volume loading were implemented. TOF patients with R-L shunting and cyanosis (“blue” TOF) function in the lower-right quadrant of the graph, with increased EDA and decreased ejection fraction. This combination implies either a large increase in afterload or systolic dysfunction related to a septal patch and/or myocardial injury. This pattern favors administration of inotropic agents for LV support in these patients.
Long-term consequences of our observations remain to be defined. For earlier populations, Abd El Rahman et al. showed decreased LV systolic function in almost 25% of patients 10 years post-TOF repair20. The same group described decreased LV systolic function and paradoxical interventricular septal motion in asymptomatic patients 11 years after TOF repair5. Studies correlating acute postoperative LV dysfunction with long-term impairment could clarify the mechanisms of long-term LV systolic dysfunction.
Regarding design weaknesses of the present study, variations in patient anatomy, surgical technique, and clinical factors are potential confounding factors. While we could not control all of these variables, the trends in our data are consistent and should be reproducible in a larger study. The validity of dividing patients based on the direction of shunting across the VSD can be challenged based on a high frequency of bidirectional shunting in TOF 13. The predominance of R-L or L-R shunting is clinically apparent in most cases, however, and reflects the degree of RV outflow obstruction and cyanosis. We confirmed the validity of our R-L shunting group, using preoperative hemoglobin as an index of chronic cyanosis. Polycythemia is also an indicator of chronic cyanosis in addition to pulse oximetry, which can be variable and sometimes unreliable. Smaller pulmonary valve diameter in the R-L shunting group also implies more RV outflow obstruction, and more right-to-left shunting across the VSD. Furthermore, the larger LV EDA in the L-R shunting group is evidence for LV volume. All these data, suggest that the two groups, as defined in this study, are well differentiated with differing shunt hemodynamics.
Effects of surgical repair techniques were reviewed. Most (8/11) patients did not require an RV infundibular or transannular patch. LV function in three patients with patches was not qualitatively different from that in patients without patches. While valve-sparing procedures were associated with residual pulmonary valvar stenosis (13-46 mmHg), we found no correlation between residual RV outflow obstruction and changes in LV systolic or diastolic function (data not shown). While a postoperative atrial communication may influence postoperative ventricular mechanics in the TOF patient, this lesion was not observed in our patients.
Additional studies using different methodologies are indicated for definition of LV mechanics in the short- intermediate- and long-term.
Summary
This study of LV mechanics in the modern surgical era revealed LV systolic and diastolic dysfunction in all patients after TOF repair. Specifically, LV SA, SW and EFa decreased, as did LV contractility as measured by PRSW. Diastolic Pressure-Area curves revealed a postoperative decrease in LV compliance as exemplified by a decrease in LV EDA at a common pre- and postoperative LVEDP. Comparison of right-to-left (“blue” TOF) and left-to-right (“pink” TOF) preoperative ventricular shunts revealed left-to-right shunting patients behaving similar to simple VSD repair (i.e. decreases in LV EDA and SA), while right-to-left shunting patients showed less ventricular eccentricity, smaller changes in SA and an increase in LV EDA. These differences imply that volume loading may be preferable in “pink” TOF patients and inotropic agents in the postoperative management of “blue: TOF patients. Postoperative LV dysfunction may reflect effects of a septal patch, and/or deficiencies of current myocardial protection strategies. Further studies elucidating the cause of LV dysfunction and its short- and long-term consequences are needed to optimize treatment of patients during and after TOF repair.
Acknowledgments
Source of Funding: This study was supported in part by NIH R01 HL 48109.
Footnotes
Disclosures: None
CLINICAL PERSPECTIVE
Tetralogy of Fallot (TOF) is a spectrum of disease, from patients with severe pulmonary blood flow restriction, to patients with simple VSD physiology and pulmonary overcirculation. Given its anatomic features, TOF is often thought of as a disease of the right heart alone, with the assumption of a normal left heart. However, this study of intraoperative left ventricular (LV) mechanics demonstrated depression of LV systolic and diastolic function in all patients after TOF repair. Mechanisms of this depressed function may involve imperfect myocardial protection and/or mechanical factors related to the VSD patch. This study also demonstrated that effects of surgery on ventricular geometry and loading varied with the direction of blood flow through the VSD. LV preload from patients with predominantly right-to-left shunts increased after repair, but preload decreased in patients with simple VSD physiology. These differences have clinical implications for the treatment of low-cardiac output in the postoperative period. The unloaded LV produced by closure of left-to-right shunts should respond favorably to volume administration. But closure of right-to-left shunts increases LV preload, suggesting inotropes as the intervention of choice. This study looked only at the immediate effects of TOF repair on the LV. Further studies are needed to define relevance of these changes to long term outcomes.
Contributor Information
Marc E. Richmond, Department of Pediatrics, Columbia University, New York, NY.
Santos E. Cabreriza, Department of Surgery, Columbia University, New York, NY.
Jason P. Van Batavia, Department of Surgery, Columbia University, New York, NY.
T. Alexander Quinn, Columbia College of Physicians & Surgeons and Department of Biomedical Engineering, Columbia University, New York, NY.
Joshua P. Kanter, Department of Pediatrics, Columbia University, New York, NY.
Alan D. Weinberg, Department of Biostatistics, Columbia University, New York, NY.
Ralph S. Mosca, Department of Surgery, Columbia University, New York, NY.
Jan M. Quaegebeur, Department of Surgery, Columbia University, New York, NY.
Henry M. Spotnitz, Department of Surgery, Columbia University, New York, NY.
References
- 1.Hoffman JI. Incidence of congenital heart disease: I. Postnatal incidence. Pediatric Cardiology. 1995;16:103–113. doi: 10.1007/BF00801907. [DOI] [PubMed] [Google Scholar]
- 2.Munkhammar P, Cullen S, Jogi P, de Leval M, Elliott M, Norgard G. Early age at repair prevents restrictive right ventricular (RV) physiology after surgery for tetralogy of Fallot (TOF): Diastolic RV function after TOF repair in infancy. J Am Coll Cardiol. 1998;32:1083–1087. doi: 10.1016/s0735-1097(98)00351-9. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso SM, Miyague NI. Right ventricular diastolic dysfunction in the postoperative period of tetralogy of Fallot. Arq Bras Cardiol. 2003;80:198–201. doi: 10.1590/s0066-782x2003000200008. [DOI] [PubMed] [Google Scholar]
- 4.Norgard G, Gatzoulis MA, Josen M, Cullen S, Redington AN. Does restrictive right ventricular physiology in the early postoperative period predict subsequent right ventricular restriction after repair of tetralogy of Fallot? Heart. 1998;79:481–484. doi: 10.1136/hrt.79.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman MYAE, Hui W, Dsebissowa F, Schubert S, Gutberlet M, Hetzer R, Lange PE, Abdul-Khaliq H. Quantitative Analysis of Paradoxical Interventricular Septal Motion Following Corrective Surgery of Tetralogy of Fallot. Pediatric Cardiology. 2005;V26:379–384. doi: 10.1007/s00246-004-0753-y. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi RR, Shore DF, Lincoln C, Mumby S, Kemp M, Brierly J, Petros A, Gutteridge JM, Hooper J, Redington AN. Acute right ventricular restrictive physiology after repair of tetralogy of Fallot: association with myocardial injury and oxidative stress. Circulation. 1999;100:1540–1547. doi: 10.1161/01.cir.100.14.1540. [DOI] [PubMed] [Google Scholar]
- 7.Garofalo CA, Cabreriza SE, Quinn TA, Weinberg AD, Printz BF, Hsu DT, Quaegebeur JM, Mosca RS, Spotnitz HM. Ventricular Diastolic Stiffness Predicts Perioperative Morbidity and Duration of Pleural Effusions After the Fontan Operation. Circulation. 2006;114:I-56–61. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 8.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. Journal of the American College of Cardiology. 1985;5:918–927. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 9.Cabreriza SE, Dean DA, Amirhamzeh MR, Jia CX, Sciacca R, Soto P, Spotnitz HM. A Method for Detecting Changes in Left Ventricular Mass During Variations in Filling Volume. Journal of the American Society of Echocardiography. 1998;11:356–364. doi: 10.1016/s0894-7317(98)70103-8. [DOI] [PubMed] [Google Scholar]
- 10.Hart JP, Cabreriza SE, Walsh RF, Printz BF, Blumenthal BF, Park DK, Zhu AJ, Gallup CG, Weinberg AD, Hsu DT. Echocardiographic analysis of ventricular geometry and function during repair of congenital septal defects. The Annals of Thoracic Surgery. 2004;77:53–60. doi: 10.1016/s0003-4975(03)01328-6. [DOI] [PubMed] [Google Scholar]
- 11.Jarmakani JMM, Graham TPJ, Canent RVJ, Jewett PH. Left Heart Function in Children with Tetralogy of Fallot before and after Palliative or Corrective Surgery. Circulation. 1972;46:478–490. doi: 10.1161/01.cir.46.3.478. [DOI] [PubMed] [Google Scholar]
- 12.Lange PE, Onnasch DGW, Bernhard A, Heintzen PH. Left and right ventricular adaptation to right ventricular overload before and after surgical repair of tetralogy of Fallot. The American Journal of Cardiology. 1982;50:786–794. doi: 10.1016/0002-9149(82)91235-8. [DOI] [PubMed] [Google Scholar]
- 13.Levin AR, Boineau JP, Spach MS, Canent RVJ, Capp MP, Anderson PAW. Ventricular Pressure-Flow Dynamics in Tetralogy of Fallot. Circulation. 1966;34:4–13. doi: 10.1161/01.cir.34.1.4. [DOI] [PubMed] [Google Scholar]
- 14.Sandor GGS, Patterson MWH, Tipple M, Ashmore PG, Popov R. Left ventricular systolic and diastolic function after total correction of tetralogy of Fallot. The American Journal of Cardiology. 1987;60:1148–1151. doi: 10.1016/0002-9149(87)90408-5. [DOI] [PubMed] [Google Scholar]
- 15.Bastos P, Campos J, Cunha D, Gomes M. Left ventricular function after total correction of tetralogy of Fallot. Eur Heart J. 1991;12:1089–1097. doi: 10.1093/oxfordjournals.eurheartj.a059843. [DOI] [PubMed] [Google Scholar]
- 16.Borow KM, Green LH, Castaneda AR, Keane JF. Left ventricular function after repair of tetralogy of fallot and its relationship to age at surgery. Circulation. 1980;61:1150–1158. doi: 10.1161/01.cir.61.6.1150. [DOI] [PubMed] [Google Scholar]
- 17.Graham TP, Jr, Cordell D, Atwood GF, Boucek RJ, Jr, Boerth RC, Bender HW, Nelson JH, Vaughn WK. Right ventricular volume characteristics before and after palliative and reparative operation in tetralogy of Fallot. Circulation. 1976;54:417–423. doi: 10.1161/01.cir.54.3.417. [DOI] [PubMed] [Google Scholar]
- 18.Cullen S, Shore D, Redington A. Characterization of Right Ventricular Diastolic Performance After Complete Repair of Tetralogy of Fallot : Restrictive Physiology Predicts Slow Postoperative Recovery. Circulation. 1995;91:1782–1789. doi: 10.1161/01.cir.91.6.1782. [DOI] [PubMed] [Google Scholar]
- 19.Quinn TA, Cabreriza SE, Blumenthal BF, Printz BF, Altmann K, Glickstein JS, Snyder MS, Mosca RS, Quaegebeur JM, Holmes JW, Spotnitz HM. Regional functional depression immediately after ventricular septal defect closure. Journal of the American Society of Echocardiography. 2004;17:1066–1072. doi: 10.1016/j.echo.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Abd El Rahman MY, Abdul-Khaliq H, Vogel M, Alexi-Meskischvili V, Gutberlet M, Hetzer R, Lange PE. Value of the new Doppler-derived myocardial performance index for the evaluation of right and left ventricular function following repair of tetralogy of fallot. Pediatric Cardiology. 2002;23:502–507. doi: 10.1007/s00246-002-1469-5. [DOI] [PubMed] [Google Scholar]


