Abstract
For more than a century, pioneering researchers have been using novel experimental and computational approaches to probe the mysteries of blood flow. Thanks to their efforts, we know that blood cells generally prefer to migrate to the axis of flow, that red and white cells segregate in flow, and that cell deformability and their tendency to reversibly aggregate contribute to the non-Newtonian nature of this unique fluid. All of these properties have beneficial physiological consequences, allowing blood to perform a variety of critical functions. Our current understanding of these unusual flow properties of blood have been made possible by the ingenuity and diligence of a number of researchers, including Harry Goldsmith, who developed novel technologies to visualize and quantify the flow of blood at the level of individual cells. Here we summarize efforts in our lab to continue this tradition and to further our understanding of how blood cells interact with each other and with the blood vessel wall.
Keywords: Blood flow, Leukocyte, Erythrocyte, Segregation, Lattice Boltzmann, Microfluidics, Margination, Aggregation
INTRODUCTION
A long history of intensive research has revealed many of the mechanisms underlying the unique fluid dynamics of blood. Pioneers in the early 20th century found that the particulate nature of blood results in non-uniform hematocrit and viscosity within the microvasculature.13,19,20,27,48 Later, others showed that the deformability of individual RBCs and their propensity to reversibly aggregate result in a shear- and conduit-dependent viscosity that minimizes flow resistance in the vasculature.2,9-12,56,64 Further careful studies have revealed the flow of individual cells in blood and their mechanical interactions with the fluid and other cells.3,25,26,28,30,33,34,41,58,59,63 Still others have examined the interactions between immune cells and the vessel wall5,43,46,47,51,58,63 or the dynamics of platelet aggregation.15,22,36,45
All of this work has made it clear that blood is a collection of cells—organized yet dynamic—interacting with each other and with their conduits to accomplish many diverse goals. Thus, blood is much like an organ in its own right, and must be approached as such. To understand the peculiarities of bulk blood flow, we need to consider how all the various components of this “organ” interact and cooperate, from the perspectives of cell biology, cell mechanics, “tissue” biophysics (i.e., how the cells synergize or cooperate with each other) and physiology (i.e., how the blood flow interacts with other organs (for example, through shear force exerted on the vessel wall or the oxygen and nutrients delivered to tissue).
Historically, advances in the field of blood flow dynamics have been driven by novel technologies. Among the most important have been intravital microscopy,4,20,38 video cinematography,23 and mathematical modeling.1,14,36,44,51,55,60 While these approaches have elucidated the rheology of individual cells and the bulk flow properties of blood, there is still much to learn about blood at the mesoscale—i.e., how the cells interact with each other and with the blood vessels. Here, we describe how recent advances in microfluidics technology and computational approaches have enabled novel studies of mesoscale blood dynamics from the standpoints of leukocyte trafficking, flow-induced cell segregation and forces on the vasculature.
RBCS AFFECT THE MOTION OF WBCS
In order to initiate rolling, circulating leukocytes must marginate (migrate radially) to contact the vessel wall. Leukocyte margination has been attributed to the ability of RBC aggregates to exclude the WBCs from the bulk solution34,49,53 and also to the interactions between individual white and red blood cells at bifurcations or postcapillary expansions.59 Using a lattice-Boltzmann mathematical model of flow in a vessel expansion, we demonstrated that a coherent rouleau of RBCs is more effective at pushing a WBC to the vessel wall than a loosely-associated group of cells. In these simulations, each cell is explicitly accounted for, and the forces between cells can be calculated.66
The results show that organized groups of RBCs induce radial motion of the leukocyte (Fig. 1). The formation of these groups depends on the relative numbers of RBCs and WBCs in the blood, the fluid dynamics at the capillary inlet, and the propensity of the RBCs to aggregate. Interestingly, the shape of the RBCs affects their ability to stay in a stacked formation. We found that a rouleau of flat RBCs behaves quite differently from a train of ellipses of the same size (Fig. 1b). Whereas flattened shapes can easily stack to form rouleau and therefore provide sufficient force to initiate WBC rolling, ellipsoidal RBCs tend to “roll” against one another, leading to disruption of the initial stacking arrangement, and therefore, less force on the marginating WBC.
FIGURE 1.
RBC rouleaux encourage leukocyte margination at postcapillary expansions. (a) Velocity field and cell positions at normalized times t = 0, 0.056, 0.333, 0.5, 0.667, and 0.944. Red blood cells are represented by different colors for tracking purposes; lighter shading of the WBC indicates that the cell is rolling on the wall. The initial stacked organization of the RBCs directly behind the WBC evolves naturally in the capillary (not shown).66 As the stack of cells exit the capillary, the parabolic flow profile causes the RBC rouleau to act as a lever, pushing the slower WBC toward the wall. The critical parameters are the size of the rouleau, the width of the expansion, and the ability of the cells to stay stacked long enough to force the cell to the wall. (b) The importance of the organization of the rouleaux becomes obvious when the RBC shape is changed from flat capsules to ellipsoids. In this case, the stack of RBCs quickly dissociates, and the leukocyte does not contact the wall. The plots c, d, e, and f show the fluctuations in the net force on the WBC in the x direction (Fx) and the WBC velocity, Vx. The velocity initially decreases dramatically as the cells enter the expansion and the RBCs push the WBC toward the wall; another dramatic speed change occurs as the WBC starts rolling through stochastic ligand–receptor binding (d) (reproduced with permission from Ref. 66).
In other studies, we demonstrated that the inclusion of reversible RBC–RBC adhesion in the rouleaux enhances the lateral force on the marginating leukocyte by maintaining the rouleau “lever.”68 Furthermore, studies in expansions in microfluidic devices at the scale of actual microvessels show that the ability of the RBCs to rapidly reorganize and aggregate in response to the change in geometry (and shear rate) at expansions greatly contributes to WBC margination.52 Thus, it is clear that the vessel geometry and shear-dependent flow anomalies of blood serve useful purposes, allowing, for example, the rolling and adhesion of WBCs in postcapillaries where adhesion molecule expression is regulated and the vessel wall is relatively thin, facilitating extravasation.
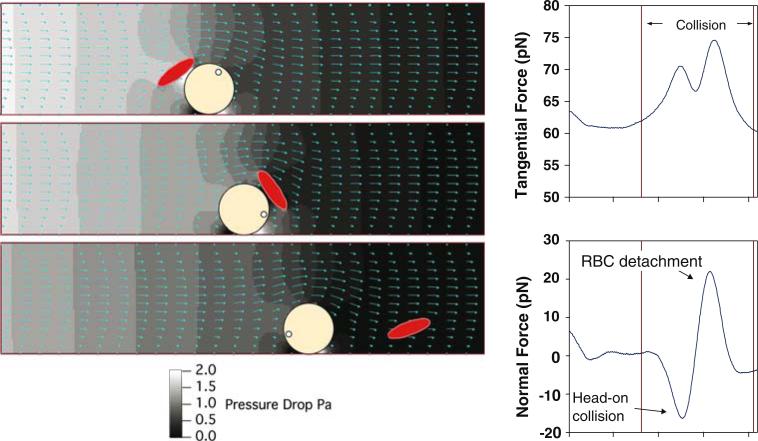
Once a WBC contacts and begins rolling on the endothelium, it is still subjected to normal and tangential forces imposed by passing blood cells. Using our modeling approach, we found that the transient forces on the rolling WBC are first positive and then negative in direction, indicating that the RBCs “bounce” the WBC against the endothelium (Fig. 2). Interestingly, the estimated magnitude of these forces were large enough for microvilli to penetrate the glycocalyx.50,73 Thus, once a WBC is rolling on the vessel wall, the unique environment of the plasma-rich zone should trap the WBC, push it along (via the enhanced tangential force), and encourage glycocalyx penetration via fluctuations in normal force. As opposed to the single RBC–WBC interaction in Fig. 2, at high hematocrit this effect would be more dramatic, as many RBCs interact with the WBC. In a real vessel network, the WBC likely escapes from the plasma-rich zone when venules converge, and when exposed to large shear rate.
FIGURE 2.
Passing RBCs “bounce” rolling leukocytes against the endothelium. Our simulations estimate the forces as RBCs interact with rolling WBCs. The normal force is first negative (directed toward the surface) and then positive (directed away from the surface). By pushing the rolling cell against the endothelium, RBCs likely encourage penetration of microvilli through the glycocalyx and engagement of adhesion molecules. Note also that the tangential force is enhanced, encouraging rolling, except where adhesion molecule density is sufficiently high (reproduced with permission from Ref. 50).
WBCS AFFECT THE MOTION OF RBCS
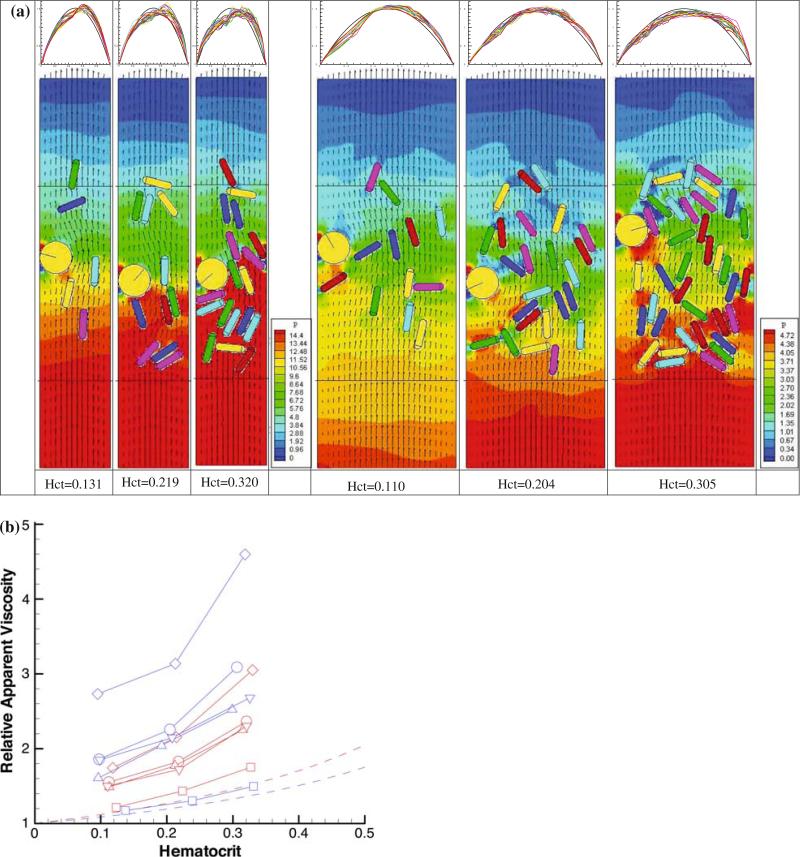
So far, we have summarized how RBCs can influence the flow behavior of WBCs, but the converse is also true. The presence of WBCs, which are larger than RBCs can greatly affect the flow resistance in the microvasculature, especially when they roll and adhere to the endothelium.6,35 To analyze this, we simulated blood flow through channels and measured the pressure field and flow velocities.67 As summarized in Fig. 3 the flow resistance depends on hematocrit, conduit dimensions, and WBC adhesion. By explicitly accounting for the cells suspended in pressure-driven flow, our simulations reproduce the Fahraeus–Lindqvist effect, which predicts that the apparent viscosity of blood decreases with decreasing diameter.27 Figure 5b shows that the simulations without WBCs (square symbols) have the same tendency as the empirical relationship (solid lines) for the 20 μm tube (blue) and 40 μm tube (red): at equivalent hematocrits, the relative apparent viscosity is higher in larger conduits than in the small conduit, in accordance with Fahraeus–Lindqvist effect. The presence of WBCs actually reverses this effect—the resistance becomes greater in the smaller tube because the large WBC occupies a larger fraction of the channel cross-section. This effect is especially important in short segments of the vasculature,67 but is exaggerated somewhat in our 2D simulations. For example, a WBC with diameter 9 μm blocks nearly 50% of the cross-sectional area of a 20 μm channel in 2D, but only 25% in a three-dimensional cylindrical tube.
FIGURE 3.
(a) WBC adhesion affects flow resistance through 20 μm (left) and 40 μm (right) channels. Flow is from bottom to top. The hematocrit for each simulation is given at bottom, and the velocity profiles across the channel at various time points are shown at top. The WBC is the yellow disk, and the RBCs are the colored capsules; background color corresponds to the local fluid pressure and the small arrows correspond to the local fluid velocity. Note the build-up of pressure behind the rolling WBC (red color), which is greater at higher hematocrit and smaller channels. (b) Relationship between relative apparent viscosity and hematocrit. The solid lines represent the data from Ref. 54. Red represents the 40 μm tube and blue the 20 μm tube (reproduced with permission from Ref. 67).
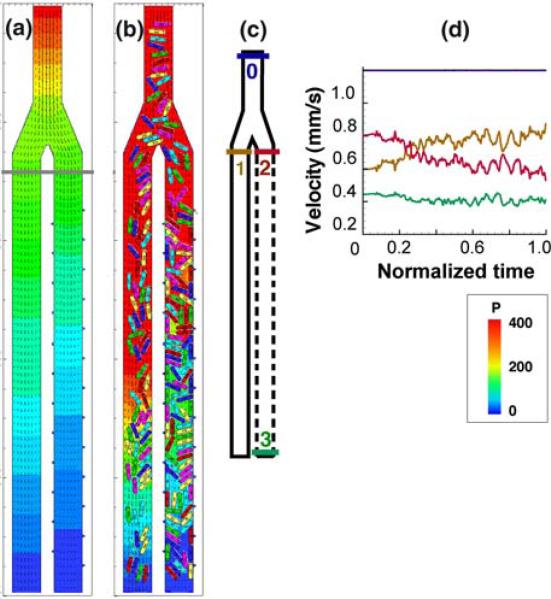
FIGURE 5.
Focal leaks cause hemoconcentration and flow diversion. In this computer simulation, plasma (a) or blood (b) flows through a simple vessel bifurcation from top to bottom. In (b) the colored capsules represent red blood cells. In both (a) and (b), the right hand daughter channel has multiple apertures that allow plasma leakage, while the left hand channel is impermeable. For the case (b), with red blood cells, the flow velocities are plotted in (d), with the colors corresponding to the locations noted in the map (c) (i.e., blue is at the inlet, orange is at the entrance to the left-hand branch, etc.). In (a) and (b) the plasma pressure coefficient [P = 2(p – pex)/ρU2, where p is local pressure, pex is pressure at the exit, U is flow velocity, and ρ is density] is represented by the color gradient and the velocity field is represented by small arrows. In (d), the system starts with no cells, and the velocities evolve with time as RBCs enter the bifurcation. Note the diversion of flow from the right to the left channel as plasma leakage and hemoconcentration increase the resistance to flow through the right hand channel (reproduced with permission from Ref. 65).
BLOOD FLOW AFFECTS BLOOD VESSELS PHYSIOLOGY AND VICE VERSA
Blood cells interact with each other, but they also impose mechanical forces on the vascular endothelium,7,21 sending important signals that affect vessel development, remodeling, and flow regulation. To investigate the interactions between cells in the blood and the vessel wall, we first imaged and digitized a portion of the vasculature in the dorsal skin of a mouse. We then performed our lattice-Boltzmann simulations of flowing RBCs and WBCs, varying initial cell configuration and RBC aggregation. The simulations show that the cells increase—transiently and dramatically—the pressure and shear stress at the vessel wall as they pass (Fig. 4). These fluctuations in force can affect vascular physiology, contributing to endothelial mitosis and angiogenesis and atherosclerosis, for example. We have also seen this effect in simulations of deformable cells at high hematocrit in three dimensions.17
FIGURE 4.
Estimated pressure fluctuations on the endothelium in the skin of a mouse due to blood cells. A and B give the flow fields and cell positions at various times for 2 different simulations. Total time of 0.8 s is normalized to 1. The pressure field is represented by colors and the velocity field is represented by arrows. Panel A: Time sequence of three RBCs following a WBC into a larger venule is shown in a–e. Blue arrows in (a) give flow direction. Panel B: simulation of six RBCs following a WBC into the right branch with diverging flow. Normalized pressure, P, at the wall positions marked by the red arrows in Panels 2A(a) and 2B(a) are shown in Panels C and D, respectively. The blue letters a–e within the plots mark the times corresponding to the panels a–e in Panels A and B. Note the large fluctuation in pressure at the wall as the WBC passes, and then the RBCs pass. In D, the pressure at the stagnation point increases significantly as the cells approach (a–d). The increase in pressure near the end of the simulation is due to the flow resistance produced by the cells in the exit segments (reproduced with permission from Ref. 69).
As in all complex physiological systems, each element affects the others in some way. So just as the blood cell dynamics affect the blood vessel wall, the blood vessel wall can affect blood flow. Smooth muscle cell contraction and dilation is one obvious example. Another is the modulation of vascular permeability and plasma extravasation. For example, excessive vessel leakiness is a hallmark of both inflammation and cancer. In inflammation, plasma extravasation and leukocyte adhesion occur in a coordinated manner to enable the immune response, but also to maintain tissue perfusion. In tumors, similar mechanisms operate, but they are not well-regulated.37 To assess how plasma leakage, blood viscosity, and vessel geometry are inter-related, we further developed our simulations to include plasma leakage from the blood vessel. As shown in Fig. 5, plasma leakage can lead to local hemoconcentration, increasing the flow resistance specifically in the leaky segments, and diverting flow to other branches.65 Indeed, our preclinical and clinical studies have shown that drugs that decrease vascular permeability may help restore uniform perfusion of tumor vasculature by providing more efficient delivery of concurrent chemotherapeutics.70,71
BIOMIMETIC SEPARATION OF BLOOD CELLS WITH A MICROFLUIDIC DEVICE
Our simulations and many experimental studies have demonstrated the natural propensity for blood cells to migrate to certain preferred locations within a conduit. With the recent availability of microfabrication techniques to design and build networks at the scale of actual blood vessels, we now have an additional tool to study and exploit the unusual segregation properties of blood.62
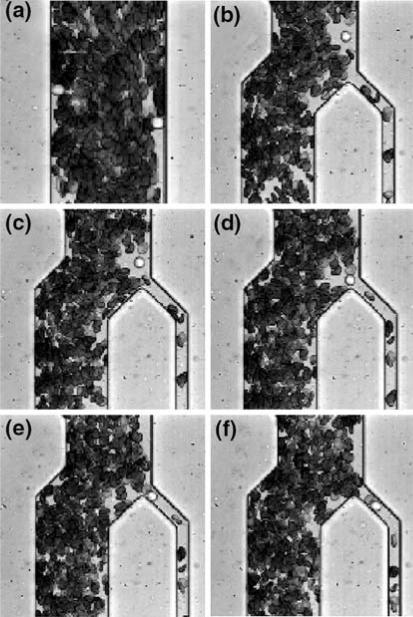
Because leukocytes comprise less than 1% of all blood cells it is necessary to concentrate or separate them as the initial step in many clinical and basic research assays. Taking advantage of the tendency for leukocytes to marginate in long channels, it is possible to design a tool for separating leukocytes directly from whole blood. The device consists of a simple network of microchannels arranged to encourage lateral migration of leukocytes and then extract them from the erythrocyte-depleted region near the sidewalls62 (Fig. 6). Providing a 34-fold enrichment of the leukocytes in a single pass, it operates on microliter samples of whole blood, provides positive, continuous flow selection of leukocytes and requires neither preliminary labeling of cells nor input of energy (except for a small pressure gradient to drive flow). This kind of separation will be the requisite initial stage in many lab-on-a-chip assays that require pure leukocytes as a starting sample.
FIGURE 6.
Microfabricated device for enriching WBCs from whole blood. Flow is from top to bottom. Blood is drawn into a long, straight channel (dimensions: 70 μm wide × 10.3 μm high × 5.5 mm long). Because of the low aspect ratio of the channel, the natural tendency of WBCs to marginate is enhanced, and most WBCs move to the sides of the channel (the end of this long channel is pictured in a). Notice that the two WBCs in this segment are both flowing adjacent to the side walls. The margination channel then feeds into a bifurcation that skims off the WBCs (time course of a WBC being extracted is shown in panels b–f). With appropriate bifurcation angle and channel dimensions, the WBCs traveling near the walls are enriched 34-fold in a single pass (reproduced with permission from Ref. 62).
TOWARDS MORE SOPHISTICATED SEPARATIONS—3D, DEFORMABLE CELLS AT PHYSIOLOGICAL HEMATOCRIT
As suggested by experiments,31,72 and by the simulations in Fig. 1, blood fluid dynamics are sensitive to cell shape. We also know that deformability of blood cells is a critical determinant of bulk blood rheology.8,16,57 For these reasons, we recently reformulated our mathematical model to include deformable, three-dimensional blood cells with realistic rest shapes.17 Our approach is to use a relatively coarse mesh for the RBCs so that the simulations (still using the lattice-Boltzmann approach) are very efficient, and finish in a reasonable time. This new model allows the investigation of more sophisticated fluid dynamics such as inertial migration of particles in pressure-driven flow.
Lateral Migration of Deformable Particles in Flow
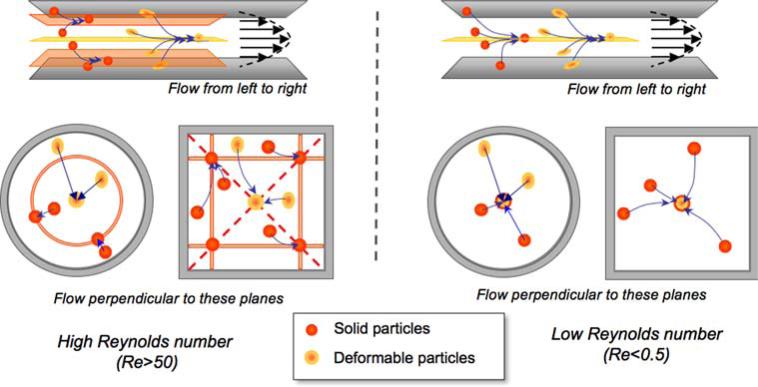
Inertial lateral migration was first reported by Segré and Silberger (SS),61 and the effect now bears their names. Careful experimentation on the flow of particles of various shapes and deformabilities has revealed its widespread importance in dilute suspension flow.29-32,40 Goldsmith and co-workers found that (i) for dilute suspensions, stiff particles settle mid-way between the channel center-plane and the nearest wall, whereas deformable particles settle at the centerplane, at Re > 124,61 and (ii) at lower Re (i.e., Re < 1), the particles migrate to the center of the channel, especially for deformable particles such as RBCs.32,42 Our simulations reproduce these results and are able to identify corresponding equilibrium positions in other geometries18 (Fig. 7).
FIGURE 7.
Equilibrium positions of spherical particles in flow depend on Reynolds number, particle stiffness, and conduit geometry. We adopt the following color convention in this paper: orange for deformable particles, red for stiff particles. At high Re (left hand side of the figure), stiff particles migrate away from the wall and away from the centerline, to a position approximately 40% away from the wall towards the center. The higher the Re, the closer to the wall is the equilibrium location. Round channels exhibit a ring of stiff particles at equidistance from the center, and rectangular geometries exhibit four distinct equilibrium positions in which stiff particles form trains.18,39 Deformable particles on the other hand, migrate towards the center. At low Re (right hand side of the figure), both stiff and deformable particles migrate towards the center of the channel.
Lateral Migration in Mixed Suspensions
In more concentrated suspensions, the Segré and Silberger effect is likely not strictly followed due to cell–cell interactions and crowding. To investigate how various particle types segregate in dense suspensions, we randomly placed a mixture of stiff (red) and deformable (orange) particles in a square micro-channel and simulated flow at high and low Re (Fig. 8). As predicted by Fig. 7, the results show that at high Re (Re = 60), (i) the stiff particles migrate toward the nearest corner equilibrium point (at normalized height and width of approximately 0.4) and (ii) the deformable particles migrate toward the channel axis (Fig. 8).
FIGURE 8.
Separation of deformable (orange) and stiff particles (red) at high Re (Re = 60, (a), (c), (e)), and low Re (Re = 0.2, (b), (d), (f)). Flow is into the plane of the figure; one of the sides of the square conduit is visible at left (checkered pattern). (a) And (b) show the initial configurations. Note that in (b), the stiff particles are initialized around the center only. (c) And (d) show the steady state configurations. (e) And (f) show the average cross-sectional distance (distance perpendicular to the direction of the flow), d, between the stiff particles (red line), deformable particle (orange line), and suspension (blue line). The particles traveled more than 10,000 times their diameter to reach steady state. The simulations required only 20 h on 5 CPUs.18
The results at high Re were not surprising, but the simulations get interesting when multiple particles with different rigidity try to assume the same equilibrium position. For example, at low Re (Re = 0.2), both stiff and deformable particles initially migrate towards the center (from t = 0 to point a in Fig. 8f), as predicted by Fig. 7. However, as the concentration increases in the growing central core (point a of Fig. 8f), the stiff particles, which were purposely initialized closer to the axis, are gradually displaced by the deformable particles (from point a to point c in Fig. 8f), and at steady state, the stiff particles all flow at the periphery, surrounding the core of deformable particles (point d in Fig. 8f). They remain in contact with the core but never invade it. The blue line of Fig. 8f shows that this process is a reorganization, rather than further migration, since the decrease in mean distance between particles, d, for the deformable particles is countered by an equivalent increase in d for the stiff particles; i.e., the blue line in Fig. 8f levels off after point b. This occurs because the magnitude of the lateral migration force for stiff particles is weaker than that for deformable particles, even though both species have the same equilibrium position.18
CONCLUSIONS
Blood cells work together to deliver oxygen, nutrients, and immune cells to tissues where they are needed. They do this by intrinsic fluid dynamics conferred by the particulate nature of blood, enhanced by cell–cell and cell–vessel interactions. Adding to the complexity and sophistication of the system, blood vessels themselves can adapt to help blood accomplish it diverse goals by dilating, constricting, sprouting or changing permeability. In order to fully understand these processes, we have to explicitly resolve the cells in flowing blood and characterize their interactions with each other and with the vessel wall. Computer simulations and microfluidic analyses are two strategies that can be used for this purpose. In the future, similar approaches could be used to understand how platelets aggregate during thrombosis and how cells interact with vessels to facilitate atherosclerosis.
ACKNOWLEDGMENTS
We would like to thank Dr. Harry Goldsmith for laying the foundation for all the work described herein. The studies were supported by NIH grant R01 HL64240 (LLM).
REFERENCES
- 1.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JJ, Nance P, Popel AS, Intaglietta M, Johnson PC. Effect of erythrocyte aggregation on velocity profiles in venules. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H222–H236. doi: 10.1152/ajpheart.2001.280.1.H222. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JJ, Popel AS, Intaglietta M, Johnson PC. Effects of erythrocyte aggregation and venous network geometry on red blood cell axial migration. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H939–H950. doi: 10.1152/ajpheart.2001.281.2.H939. [DOI] [PubMed] [Google Scholar]
- 4.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat. Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Lewinsohn D, Duijvestijn A, Bargatze R, Wu N, Jalkanen S. Interactions between endothelial cells and leukocytes. J. Cell. Biochem. 1986;30:121–131. doi: 10.1002/jcb.240300204. [DOI] [PubMed] [Google Scholar]
- 6.Chapman GB, Cokelet GR. Flow resistance and drag forces due to multiple adherent leukocytes in post-capillary vessels. Biophys. J. 1998;74:3292–3301. doi: 10.1016/S0006-3495(98)78036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol. Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Chien S. The Benjamin W. Zweifach award lecture. Blood cell deformability, interactions: from molecules to micromechanics and microcirculation. Microvasc. Res. 1992;44:243–254. doi: 10.1016/0026-2862(92)90084-3. [DOI] [PubMed] [Google Scholar]
- 9.Chien S, Usami S, Dellenback RJ, Gregersen MI. Blood viscosity: influence of erythrocyte deformation. Science. 1967;157:827–829. doi: 10.1126/science.157.3790.827. [DOI] [PubMed] [Google Scholar]
- 10.Chien S, Usami S, Dellenback RJ, Gregersen MI, Nanninga LB, Guest MM. Blood viscosity: influence of erythrocyte aggregation. Science. 1967;157:829–831. doi: 10.1126/science.157.3790.829. [DOI] [PubMed] [Google Scholar]
- 11.Chien S, Usami S, Skalak R. Blood flow in small tubes. In: Renkin EM, Michel CC, editors. Handbook of Physiology—The Cardiovascular System IV. American Physiological Society; Bethesda, MD: 1984. [Google Scholar]
- 12.Cokelet GR. The rheology and tube flow of blood. In: Skalak R, Chien S, editors. Handbook of Bioengineering. McGraw-Hill; New York: 1987. pp. 14.1–14.17. [Google Scholar]
- 13.Cokelet GR, Goldsmith HL. Decreased hydrodynamic resistance in the two-phase flow of blood through small vertical tubes at low flow rates. Circ. Res. 1991;68:1–17. doi: 10.1161/01.res.68.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Das B, Johnson P, Popel A. Computational fluid dynamic studies of leukocyte adhesion effects on non-Newtonian blood flow through microvessels. Biorheology. 2000;37:239–258. [PubMed] [Google Scholar]
- 15.Dewitz TS, Martin RR, Solis RT, Hellums JD, McIntire LV. Microaggregate formation in whole blood exposed to shear stress. Microvasc. Res. 1978;16:263–271. doi: 10.1016/0026-2862(78)90059-6. [DOI] [PubMed] [Google Scholar]
- 16.Dong C, Skalak R. Leukocytes deformability: finite element modeling of large viscoelastic deformation. J. Theor. Biol. 1992;158:173–193. doi: 10.1016/s0022-5193(05)80716-7. [DOI] [PubMed] [Google Scholar]
- 17.Dupin MM, Halliday I, Care CM, Alboul L, Munn LL. Modeling the flow of dense suspensions of deformable particles in three dimensions. Phys. Rev. E. 2007;75:066707. doi: 10.1103/PhysRevE.75.066707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupin MM, Munn LL. Lateral Migration of Blood Cells in Flow Depends on Rheology and Size: Insights from Simulations. Biomedical Engineering Society; Los Angeles, CA: 2007. p. 4.56. [Google Scholar]
- 19.Fahraeus R. The suspension stability of the blood. Physiol. Rev. 1929;9:241–274. [Google Scholar]
- 20.Fahraeus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. Am. J. Physiol. 1931;96:562–568. [Google Scholar]
- 21.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 22.Giorgio TD, Hellums JD. A cone and plate viscometer for the continuous measurement of blood platelet activation. Biorheology. 1988;25:605–624. doi: 10.3233/bir-1988-25402. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith HL. Microscopic flow properties of red cells. Fed. Proc. 1967;26:1813–1820. [PubMed] [Google Scholar]
- 24.Goldsmith HL. Red cell motions and wall interactions in tube flow. Fed. Proc. 1971;30:1578–1588. [PubMed] [Google Scholar]
- 25.Goldsmith HL, Bell DN, Braovac S, Steinberg A, McIntosh F. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys. J. 1995;69:1584–1595. doi: 10.1016/S0006-3495(95)80031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldsmith HL, Bell DN, Spain S, McIntosh FA. Effect of red blood cells and their aggregates on platelets and white cells in flowing blood. Biorheology. 1999;36:461–468. [PubMed] [Google Scholar]
- 27.Goldsmith HL, Cokelet GR, Gaehtgens P. Robin Fahraeus: evolution of his concepts in cardiovascular physiology. Am. J. Physiol. 1989;257:H1005–H1015. doi: 10.1152/ajpheart.1989.257.3.H1005. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith HL, Karino T. Interactions of human blood cells with the vascular endothelium. Ann. N. Y. Acad. Sci. 1987;516:468–483. doi: 10.1111/j.1749-6632.1987.tb33067.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith HL, Marlow JC. Flow behaviour of erythrocytes. I. Rotation and deformation in dilute suspensions. Proc. R. Soc. Lond. B. 1972;182:351–384. [Google Scholar]
- 30.Goldsmith HL, Marlow JC. Flow behavior of erythrocytes II. Particle motions in concentrated suspensions of ghost cells. J. Colloid Interface Sci. 1979;71:383–407. [Google Scholar]
- 31.Goldsmith HL, Mason SG. The flow of suspensions through tubes. I. Single spheres, rods and discs. J. Colloid Sci. 1962;17:448–476. [Google Scholar]
- 32.Goldsmith HL, Mason SG. The flow of suspensions through tubes II. Single large bubbles. J. Colloid Sci. 1963;18:237–261. [Google Scholar]
- 33.Goldsmith HL, Mason SG. Further comments on the radial migration of spheres in Poiseuille flow. Biorheology. 1965;3:33–36. [PubMed] [Google Scholar]
- 34.Goldsmith HL, Spain S. Margination of leukocytes in blood flow through small tubes. Microvasc. Res. 1984;27:204–222. doi: 10.1016/0026-2862(84)90054-2. [DOI] [PubMed] [Google Scholar]
- 35.Helmke BP, Bremne SN, Zweifach BW, Skalak R, Schmid-Schonbein GW. Mechanisms for increased blood flow resistance due to leukocytes. Am. J. Physiol. 1997;273:H2884–H2890. doi: 10.1152/ajpheart.1997.273.6.H2884. [DOI] [PubMed] [Google Scholar]
- 36.Hubbell JA, McIntire LV. Platelet active concentration profiles near growing thrombi. A mathematical consideration. Biophys. J. 1986;50:937–945. doi: 10.1016/S0006-3495(86)83535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 38.Jain RK, Munn LL, Fukumura D. Transparent window models and intravital microscopy. In: Teicher BA, editor. Tumor Models in Cancer Research. Humana Press Inc; Totowa: 2001. pp. 647–671. [Google Scholar]
- 39.Jiang Y, Myers MN, Giddings JC. Separation behavior of blood cells in sedimentation field-flow fractionation. J. Liq. Chromatogr. Relat. Technol. 2005;22:1213–1234. [Google Scholar]
- 40.Karino T, Goldsmith HL. Flow behaviour of blood cells and rigid spheres in an annular vortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977;279:413–445. doi: 10.1098/rstb.1977.0095. [DOI] [PubMed] [Google Scholar]
- 41.Karino T, Goldsmith HL, Motomiya M, Mabuchi S, Sohara Y. Flow patterns in vessels of simple and complex geometries. Ann. N. Y. Acad. Sci. 1987;516:422–441. doi: 10.1111/j.1749-6632.1987.tb33063.x. [DOI] [PubMed] [Google Scholar]
- 42.Karnis A, Goldsmith HL, Mason SG. Axial migration of particles in Poiseuille flow. Nature. 1963;200:159–160. [Google Scholar]
- 43.Konstantopoulos K, McIntire LV. Effects of fluid dynamic forces on vascular cell adhesion. J. Clin. Invest. 1996;98:2661–2665. doi: 10.1172/JCI119088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konstantopoulos K, Neelamegham S, Burns AR, Hentzen E, Kansas GS, Snapp KR, Berg EL, Hellums JD, Smith CW, McIntire LV, Simon SI. Venous levels of shear support neutrophil–platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation. 1998;98:873–882. doi: 10.1161/01.cir.98.9.873. [DOI] [PubMed] [Google Scholar]
- 45.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 46.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiological flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 47.Lipowsky HH, House SD, Firrell JC, editors. Vascular Endothelium in Health and Disease. Plenum Press; New York: 1988. Leukocyte Endothelium Adhesion and Micro-vascular Hemodynamics. [Google Scholar]
- 48.Maude AD, Whitmore RL. Theory of the flow of blood in narrow tubes. J. Appl. Pysiol. 1958;12:105–113. doi: 10.1152/jappl.1958.12.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Mayrovitz HN, Kang SJ, Herscovici B, Sampsell RN. Leukocyte adherence initiation in skeletal muscle capillaries and venules. Microvasc. Res. 1987;33:22–34. doi: 10.1016/0026-2862(87)90004-5. [DOI] [PubMed] [Google Scholar]
- 50.Migliorini C, Qian Y, Chen H, Brown E, Jain R, Munn L. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys. J. 2002;83:1834–1841. doi: 10.1016/S0006-3495(02)73948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munn LL, Melder RJ, Jain RK. Role of erythrocytes in leukocyte–endothelial interactions: mathematical model and experimental validation. Biophys. J. 1996;71:466–478. doi: 10.1016/S0006-3495(96)79248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munn L, Mulivor AW, Dupin M, Shevkoplyas SS, Sun C. Tumor vessel abnormalities affect blood cell dynamics and flow distribution. J. Biomech. 2006;39:S396. [Google Scholar]
- 53.Pearson MJ, Lipowsky HH. Influence of erythrocyte aggregation on leukocyte margination in postcapillary venules of rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1460–H1471. doi: 10.1152/ajpheart.2000.279.4.H1460. [DOI] [PubMed] [Google Scholar]
- 54.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am. J. Physiol. 1992;263:H1770–H1778. doi: 10.1152/ajpheart.1992.263.6.H1770. [DOI] [PubMed] [Google Scholar]
- 55.Pries AR, Secomb TW. Microcirculatory network structures and models. Ann. Biomed. Eng. 2000;28:916–921. doi: 10.1114/1.1308495. [DOI] [PubMed] [Google Scholar]
- 56.Reinke W, Gaehtgens P, Johnson PC. Blood viscosity in small tubes: effect of shear rate, aggregation, and sedimentation. Am. J. Physiol. 1987;253:H540–H547. doi: 10.1152/ajpheart.1987.253.3.H540. [DOI] [PubMed] [Google Scholar]
- 57.Sasaki A, Jain RK, Maghazachi AA, Goldfarb RH, Herberman RB. Low deformability of lymphokine-activated killer cells as a possible determinant of in vivo distribution. Cancer Res. 1989;49:3742–3746. [PubMed] [Google Scholar]
- 58.Schmid-Schoenbein GW, Fung Y-C, Zweifach BW. Vascular endothelium–leukocyte interaction. Sticking shear force in venules. Circ. Res. 1975;36:173–184. doi: 10.1161/01.res.36.1.173. [DOI] [PubMed] [Google Scholar]
- 59.Schmid-Schoenbein GW, Usami S, Skalak R, Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc. Res. 1980;19:45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- 60.Secomb TW, Styp-Rekowska B, Pries AR. Two-dimensional simulation of red blood cell deformation and lateral migration in microvessels. Ann. Biomed. Eng. 2007;35:755–765. doi: 10.1007/s10439-007-9275-0. [DOI] [PubMed] [Google Scholar]
- 61.Segre G, Silberger A. Radial particle displacements in Poiseuille flow of suspensions. Nature. 1961;189:209–210. [Google Scholar]
- 62.Shevkoplyas SS, Yoshida T, Munn LL, Bitensky MW. Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 2005;77:933–937. doi: 10.1021/ac049037i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 64.Skalak R, Chien S. Handbook of Bioengineering. McGraw-Hill; New York: 1987. [Google Scholar]
- 65.Sun C, Jain RK, Munn LL. Non-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusion. Ann. Biomed. Eng. 2007;35:2121–2129. doi: 10.1007/s10439-007-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun CH, Migliorini C, Munn LL. Red blood cells initiate leukocyte rolling in postcapillary expansions: a lattice Boltzmann analysis. Biophys. J. 2003;85:208–222. doi: 10.1016/S0006-3495(03)74467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun CH, Munn LL. Particulate nature of blood determines macroscopic rheology: a 2-D lattice Boltzmann analysis. Biophys. J. 2005;88:1635–1645. doi: 10.1529/biophysj.104.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun CH, Munn LL. Influence of erythrocyte aggregation on leukocyte margination in postcapillary expansions: a lattice-Boltzmann analysis. Physica A. 2006;362:191–196. [Google Scholar]
- 69.Sun CH, Munn LL. Lattice Boltzmann simulation of blood flow in digitized vessel networks. Comput. Math. Appl. doi: 10.1016/j.camwa.2007.08.019. doi:10.1016/j.camwa.2007.08.019:2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases [see comment]. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Yu SK, Goldsmith HL. Behavior of model particles and blood cells at spherical obstructions in tube flow. Microvasc. Res. 1973;6:5–31. doi: 10.1016/0026-2862(73)90003-4. [DOI] [PubMed] [Google Scholar]
- 73.Zao Y, Chien S, Weinbaum S. Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys. J. 2001;80:1124–1140. doi: 10.1016/S0006-3495(01)76090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]