Abstract
Physical interactions between circulating cells and the vascular wall play a central role in inflammation, metastasis, atherosclerosis, and therapeutic cell delivery. Unfortunately, traditional in vitro flow assays cannot be used to visualize the details of cell-surface interactions in blood flow because of inappropriate geometry and the poor penetration of light in erythrocyte solutions. To overcome these obstacles, we have developed an agarose-cast cylindrical vessel system to examine the profiles of cells interacting with surfaces under flow conditions. This design allows observation and quantification of cell deformation as cells adhere to surfaces under dynamic flow conditions without modifying the microscope or optical path. Furthermore, our flow system is uniquely suited for monitoring the profiles of adherent leukocytes deforming in response to erythrocyte suspension flow. We have used this flow system to study the role of erythrocytes in leukocyte-substrate interactions. Our results show that the cell deformation index (the ratio of the cell length to cell height) is higher in erythrocyte solutions compared to erythrocyte-free saline. This novel lateral view flow system provides a powerful technique for visualizing and quantifying the morphological changes of cells in contact with substrates exposed to shear stress.
INTRODUCTION
The parallel plate flow chamber has been used by many investigators to study the adhesive forces involved in cell-substrate binding and rolling (1,14,18,21,26). But some aspects of cellular trafficking in blood flow are best studied from a vantage point parallel to the surface. Lateral views of cells in contact with the substrate provide more detailed morphological information regarding cell contact, rolling, and deformation.
Our previous in vitro and in vivo studies of lymphocyte adhesion to tumor necrosis factor-α (TNF-α)-activated endothelium suggest that red blood cells (RBCs) are an essential contributor to the capture process (20,21). Recently, Rinker et al. (22) have also shown that the presence of erythrocytes is important in stabilization and enhancement of monocyte recruitment to endothelial cells exposed to flow at physiological shear rates. We hypothesized that RBCs can increase the overall efficiency of lymphocyte capture by increasing the frequency of collision and by imparting additional normal force to lymphocytes as they interact with the endothelium. To further characterize these interactions, visualization of the lateral perspective of cells interacting with the substrate is needed. To date, lateral profile images have been collected either by growing cells on a horizontal substrate and then turning the specimen for viewing or by using 45° prisms to obtain two perpendicular views (3,4,15,16).
However, difficulties exist in obtaining lateral view images of cells in RBC suspensions using existing techniques. The first difficulty lies in the high absorbance of visible light in RBC solutions, which limits the depth of light penetration and renders the long optical path of the prism system developed by Cao et al. impractical (4). The problem is one of geometry, and we concluded that the optimal solution would be to visualize cells adherent at the wall in a cylindrical tube. Observing cells at the edge of the cross-section perpendicular to illuminating light (Figure 1) allows the light to avoid the bulk of the RBCs as it passes from the condenser to the objective.
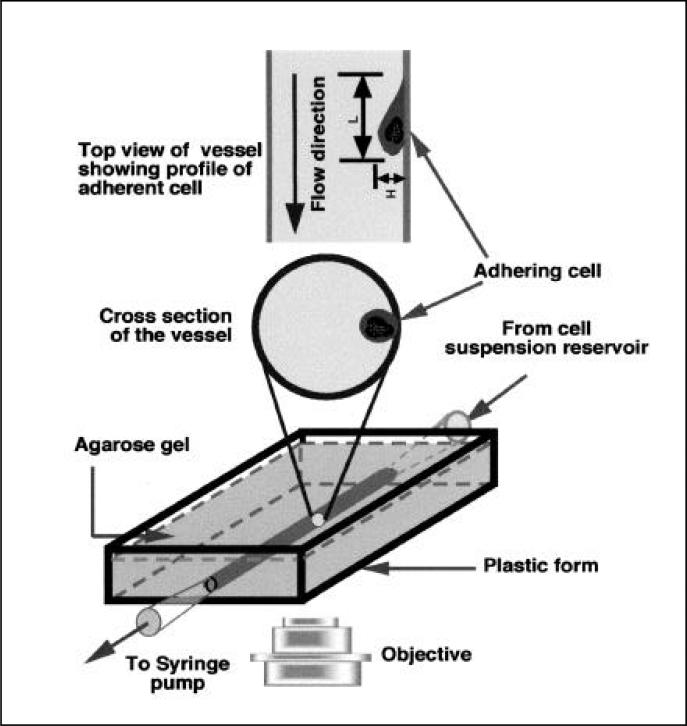
Figure 1. Schematic of the experimental setup of the agarose-cast vessel and experimental setup.
An artificial vessel is formed in an agarose gel with the input port connected to the cell suspension reservoir and the output port connected to the syringe pump. The vessel surface is first treated with polyethylenimine and glutaraldehyde and then coated with fibronectin and E-selectin. (Top) Profile of the vessel and adherent cell observed in the objective. (Middle) Cross-section of the vessel with an adherent cell.
This leads to the second problem in design, which involves the refraction of glass and plastic capillary tubes commonly used to investigate cells under flow conditions. Capillaries modeled from glass and polymers have been used to investigate interactions between leukocytes and adhesion molecules (2,8,17) and RBC behavior at vessel junctions (7,10). Studies such as these were able to use tube geometry successfully because their observations were restricted to cells near the center of the projected image of the tube, where distortion due to refraction is minimal. These studies yielded neither cell profiles nor visualization of white cells within a physiologic solution of erythrocytes. Unfortunately for our purposes, light refraction is dramatic at both the air-capillary and medium-capillary interfaces because of the curvature of the tube wall, making it impossible to image undistorted profiles of bound cells at the edge of the projected capillary where the RBC light absorption is minimal.
To overcome these problems, we have developed a lateral view flow system with appropriate geometry for minimizing the path length through the flow stream and refraction of light at the interfaces (Figure 1). Cell interactions with the substrate can be viewed at high shear rates (>100/s) even in the presence of physiologic concentrations of RBCs (hematocrit = 30%). To demonstrate the potential of this system, we measured neutrophil deformation under laminar flow in various suspending solutions. The lateral view flow system presented in this study allowed visualization of both cell deformation and cell-substrate contact length in shear flow. This system provides a novel technique for investigating cell rolling, deformation, and adhesion under defined flow conditions.
MATERIALS AND METHODS
Preparation of Neutrophil, Erythrocyte, and Dextran Solutions
Normal human neutrophils were isolated from sodium heparin anti-coagulated fresh blood collected from healthy donors using Polymorphprep™ (Nycomed Pharma As, Oslo, Norway). After centrifugation, erythrocytes were pelleted, washed three times in Hank's balanced salt solution (HBSS), and stored at 4°C for subsequent use. The polymorphonuclear fraction was washed three times with RPMI and then resuspended in RPMI with 5% (v/v) autologous donor plasma. To increase cell deformability, and thus the amount of observable membrane deflection, neutrophils were incubated with cytochalasin B (10 μM; Sigma, St Louis, MO, USA) for 15 min before use. Viscosities were measured from the pressure drop across a parallel plate flow chamber.
Cylindrical Agarose-Cast Vessel
The following solutions were prepared with Dulbecco's phosphate-buffered saline (D-PBS; Sigma): 1% agarose (Fisher Scientific, Pittsburgh, PA, USA), 0.5% polyethylenimine (Sigma), 3% glutaraldehyde (Poly-science, Warrington, PA, USA), 10 μg/mL fibronectin (Sigma), and 10 ng/mL E-selectin (R&D Systems, Minneapolis, MN, USA). A 1% agarose concentration was selected based on its mechanical rigidity and suitability for polyethylenimine uptake and cross-linking. The cylindrical agarose-cast vessels were made by injecting melted agarose (1%) into a plastic form (6 × 0.5 × 3 cm) around a polyethylene tube (PE10, OD 0.61 mm; Becton Dickinson, Franklin Lakes, NJ, USA). After the agarose cooled, the polyethylene tube was carefully removed, leaving a cylindrical channel in the gel with dimensions of 5 cm (length) × 0.6 mm (diameter) (Figure 1). Polyethylenimine solution was injected into the vessel, which was then held at 60°C for 1 h and gently washed three times with 1 mL D-PBS. A 3% glutaraldehyde solution was injected into the vessel to cross-link polyethylenimine for 5 min at 20°C–25°C, and the vessel was again washed three times with D-PBS. This process activated the vessel surface, promoting protein attachment to reactive sites (24).
To provide a surface conducive for neutrophil adhesion, the vessel was coated with 10 μg/mL fibronectin (5) and 10 ng/mL E-selectin (19) for 4 h and finally washed with 0.1% bovine serum albumin to block nonspecific binding. Even at the highest volumetric flow rate in this study (2.0 mL/min), the flow was laminar (Reynolds number approximately 70). The entrance length (Le = 0.07 rRe, where r = radius) of 0.14 cm in our system was negligible compared to the vessel length of 5 cm. Although agarose is technically a viscoelastic material, the cast vessels were not significantly deformed by small pressure gradients produced by the flow: the maximum change in diameter of the vessel due to perfusion was less than 2% of the static diameter (608 ± 12 μm at static and 599 ± 13 μm during perfusion).
Data Acquisition
A computer-driven, variable speed syringe pump (Harvard Apparatus, South Natick, MA, USA) pulled cells suspended in RPMI, with or without RBCs, from a reservoir through the agarose-cast vessel. An IBM PC controlled the operation of the pump. Images of cells bound to the edge of the vessel were observed using a video camera (5000 Series; COHU, San Diego, CA, USA) attached to the microscope (Diaphot; TMD Nikon, Melville, NY, USA). The images were recorded on a video recorder (Panasonic AG-6730; Matsushita Electronic, Osaka, Japan) for offline analysis.
Image Analysis
The recorded images were analyzed using NIH Image 1.61 software (available via download at http://rsb.info.nih.gov/nih-image). The vessel diameter (DV) and length in the observed field (LV), projected cell length (L), and cell height (H, side view) were measured. Lateral cell deformation indices (ψ) were calculated as the cell length divided by cell height.
Statistical Analysis
Statistical differences between the means of cell deformation were analyzed using a Student's t-test. Data are presented as mean ± the standard error of the mean (sem).
RESULTS
For Newtonian solutions such as saline, the velocity profiles can be calculated using the Navier-Stokes equations. The maximum fluid velocity (i.e., at r = 0) is 5.7 and 11.4 mm/s for volumetric flow rates of 0.5 and 1.0 mL/min, respectively. These values are not significantly different from experimental measurements in saline of the maximum velocities using fluorescent beads and stroboscopic illumination, which were 5.9 ± 0.3 and 11.0 ± 0.5 mm/s, respectively. The experimental flow profiles fit the parabolic equation well (R2 = 0.95 at Q = 0.5 mL/min, R2 = 0.96 at Q = 1.0 mL/min). The maximum relative error of the calculated shear rate was ± 15%, which is due to uncertainties in the locations of the beads caused by the finite depth of focus (approximately 13 μm). Correspondingly, the data analyses in this study assumed an error of 15% in the shear rate.
Additional considerations apply in the analysis of erythrocyte flow, which is non-Newtonian. The velocity profiles of erythrocyte suspensions are more blunt and have a RBC-poor zone near the vessel wall. To describe the shape of the flow profile of RBC suspensions and the deviation from parabolic flow, the following empirical equation has been proposed (23):
| [Eq. 1] |
where Vmax is the maximum flow velocity in the vessel and k is a parameter that depends on the hematocrit, flow rate, and geometry. For k = 2, a parabolic velocity distribution is obtained; increasing k produces a progressively flatter velocity profile. Velocity profiles of RBC suspensions have been measured both in vitro in D = 75-μm polypropylene tubes (12) and in arterioles of the rabbit mesentery (23,25). In the studies of Tangelder et al. (25), k values ranged from 2.3 to 4.0 in arterioles of the rabbit mesentery (D = 17−32 μm). Our curve fitting of the in vitro data of Goldsmith et al. (13) resulted in k = 3.
Observation of Cell Deformation Using the Agarose-Cast Vessel
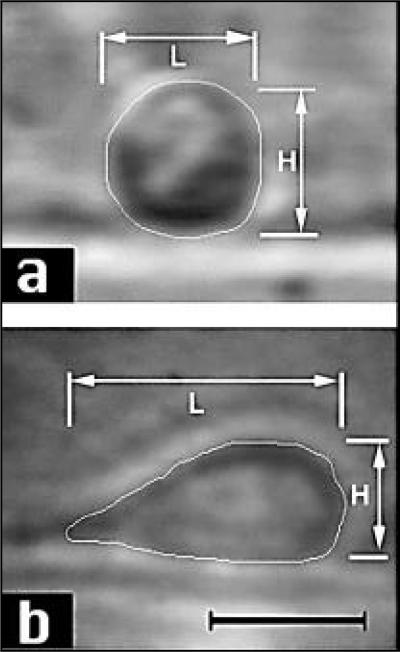
Figure 2 shows representative video micrographs of lateral profiles of neutrophils attached to the substrate and deforming under the influence of flow. In static conditions or low shear rates (<50/s), cells were round and undeformed (Figure 2a). As the flow rate increased, cells elongated along the direction of flow and assumed a teardrop shape (Figure 2b). Some cells detached from the surface at the high shear rates. An edge detection image processing algorithm was applied to delineate the cell boundary.
Figure 2. Images of neutrophil deformation in shear flow.
(a) Lateral view image of a neutrophil collected from the agarose-cast vessel with RPMI as the medium at a flow rate of 0.05 mL/s (shear rate 12/s, shear stress 0.12 dyn/cm2, deformation index, ψ = 1.0). (b) Lateral view image of neutrophils in the vessel in the presence of erythrocytes (10%) when the flow rate was 2.0 mL/s (shear rate 1500/s, shear stress 15 dyn/cm2, ψ = 2.3). Bars represent 10 μm.
Cell Deformation in the Presence of RBCs
We next used the agarose-cast vessel to quantify the influence of RBCs on cell-substrate interactions. After perfusing the vessel with neutrophils suspended in RPMI for 5 min at 0.02 mL/min, 10% (v/v) erythrocytes were added to the suspension. In the current study, a 10% RBC concentration was chosen to demonstrate the role of RBCs in cell adhesion while maintaining a significant RBC-free layer at the vessel periphery. At low shear rates (<50/s), few adherent neutrophils could be observed due to RBCs absorbing the visible light near the wall. But at higher shear rates (>100/s), an RBC-free layer approximately 10 μm thick formed at the vessel periphery due to the redistribution of RBCs, and the profiles of adhering cells could be observed (Figure 2).
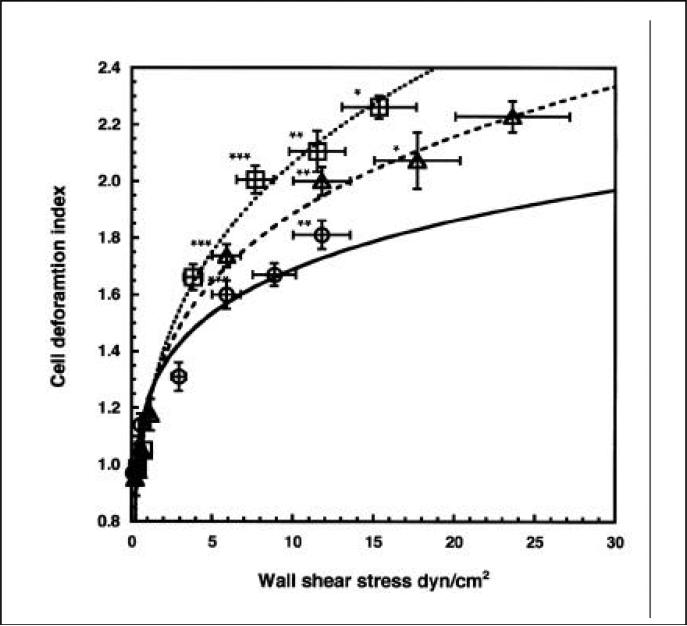
We examined the deformation of neutrophils suspended in RPMI, dextran solution (MW: 162 000, 2.5%; Sigma), or RBC suspension (10%) under flow conditions. The bulk viscosity of the 2.5% dextran solution was approximately equal to that of a 10% RBC suspension (1.95 cP) and constant over the range of shear rates in the study. The deformation index of neutrophils was significantly higher in RBC solution compared to RPMI or dextran at wall shear stress greater than 5 dyn/cm2 (Figure 3), indicating a contribution of RBCs to neutrophil adhesion that cannot be explained by viscosity-induced shear stress alone. For this comparison, the wall shear stresses were calculated from a parabolic profile in the case of RPMI and saline and from Equation 1 in the case of RBC solution.
Figure 3. Comparison of neutrophil deformation index in shear flow in different suspending media.
Neutrophils were suspended in RPMI (circle), 25% dextran in RPMI (triangle), and 10% RBCs in RPMI (square). The shear rate profile in the presence of RBC is calculated from Equation 1 with k = 3 (inset). The shear rate profiles for RPMI and 25% dextran are assumed parabolic. The y-axis error bars are sem(16 < n < 40), and the x-axis error bars are the maximum error in estimating the flow velocities (15% of the estimated flow velocity). Within each range of shear stress 5 < τ < 10, 10 < τ < 15, and 15 < τ < 20 dyn/cm2, there were significant differences in cell deformation between cells in RPMI or dextran and those in RBCs (P < 0.05).
DISCUSSION
The leukocyte deformation index observed in our in vitro system is comparable to results published by other groups. Damiano et al. (9) observed increases in the deformation index from 1.2 to 1.6 in vivo as wall shear rates increased from 500 to 3100/s. Using rat mesenteric venules, Firrell and Lipowsky (11) investigated leukocyte deformation in vivo. The deformation index was defined as the difference between length of a deformed white blood cell (WBC) and its initial length. As wall shear rate increased from 0 to 800/s, the deformation index increased from 0 to 0.4. This would correspond to 1.4 by our definition of L/H (11). Note, however, that these two in vivo studies used rat leukocytes and did not use cytochalasin B to increase cell deformability. When using neutrophils not treated with cytochalasin B, the deformation indices in our system were 1.48 ± 0.1 without RBCs and 1.80 ± 0.1 with RBCs at a wall shear stress of 1180/s. These results are therefore consistent with previous studies of normal neutrophils.
The viscosity of an RBC suspension is a nonlinear function of hematocrit, radial position in the conduit, and fluid velocity (6). The shear stress cannot be calculated assuming a uniform viscosity and parabolic velocity profile. Consequently, the forces experienced by cells adhering at the wall are difficult to assess in these particulate non-Newtonian solutions and must be estimated from empirical results in the literature. Assuming k = 3 for our RBC suspension velocity profile (see Materials and Methods), the wall shear rate in the profile calculated with Equation 1 is 25% higher than that of the parabolic profile. However, because of the RBC-free layer at the vessel wall, the viscosity near the wall should be much lower than that of the bulk solution (because there are few RBCs there). Thus, at equivalent wall shear rates, the wall shear stress on an adherent cell in the RBC suspension is approximately 25% higher than that in saline but actually 35% lower than that in dextran solution.
Based on these calculations, the neutrophil deformation in the presence of RBCs is significantly higher than that in dextran solution with matched bulk viscosity, suggesting that bulk viscosity may not be an accurate predictor of wall shear stress in RBC-containing solutions. Dextran solutions increased the shear stress, and thus cell deformation, but did not increase the probability of cell rolling or adhesion (21). This provides further support for the hypothesis that the unique fluid dynamics near the vessel wall in blood provides a privileged environment for cell wall interactions.
The agarose-cast vessel described here has the advantage of cylindrical geometry; also, the gel defining the vessel is viscoelastic, similar to blood vessels in vivo. One of the limitations of the agarose-cast vessel is that the agarose gel produces a strong fluorescent background when observed under epifluorescence illumination because of chemical changes induced during cross-linking. This makes fluorescence microscopy in the current system impractical and negates the use of fluorescently labeled neutrophils to increase their visibility against the RBC background. New casting materials and techniques must be tested to reduce the autofluorescence and increase the clarity in brightfield illumination. Another possible extension of this method would involve growing endothelial cell monolayers on the cast vessel wall. This would allow the assessment of cell adhesion to endothelial monolayers in cylindrical geometry.
In summary, we have designed an in vitro lateral view flow system and demonstrated its utility in studies of blood cell interactions with adhesion molecule-coated surfaces. This lateral view flow system provides a new technique for visualizing and quantifying the morphological changes of cells in contact with substrates under shear stress. The system may be applied to in vitro studies of inflammation, tumor metastasis, and atherosclerosis where cells interact with adhesion molecules under controlled conditions.
ACKNOWLEDGMENTS
This study was supported by the National Science Foundation grant no. BES-9529673 and the National Institutes of Health grant no. RO1-HL64240. The authors thank Dr. Cheng Dong (Bioengineering, Pennsylvania State University) for his help in these studies. The authors thank Drs. Kevin Burton, Randy Dull, Eugene Eckstein, Ananth Kadambi, Gerald Koenig, and Saroja Ramanujan for their helpful discussions and advice.
REFERENCES
- 1.Alon R, Chen S, Fuhlbrigge R, Puri KD, Springer TA. The kinetics and shear threshold of transient and rolling interactions of L-selectin with its ligand on leukocytes. Proc. Natl. Acad. Sci. USA. 1998;95:11631–11636. doi: 10.1073/pnas.95.20.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J. Exp. Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boocock CA, Brown AF, Dunn GA. A simple chamber for observing microscopic specimens in both top and side views. J. Microsc. 1985;137:29–34. doi: 10.1111/j.1365-2818.1985.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Usami S, Dong C. Development of a side-view chamber for studying cell-surface adhesion under flow conditions. Ann. Biomed. Eng. 1997;25:573–580. doi: 10.1007/BF02684196. [DOI] [PubMed] [Google Scholar]
- 5.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 6.Chien S, Usami S, Dellenback RJ, Gregersen MI. Blood viscosity: influence of erythrocyte deformation. Science. 1967;157:827–829. doi: 10.1126/science.157.3790.827. [DOI] [PubMed] [Google Scholar]
- 7.Cokelet GR, Soave R, Pugh G, Rathbun L. Fabrication of in vitro microvascular blood flow systems by photolithograph. Microvasc. Res. 1993;46:394–400. doi: 10.1006/mvre.1993.1062. [DOI] [PubMed] [Google Scholar]
- 8.Cooke BM, Usami S, Perry I, Nash GB. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvasc. Res. 1993;45:33–45. doi: 10.1006/mvre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 9.Damiano ER, Westheider J, Tozeren A, Ley K. Variation in the velocity, deformation, and adhesion energy density of leukocytes rolling within venules. Circ. Res. 1996;79:1122–1130. doi: 10.1161/01.res.79.6.1122. [DOI] [PubMed] [Google Scholar]
- 10.Fenton BM, Carr RT, Cokelet GR. Nonuniform red cell distribution in 20 to 100 μm bifurcations. Microvasc. Res. 1985;29:103–126. doi: 10.1016/0026-2862(85)90010-x. [DOI] [PubMed] [Google Scholar]
- 11.Firrell JC, Lipowsky HH. Leukocyte margination and deformation in mesenteric venules of rat. Am. J. Physiol. 1989;256:H1667–H1674. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith HL. Microscopic flow properties of red cells. Fed. Proc. 1967;26:1813–1820. [PubMed] [Google Scholar]
- 13.Goldsmith HL, Karino T. Microscopic considerations: the motions of individual particles. Ann. NY Acad. Sci. 1977;283:241–255. [Google Scholar]
- 14.Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys. J. 1992;63:35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hlinka J, Sanders FK. Real and reflected images of cells in profile. J. Cell Sci. 1972;11:221–231. doi: 10.1242/jcs.11.1.221. [DOI] [PubMed] [Google Scholar]
- 16.Ingram VM. A side view of moving fibroblasts. Nature. 1969;222:641–644. doi: 10.1038/222641a0. [DOI] [PubMed] [Google Scholar]
- 17.Jutila MA, Wilson E, Kurk S. Characterization of an adhesion molecule that mediates leukocyte rolling on 24 h cytokineor lipopolysaccharide-stimulated bovine endothelial cells under flow conditions. J. Exp. Med. 1997;186:1701–1711. doi: 10.1084/jem.186.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence MB, McIntire LV, Eskin SG. Effects of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 19.Ley K, Tedder TF. Leukocyte interactions with vascular endothelium: new insights into selectin-mediated attachment and rolling. J. Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 20.Melder RJ, Munn LL, Yamada S, Ohkubo C, Jain RK. Selectin- and integrin-mediated T-lymphocyte rolling and arrest on TNF-α-activated endothelium: augmentation by erythrocytes. Biophys. J. 1995;69:2131–2138. doi: 10.1016/S0006-3495(95)80087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn LL, Melder RJ, Jain RK. Role of erythrocytes in leukocyte-endothelial interactions: mathematical model and experimental validation. Biophys. J. 1996;71:466–478. doi: 10.1016/S0006-3495(96)79248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinker KD, Prabhakar V, Osborn EA, Truskey GA. Effects of fluid viscosity and erythrocytes on monocyte adhesion.. Proceedings of the First Joint BMES/EMBS Conference Atlanta, GA.1999. [Google Scholar]
- 23.Roevros J. Analogue processing of CW Doppler flowmeter signals to determine average frequency shift momentaneously without the use of a wave analyser. In: Reneman R, editor. Cardiovascular Applications of Ultrasound. North-Holland Publishing Company; Amsterdam: 1974. pp. 43–54. [Google Scholar]
- 24.Stubbings D, Bubb MO, Conradie JD. Two methods for the introduction of amino groups into agarose-based matrices: their use in immunoaffinity chromatograph. Anal. Biochem. 1993;210:159–162. doi: 10.1006/abio.1993.1167. [DOI] [PubMed] [Google Scholar]
- 25.Tangelder GJ, Slaaf DW, Muijtjens AM, Arts T, oude Egbrink MG, Reneman RS. Velocity profiles of blood platelets and red blood cells flowing in arterioles of the rabbit mesentery. Circ. Res. 1986;59:505–515. doi: 10.1161/01.res.59.5.505. [DOI] [PubMed] [Google Scholar]
- 26.Truskey GA, Pirone JS. The effect of fluid shear stress upon cell adhesion to fibronectin-treated surfaces. J. Biomed. Mater. Res. 1990;24:1333–1353. doi: 10.1002/jbm.820241006. [DOI] [PubMed] [Google Scholar]