Abstract
Both the dorsal and ventral striatum have been demonstrated to have a critical role in reinforcement learning and addiction. Dissecting the specific function of these striatal compartments and their associated nigrostriatal and mesoaccumbens dopamine pathways, however, has proved difficult. Previous studies using lesions to isolate the contribution of nigrostriatal and mesoaccumbens dopamine in mediating the locomotor and reinforcing effects of psychostimulant drugs have yielded inconsistent and inconclusive results. Using a naturally occurring mutant mouse line, aphakia, that lacks a nigrostriatal dopamine pathway but retains an intact mesoaccumbens pathway, we show that the locomotor activating effects of cocaine, including locomotor sensitization, are dependent on an intact nigrostriatal dopamine projection. In contrast, cocaine reinforcement, as measured by conditioned place preference and cocaine sensitization of sucrose preference, is intact in these mice. In light of the well-established role of the nucleus accumbens in mediating the effects of psychostimulants, these data suggest that the nigrostriatal pathway can act as a critical effector mechanism for the nucleus accumbens highlighting the importance of intrastriatal connectivity and providing insight into the functional architecture of the striatum.
Keywords: cocaine, conditioned place preference, Pitx3, locomotor sensitization, dorsal striatum, nucleus accumbens
Introduction
The architecture of the striatum with its modular compartments and complex cortical and subcortical loops has been difficult to functionally dissect. Studies of drugs of abuse, addiction, and reward processing have provided considerable insight into the function of the basal ganglia and have highlighted the importance of dopamine as a neuromodulator mediating the acquisition and expression of motivated behavior (Cagniard et al, 2006; Kelley, 2004; O'Doherty et al, 2004; Robinson et al, 2005, 2006; Robinson and Berridge, 1993, 2003; Salamone and Correa, 2002; Schultz, 2002; Schultz et al, 1997). The reinforcing properties of drugs of abuse are believed to act on the brain's reward system, particularly dopamine, and facilitate neuroadaptations and maladaptive learning that establish a repertoire of drug-related behaviors (Anderson and Pierce, 2005; Di Chiara and Bassareo, 2007; Everitt et al, 2001; Hyman et al, 2006; Pierce and Kalivas, 1997; Robbins and Everitt, 2002). Both the nucleus accumbens (NAc) and the dorsal striatum (DSt) are integral components of reward learning and motivation and have been hypothesized to be distinctly involved in the acquisition and maintenance of addiction (Everitt and Robbins, 2005). The NAc is believed to be critical during early stages of addiction, whereas the DSt, mediating habitual stimulus-response (S-R) behaviors, may be important to later stages and underlie both craving and compulsion to seek drugs (Gerdeman et al, 2003; Letchworth et al, 2001; Porrino et al, 2004; Vanderschuren et al, 2005; Volkow et al, 2006). The functional interaction between the dorsal and ventral compartments of the striatum and their associated mesoaccumbens and nigrostriatal pathways, however, is poorly understood.
Administered to rodents, cocaine increases locomotion and is highly effective as a reinforcer. The increased locomotion induced by cocaine is the primary behavioral measure used to study psychostimulant drug effects (Flagel and Robinson, 2007), including sensitization to repeated exposures to cocaine. However, the relationship between the psychostimulant action of cocaine and its reinforcing properties with respect to the underlying neural substrates that mediate these effects remains uncertain. Although the nigrostriatal pathway is generally associated with regulating motor output and the mesolimbic pathway with reward learning, the NAc is often emphasized in mediating both the rewarding and locomotor stimulating effects of psychostimulants (Everitt and Robbins, 2005; Li et al, 2004; Sellings and Clarke, 2006). The role of the DSt, in contrast, has never been unambiguously resolved. During the 1970s, using lesion studies, an intensive effort was made to determine whether the nigrostriatal or mesoaccumbens pathway mediates the locomotor stimulating effects of psychostimulants (Costall et al, 1977; Creese and Iversen, 1975; Fink and Smith, 1979, 1980; Jeste and Smith, 1980; Kelly et al, 1975; Pijnenburg et al, 1975). However, due to the difficulties in controlling the precise scope of experimental lesions, combined with the need for lesions of dopamine nuclei to be greater than 90% complete to be effective (Costall et al, 1977; Creese and Iversen, 1972; Fink and Smith, 1980), these studies were inconclusive and the relative importance of the DSt as a substrate mediating the locomotor stimulating effects of psychostimulants remains uncertain (see Supplementary Materials for a brief summary of these studies).
We take advantage of a mutant mouse line with selective loss of the nigrostriatal dopaminergic pathway to ask its contribution to the reinforcing and psychostimulant effects of cocaine. Aphakia mice were first identified with a blind phenotype resulting from a failure of lens development (Varnum and Stevens, 1968), subsequently attributed to a 652 bp deletion in the promoter region of the Pitx3 gene that encodes the homeodomain containing transcription factor Pitx3 (Semina et al, 2000). Rieger et al (2001) further identified an additional deletion that eliminates exon 1 of Pitx3 itself. The mutation is recessive and heterozygote mice are normal (Kas et al, 2008; Maxwell et al, 2005; Semina et al, 2000). Within the central nervous system, Pitx3 is only expressed within A9 and A10 dopamine cells (Maxwell et al, 2005). At birth, Pitx3-deficient mice have a virtually complete loss of A9 substantia nigra (SN) dopamine neurons, resulting in a 90% reduction in dorsal striatal dopamine ((Hwang et al, 2003; Nunes et al, 2003; Smits et al, 2005; van den Munckhof et al, 2003); see also Figure 1). Although normal at birth, the ventral tegmental area (VTA) is progressively affected postnatally with a noticeable decline in dopamine cells by P100 (Kas et al, 2008; van den Munckhof et al, 2003). No other brain regions, including other dopamine nuclei, are affected (Hwang et al, 2003; Nunes et al, 2003). Despite the moderate, progressive loss of VTA neurons, both qualitatively and quantitatively the mutation is comparatively selective for the nigrostriatal pathway (Hwang et al, 2003; Hwang et al, 2005; Kas et al, 2008; Maxwell et al, 2005; Nunes et al, 2003; Smits et al, 2005; Smits et al, 2008; van den Munckhof et al, 2003). The nigrostriatal pathway is catastrophically affected: (1) dopamine neurons in the ventral SN compacta are essentially eliminated, (2) dopamine content in the DSt as measured by tyrosine hydroxylase (TH) reactivity and HPLC is reduced by approximately 90%, (3) dopamine release as measured by fast-scan cyclic voltammetry is nearly eliminated in the DSt (Kas et al, 2008; Smits et al, 2005), (4) abnormal c-FOS induction in the DSt is observed in response to l-dopa (Hwang et al, 2005), and (5) abnormally high levels of serotonergic innervation are observed in the DSt (Smits et al, 2008), similar to that observed with neonatal 6-OHDA lesions of the SN (Snyder et al, 1986; Stachowiak et al, 1984). In contrast, the mesolimbic dopamine cells are only moderately affected and the NAc exhibits none of these dramatic changes. It has been suggested that the progressive loss of mesolimbic dopamine neurons may be compensated for by a greater percentage of neurons in the VTA being active rather than silent (Smits et al, 2005). Using these mice, we show that the psychostimulant effects of cocaine, both acute and sensitized, are dependent on an intact nigrostriatal pathway, whereas the reinforcing effects are not.
Figure 1.
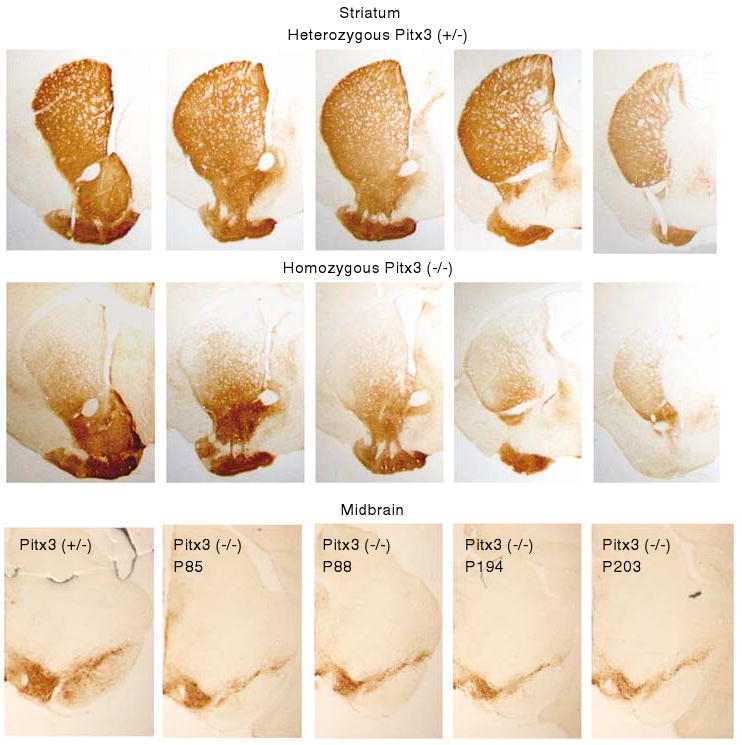
Tyrosine hydroxylase immunoreactivity in serial coronal sections of the striatum in mice heterozygote (top panel) and homozygote (middle panel) for the mutation in the promoter region of the Pitx3 gene. Bottom panel shows midbrain sections from one heterozygote (left) and four homozygote mice from two age groups (right).
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at The University of Chicago. Mice were group housed in standard conditions with ad libitum access to food and water, except during the sucrose preference test when they were singly housed. To obtain Pitx3-deficient homozygote experimental animals and heterozygote littermate controls, Pitx3-deficient homozygotes (Pitx3ak/2J; Jackson Laboratories, Bar Harbor) were bred with C57BL/6 wild-type mice. The heterozygote offspring were then bred with homozygotes, yielding homozygote Pitx3-deficient mice and littermate heterozygote control mice. All experiments were carried out during the light period (0600–1800 hours) except the sucrose preference, which was conducted in the home cage across a period of 30 days.
Different laboratories have chosen various blind controls as comparison groups for the Pitx3-deficient mice, including other mutant lines that are blind or specifically blinding wild-type mice on a comparable genetic background. Use of different control groups may contribute to some of the discrepancies and controversies between studies, as either genetic background is not sufficiently well matched or the method of blinding the control mice may not have effects equivalent to early failure of lens development observed in the Pitx3-deficient mice. We elected to prioritize control for genetic background by using littermate heterozygote controls and address the potential confound of blindness as needed in interpretation of the data (see (Kas et al, 2008) for discussion). As shown in our results, as long as a task does not have a requirement for explicit use of visual cues, the Pitx3-deficient mice perform remarkably similar to wild-type littermates. Further, Kas et al (2008) have shown that blindness does not account for the activity phenotype of the Pitx3-deficient mice.
Because the VTA in Pitx3-deficient mice, though intact at birth, shows progressive decline with age observable at P100 (Kas et al, 2008; van den Munckhof et al, 2003) we included two age groups in several experiments: mice less than 100-day old (between 60 and 90 days) and mice greater than 100 days (between 150 and 200 + days). The average age and number of animals in each age group varies by experiment and is described in the results where this is applicable.
Immunohistochemistry
Mice were perfused with a buffered 4% paraformaldehyde solution and removed brains were postfixed for 24 h and cryoprotected in 25% sucrose for another 48 h. Brains were cut into 50-μm coronal cross-sections encompassing the midbrain and the entire striatum using a cryostat. Free-floating sections were collected for immunohistochemistry as separate sets so that each set contained every sixth serial section. One set of sections was immunostained for TH. Rostrocaudal position of sections was assessed with the aid of a mouse brain atlas (Paxinos and Franklin, 2001). Immunostaining was performed using an avidin–bitoin–peroxidase complex method (Vectastain ABC Elite Kit, Vector Laboratories). TH immunoreactivity was visualized by using the diaminobenzine (DAB, Sigma Fast 3,3′-diaminobenzine tablets) method. The primary rabbit anti-TH antibody (Pel-Freez Biologicals) was diluted at a ratio of 1 : 1000 for 48 h. The secondary biotinylated goat anti-rabbit IgG (Jackson Immunoresearch Laboratories) was diluted at a ratio of 1 : 500. N = 3 (Pitx3 deficient, <100 days), 4 (Pitx3 deficient, >100 days), 2 (Pitx3 heterozygote, ages 85, 88 days), and 3 (wild-type C57BL/6, age 150 days).
Quantifying tyrosine hydroxylase reactivity
All sections were prepared in a single batch as described above and photographed in a single session using the same microscope settings. Corresponding sections were identified and, using Adobe Photoshop, the images were aligned against a reference section using the anterior commissure as an anchor point to ensure each section was situated in the frame identically. ImageJ (http://rsb.info.nih.gov/ij/) was used for all quantification. To assess the gradient of denervation, the matched sections for each mouse within a genotype were opened into a stack and combined into a composite section (‘z-project’ in ImageJ), which was used for quantification. Reference lines were drawn onto the composite images to quantify the intensity of tyrosine hydroxylase reactivity (measured as 256 levels of brightness) either along the extent of the line to assess gradient (shown in Figures 2a and b) or taking the average of each line to assess an angular gradient (shown in Figure 2d). The anterior commissure was used as an origin for dorsal to ventral reference lines; the medial to lateral reference lines were drawn parallel to the tilt of the DSt. A rectangular region of interest was drawn to quantify TH reactivity in the NAc in the composite images (shown in Figures 2a and b). In quantifying a rectangle, ImageJ calculates the average intensity of vertical lines moving across the horizontal extent of the rectangle. To calculate statistics and compare <100 and >100 days old Pitx3-deficient mice with wild-type and heterozygote controls (data shown in Figure 2h), a polygon region of interest was drawn around the DSt and the NAc in each individual section and the average intensity of the area within these polygons calculated (each section used with the drawn regions of interest are available in Supplementary Materials, Figures S1–S3). Heterozygote Pitx3 (+/−) and wild-type C57BL/6 controls were pooled as previous studies have shown the heterozygote mice are unaffected (Kas et al, 2008; Maxwell et al, 2005; Semina et al, 2000; see also Supplementary Figure S1).
Figure 2.
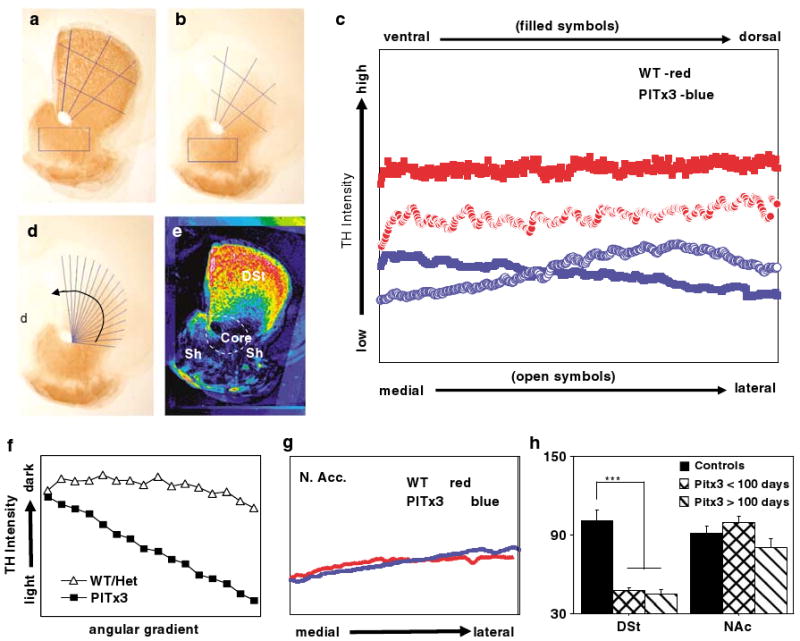
Quantification of dopamine denervation in the striatum. Composite images averaging the intensity of tyrosine hydroxylase (TH) reactivity from overlaid, matched, and aligned sections for (a) control (N = 2, heterozygote, N = 3 wild-type) and (b) Pitx3-deficient mice (N = 7). Lines used to quantify the gradient in the dorsal striatum (DSt) from ventral to dorsal (3) and from medial to lateral (2) are shown. The rectangular regions used to quantify TH reactivity in the nucleus accumbens (Nac) are also shown. (c) The mean of TH reactivity measured along the reference lines (filled symbols plot ventral to dorsal measurements; open symbols plot medial to lateral; genotypes represented by color). (d) Radiating reference lines used to compute angular gradient of dopamine denervation are shown on the Pitx3-deficient composite image. (e) Pseudo-colored difference map resulting from subtracting the Pitx3-deficient composite from the control composite (hot colors (red) show greatest difference, cool colors (blue) least difference) with DSt and the NAc shell and core labeled according to standard mouse atlas (Paxinos, 2nd edn.). (f) Average intensity of TH-reactivity for each spoke of the radiating reference lines plotted in the direction of the arrow shown in (d). (g) TH reactivity in the NAc plotted from medial to lateral based on rectangular region of interest shown in (a) and (b). Each point represents the average of a vertical column within the rectangle. (h) Group averages of TH immunoreactivity in the DSt and NAc from sections quantified individually (rather than as composite) using a polygon regions of interest drawn around the DSt and NAc. The Pitx3-deficient mice were divided into young (< 100 days, N = 3) and old (> 100 days, N = 4). ***p<0.001.
Behavior Tests
Open field
Each mouse was placed in an acrylic open-field chamber of 40 (l) × 40 (w) × 37 cm(h) (Med Associates, St Albans, VT). Illumination was 21 lx. Infrared beams recorded the animals' locations and paths (locomotor activity) as well as the number of rearing movements (vertical activity). Data were collected in 5-min bins during 60-min trials. The chambers were cleaned with 70% ethanol between all trials.
Stereotypy
All sessions in the locomotor sensitization experiment were videotaped. To manually assess stereotypy, the videotapes for the first conditioning session, the last conditioning session, the cocaine challenge and saline control sessions were reviewed. For each session, five 1-min observation points were randomly selected and observed. If the mouse displayed stereotypic behaviors (head bobbing, circling, gnawing, continuous sniffing, or climbing) for greater than 50% of the observation minute, that observation was scored 1, otherwise, 0. The five observations within each session were then averaged for each mouse. To test stereotypy, an apomorphine challenge was administered using a within-subject design. Mice were tested in two 30-min sessions on 2 consecutive days, receiving saline in session one and 2 mg/kg of apomorphine on session two. Stereotypy was measured as described above.
Conditioned place preference
A conditioned place preference (CPP) insert placed in the open-field chambers described above (Med Associates) divides the open-field boxes into two chambers with access between the two chambers controlled by the experimenter (ie a gate). The flooring was different in each chamber, one having wire mesh bottom, the other a bar grid bottom. Light was maintained at 21 lx. An unbiased, two-chamber design was used. For conditioning sessions, access between the chambers was closed. Mice were provided five conditioning sessions for each chamber alternating between saline and cocaine (10 mg/kg) for a total of 10 consecutive conditioning sessions. Mice were placed in the chambers immediately after injection. The possible pairings between drug and the two different chambers were counterbalanced to control for potential unconditioned preference for one chamber over the other. Activity was recorded during the conditioning sessions for analysis of locomotor sensitization. During the test phase, access between the chambers was opened and mice were placed in the chambers drug free and their locomotor activity and position monitored for 30 min. The start chamber into which the mice were placed was counterbalanced. Place preference was calculated as the time spent in the cocaine-paired chamber divided by the total time (30 min).
Locomotor sensitization
For preexposure, mice of each genotype were administered five sessions of either cocaine (10 mg/kg), or saline (0.9%) and placed in the open-field boxes for 1 h. Preexposure sessions were separated by 72 h. Two weeks after the last preexposure administration, all mice were challenged with a low dose of cocaine (5 mg/kg) and saline in two 30-min sessions separated by 48 h. In addition to raw distance traveled during the challenge sessions, we calculated the percent increase observed in the cocaine over the saline challenge sessions for all groups.
Sucrose preference
Mice were singly housed in cages that included two identical water bottles (15-ml round bottom polypropylene tubes with rubber stopper on the mouth and a sipper tube with ball bearing), one of which contained sucrose solution at varying concentrations in the course of the experiment (0, 0.2, 5, 10, and 15%). Each concentration of sucrose was provided for 6 days. The positions of the bottles were rotated daily to counterbalance potential position preferences. The bottles were weighed daily and the amount consumed from each recorded. A cage without mice was maintained and weighed daily to track spillage, which remained minimal and is not reported. Preference as a percent was calculated by dividing the amount consumed from the sucrose bottle from the total amount consumed from both bottles.
Drug Administration
Cocaine hydrochloride (Sigma) or apomorphine was dissolved in 0.9% saline at a concentration resulting in administration of 0.01 μl/g of body weight to achieve the concentration indicated in the results. Saline (0.9%) was administered at 0.01 μl/g of body weight.
Data Analysis
Unless otherwise indicated, analysis of variance was used for analysis of statistical significance (Statview, SAS Institute, Cary, NC).
Results
Altered Dopaminergic Innervation in Pitx3-Deficient Mice
Consistent with previous reports (Hwang et al, 2003; Nunes et al, 2003; Smits et al, 2005; van den Munckhof et al, 2003), we observe a dramatic loss of dopamine innervation in the DSt of Pitx3-deficient mice, whereas the ventral striatum is relatively unaffected (Figure 1, top panels). As previously reported, both the shell and core of the NAc are equally spared (Hwang et al, 2003; Nunes et al, 2003). In the midbrain, we observe a reduction of dopamine cells (Figure 1, bottom panel). Previous studies have shown that this loss is predominant in the ventral tier of the SN compacta, whereas the dorsal tier is only moderately affected (van den Munckhof et al, 2003). As slow, progressive loss of dopamine cells in the VTA has been previously reported, becoming evident at P100 (van den Munckhof et al, 2003), we show representative sections from mice at both younger and older ages (Figure 1, bottom). In the striatum, the dopamine denervation appears to be graded. We characterize this gradient in Figure 2. Taking equivalent sections from several mice (N = 5 control; N = 7 Pitx3 deficient) we constructed composite images (Figures 2a and b). We drew three ventral to dorsal and two medial to lateral reference lines and quantified the intensity of TH reactivity along those lines. Plotting the average of the line sets (Figure 2c) shows clearly reduced TH intensity in Pitx3-deficient mice compared to controls. In addition, the ventral to dorsal axis shows a gradual decreasing gradient (ie decreasing TH), whereas the medial to lateral axis shows a more pronounced increasing gradient (ie increasing TH). This suggested that the denervation may be best characterized as an angular gradient. To test this, radiating reference lines were drawn at equally spaced intervals from an origin on the anterior commissure (Figure 2d) and the average intensity along each line was calculated and plotted (Figure 2f) showing a clear angular gradient in the striatal denervation of the Pitx3-deficient mice. A pseudo-colored difference map between controls and Pitx3-deficient mice helps visualize this difference and shows that the lost dopamine innervation forms a large cap on the striatum that extends ventrally at its medial and lateral extremes (Figure 2e). TH reactivity in the NAc was quantified using a rectangular region of interest (shown on composite sections in Figures 2a and b) and showed no difference between the genotypes (Figure 2g).
To statistically analyze TH differences between the control mice and two age groups of Pitx3-deficient mice, the sections contributing to the composite images were individually analyzed by enclosing the entire DSt or NAc in a polygon region of interest and calculating the average intensity (each section used and the regions of interest drawn are available in Supplementary Figures S1–S3). Figure 2h shows that TH reactivity is dramatically reduced in the DSt of Pitx3-deficient mice compared to controls (F2,12 = 28.3, p = 0.0001) and no difference between the <100- and >100-day Pitx3 mice (Fisher's PSLD pairwise comparison, p = 0.83). In contrast, there is no significant difference between the groups in the NAc (F2,12 = 28.3, p = 0.154). Comparing the young and old Pitx3-deficient mice, there appears to be a trend toward a mild decrease in NAc TH reactivity in the older group, but this does not reach statistical significance (Fisher's PSLD, p = 0.077) and is clearly on a different scale from the loss observed in the DSt.
The profound dopamine denervation in the DSt and the observed gradient presumably reflect the projection pattern of primarily the ventral tier of the SNc that is clearly lost in the Pitx3-deficient mice (van den Munckhof et al, 2003). The spared regions may reflect projections from the dorsal SNc, the VTA (A10) or the retrorubral area (A8) (Joel and Weiner, 2000; Yue et al, 1999).
Baseline Open-Field Activity
It remains controversial whether the Pitx3-deficient mice are hypokinetic (Smidt et al, 2004) or hyperkinetic (Hwang et al, 2005; Smits et al, 2008), with different groups reporting conflicting results (Hwang et al, 2003; Nunes et al, 2003; Smidt et al, 2004; van den Munckhof et al, 2003). We have conducted open-field experiments with independent cohorts in different experiments, each of which includes establishing a baseline response using 1-h sessions on 3 consecutive days (66 littermate controls; 67 Pitx3 deficient). The combined baseline data indicate that the Pitx3-deficient mice are mildly hyperactive in the open field (Figure 3a, inset; F1,131 = 9.21, p = 0.0029), consistent with the findings of Hwang et al (2005). However, looking at activity binned into 5-min blocks indicates that the significant difference between the two groups is accounted for entirely by elevated activity in the Pitx3-deficient mice during the initial 5 min of an open-field session, an observation we find consistently across individual experiments (Figure 3a, block 1: F1,131 = 138.4, p<0.0001; blocks 2–12: F1,131 = 0.573, p = 0.45). The precise reason for this initial elevation in activity is uncertain. One possibility is that Pitx3-deficient mice rely more on nonvisual senses to survey a novel environment. Despite this alteration in initial response, during the remaining 55 min of open field, there are no differences between phenotypically normal heterozygotes and the homozygote Pitx3-deficient mice, consistent with recent findings controlling for genetic background in the same manner used here (Kas et al, 2008). As we are measuring the acute locomotor response to cocaine, in which the pharmacological actions of the drug span the open-field session peaking well after the initial 5 min, we conclude for purposes of this study that the Pitx3-deficient and heterozygote control mice show essentially the same baseline locomotor activity.
Figure 3.
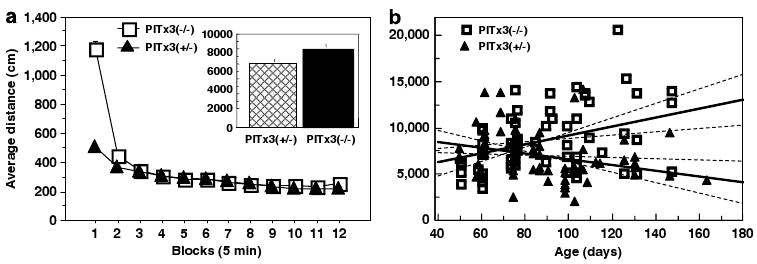
Baseline activity of Pitx3 and littermate heterozygote controls. (a) Distance traveled (mean ± SEM) during 5-min bins averaged across baseline sessions. Inset: average distance (mean ± SEM) traveled during baseline sessions. N = 66 (Pitx3 +/−), 67 (Pitx3 −/−). (b) Scatter plot showing distance traveled plotted against age. Regression lines in bold for each group with 95% confidence for slope shown as dashed lines.
Using the same data set, we analyzed the correlation between age and activity and found a clear difference between groups (genotype × age interaction, F1,129 = 8.87, p = 0.0035; Figure 3b). Control mice tend to exhibit decreased exploratory activity with age, whereas the Pitx3-deficient mice tend to show mildly increased exploratory activity, likely attributable to compensatory activity in the NAc in response to either decreasing dopaminergic input from the VTA (de Rover et al, 2006; Smits et al, 2005; van den Munckhof et al, 2006) or as a mesolimbic compensation for the loss of nigrostriatal signaling (van Oosten et al, 2005). Alternatively, a shifted balance between dopaminergic and serotonergic signaling in the DSt (Smits et al, 2008) may contribute to the observed increase in activity. As a result, differences in exploratory activity between the Pitx3-deficient mice and littermate controls become more pronounced as the mice age, although the general pattern remains with only the first 5 min showing a statistically significant difference between genotypes. Finally, it has been suggested that the Pitx3-deficient mice exhibit abnormal circadian rhythms (Hwang et al, 2005); however, we and others have demonstrated that circadian phase does not impact exploratory response in short open-field sessions (Beeler et al, 2006; Valentinuzzi et al, 2000). Consequently, this potential difference should not impact our study.
Acute Cocaine-Induced Locomotor Response
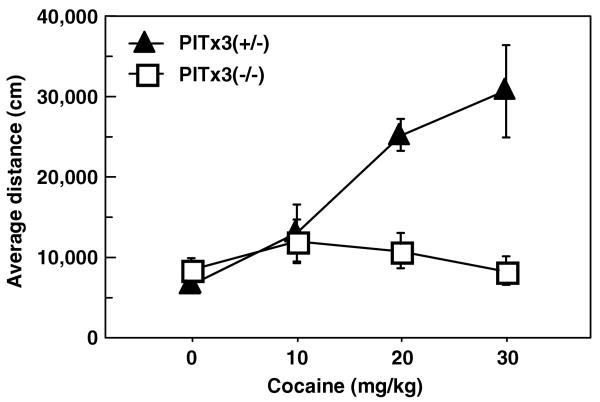
We tested the acute locomotor response to cocaine using the open field to measure locomotor activity. The average of three baseline sessions shows no significant difference between the Pitx3 (−/−) and heterozygote controls (F1,30 = 1.4, p = 0.245; data not shown). A between-subjects design was used to construct a dose–response curve. The heterozygote controls show the expected dose-dependent increase in locomotor activity; however, the Pitx3-deficient mice show a dramatically diminished acute locomotor response (genotype × dose interaction, F3,24 = 7.58; p = 0.001; Figure 4). When Pitx3-deficient mice are analyzed alone, cocaine dose does not significantly affect locomotion (F3,12 = 0.742, p = 0.547). The lack of acute locomotor response to cocaine in the Pitx3 mice was not due to increased stereotypy. Stereotypy scores showed no significant differences between genotype (F1,24 = 0.067, p = 0.797) nor genotype × dose interaction (F3,24 = 1.53, p = 0.23). This was confirmed by visually scored videotapes of sessions where minimal stereotypy was observed with no differences between experimental and control animals (genotype main effect, F1,20 = 0.298, p = 0.59; data not shown). The average age of the mice in this experiment was 86.4 and 88.4 days for the experimental and control groups, respectively, ranging from 50 to 174 days with ages distributed equally across groups. Age showed no significant correlation with cocaine response (genotype × age interaction F1,28 = 0.661, p = 0.423; data not shown). These data indicate a nearly complete loss of the acute locomotor stimulant response to cocaine in the Pitx3-deficient mice. The residual response to acute cocaine could be due to residual dopamine in the DSt arising from either a thin layer of dorsal neurons spared in the SNc (van den Munckhof et al, 2003) or, alternatively, from the retrorubral dopamine cell projections (A8) (Nunes et al, 2003). Finally, to test whether the Pitx3-deficient mice have the postsynaptic and structural mechanisms necessary to exhibit stereotypy, we challenged Pitx3-deficient and control mice with apomorphine (2 mg/kg). Both groups exhibited stereotypy in response to the apomorphine (Supplemental Figure S4; main effect of apomorphine, F1,10 = 23.226, p = 0.0007; main effect genotype, F1,10 = 0.541, p = 0.479, genotype × apomorphine interaction, F1,10 = 2.58, p = 0.139).
Figure 4.
Cocaine dose–response curve. Average distance traveled (mean ± SEM) in response to cocaine administration (N = 4 per genotype/dose).
Reinforcing Properties of Cocaine
Using the CPP paradigm with two chambers and an unbiased design, we examined the reinforcing properties of cocaine in the Pitx3 mice. We provided 10 conditioning sessions on consecutive days alternating between the cocaine- and saline-paired chambers for a total of five conditioning sessions per chamber. Mice were placed in the chambers immediately following injection. On the test day, mice were placed in the apparatus without any injection and given access to both chambers. Both groups equally exhibited a preference for the cocaine-paired chamber indicating that the reinforcing properties of cocaine are unaffected in the Pitx3 (−/−) mice (Figure 5a). Two age cohorts were included in the CPP study, an older group (N = 6, average age 214 and 219 days, Pitx3-deficient and heterozygote controls, respectively) and a younger group (N = 8, average age 60.3 and 61.8 days, Pitx3-deficient and heterozygote controls, respectively). There was no difference in preference for the drug-paired chamber between ages (age cohort main effect, F1,24 = 2.56, p = 0.1226; genotype × age cohort interaction, F1,24=1.04, p = 0.317; data not shown). These data suggest the reinforcing properties of cocaine are intact in the Pitx3-deficient mice.
Figure 5.
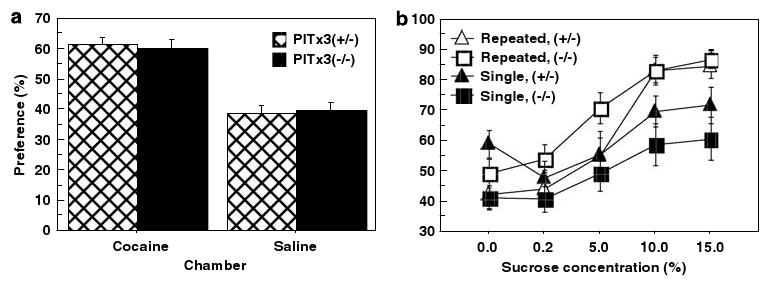
Conditioned place preference and sucrose preference. (a) Percent of time (mean ± SEM) spent in the cocaine- and saline-paired chambers during 30-min test session (N = 14 per genotype). (b) Pitx3-deficient and Pitx3 heterozygote controls were administered repeated cocaine (six exposures, 10 mg/kg, open symbols) or a single cocaine exposure (10 mg/kg, filled symbols) and placed in home cages with a choice of sucrose or water. Sucrose consumption as a percentage of total consumption (mean ± SEM) reflects preference and is shown at five increasing sucrose concentrations, averaged over 6 days for each concentration (N = 4 per genotype/preexposure condition).
It has been demonstrated that the reinforcing properties of cocaine are subject to sensitization as a result of repeated exposures to drug and that this sensitization can be observed in CPP (Shippenberg and Heidbreder, 1995). As both Pitx3-deficient mice and controls showed identical preference for the cocaine-paired chamber, this implies that sensitization of the reward system occurs in the Pitx3-deficient mice; otherwise, we would expect a difference in preference on testing. In an additional experiment, we conducted a sucrose preference test comparing mice of both genotypes that had previously experienced either six repeated or a single cocaine injection (10 mg/kg; Figure 5b). At the 10 and 15% concentrations, the mice previously exposed to repeated cocaine injections show enhanced preference compared to mice exposed to a single prior cocaine injection (sucrose concentration × cocaine prior exposure F4,48 = 2.57, p = 0.049) with no differences between genotypes (genotype F1,12 = 0.034, p = 0.856, genotype × prior exposure interaction F4,48 = 0.207, p = 0.993). These data suggest that previous, repeated exposure to cocaine sensitizes the reward pathway and that this effect is intact in the Pitx3-deficient mice (average age of Pitx3-deficient and control mice, 142 and 136 days, respectively).
Sensitization of Cocaine-Induced Locomotor Response
By measuring locomotor activity during conditioning sessions in the CPP test, we obtained data on the locomotor sensitizing effects of repeated cocaine exposure in these mice (Shimosato and Ohkuma, 2000). Control mice clearly exhibit sensitization of their locomotor response to cocaine (repeated measures of cocaine sessions, heterozygotes only, F4,52 = 11.71, p<0.0001), whereas the Pitx3 (−/−) did not (repeated measures of cocaine sessions, homozygotes only, F4,52 = 0.815, p = 0.521; Figure 6a). Locomotor activity in the Pitx3 (−/−) mice in response to repeated cocaine injections appears to mirror repeated saline injections, suggesting changes in activity across sessions are being mediated primarily by habituation processes rather than sensitization. Consistent with the acute locomotor response observed in the dose–response curve, a residual locomotor response to acute cocaine is evident (cocaine main effect in homozygotes, F1,52 = 23.7, p = 0.03), although this residual response does not exhibit sensitization. This residual locomotor response in the Pitx3-deficient mice is most likely attributable to retrorubral (A8) dopamine projections to the DSt that are retained in these mice (Nunes et al, 2003) or to the spared dorsal tier SN projections (Figure 1, bottom; (van den Munckhof et al, 2003)). As described above, two age cohorts were used in this experiment. Heterozygote control mice in both age cohorts show significant sensitization of locomotor activity across cocaine conditioning sessions and Pitx3-deficient mice in both age cohorts do not.
Figure 6.
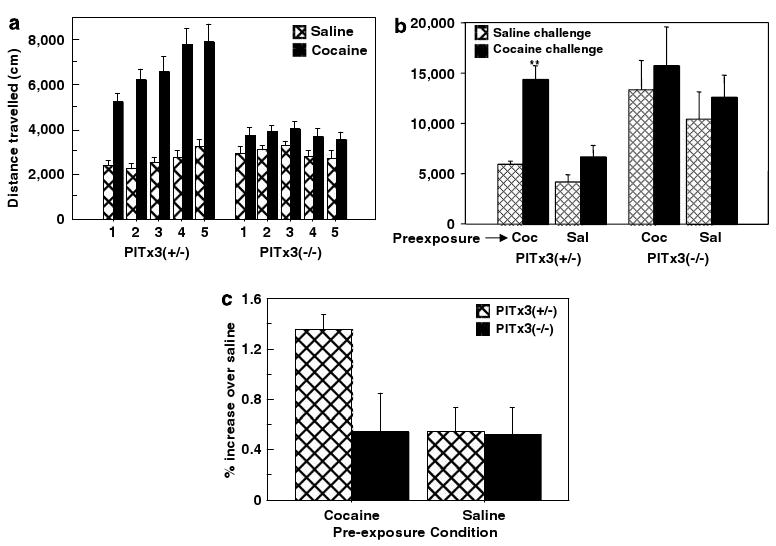
Locomotor sensitization to repeated exposures of cocaine. (a) Ambulatory activity (mean ± SEM) during 30-min conditioning sessions alternating saline and cocaine pairing in the conditioned place preference test (N = 14 per genotype). (b) Mice preexposed to either 5 days of cocaine (10 mg/kg) or saline (72 h between exposures) and then challenged with cocaine (5 mg/kg, solid bars) and saline (hatched bars) in 30 min open-field sessions on consecutive days (mean distance ± SEM, N = 6 per genotype/preexposure condition). (c) Response to cocaine challenge normalized as percent increase over saline challenge (mean ± SEM).
Some aspects of sensitization, such as increased dopamine release in the NAc, are not observed immediately following the last dose of a sensitization regimen but develop over time and are observed in subsequent weeks (Paulson and Robinson, 1995). It is possible that the Pitx3-deficient mice would exhibit sensitization after a period of elapsed time following the last preexposure administration if tested later. To test this possibility, we conducted a sensitization protocol consisting of either five cocaine (10 mg/kg) or saline preexposure injections separated by at least 72 h. Two weeks after the final administration, all mice were given two challenge sessions with cocaine (5 mg/kg) and saline (Figure 6b). The heterozygote control mice preexposed to cocaine show enhanced locomotor response to the cocaine challenge (cocaine × preexposure, F1,10 = 24.01, p = 0.0006), whereas the Pitx3-deficient mice show no difference as the result of preexposure to repeated cocaine administration (cocaine × preexposure, F1,11 = 0.002, p = 0.9617; average age of Pitx3-deficient and control mice, 118 and 129 days, respectively). The sensitized response is summarized in Figure 6c as the percentage increase in the cocaine compared to saline challenge sessions for all groups. Notably, the Pitx3-deficient mice show higher overall activity consistent with their ages as predicted by the scatter plot regression lines in Figure 3b. Consistent with the results above, cocaine did not have a statistically significant effect on the Pitx3-deficient regardless of preexposure condition (F1,10 = 1.61, p = 0.23). These data, together with the analysis of the CPP conditioning sessions suggest that the Pitx3-deficient mice do not exhibit sensitization of the locomotor response to cocaine.
Discussion
Using Pitx3-deficient mice that lack a nigrostriatal pathway, we report here that cocaine-induced increases in locomotor activity and sensitization of locomotor activity are dependent on an intact nigrostriatal pathway. In contrast, cocaine CPP, sucrose preference, and enhanced sucrose preference subsequent to repeated cocaine exposures are intact, suggesting these are independent of the nigrostriatal pathway. The pattern of denervation observed (Figures 1 and 2) arises primarily from the loss of ventral tier SNc neurons (van den Munckhof et al, 2003), suggesting these dopamine neurons and their projections to the DSt have a critical role within the nigrostriatal pathway in mediating locomotor activity in response to cocaine. Although the spared behavioral functions are likely mediated by the mesolimbic pathway, which is only mildly affected, the potential contribution of the remaining dorsal tier SNc projections remains unknown. It has been suggested that the anatomic division of A9 and A10 dopamine cells into SNc and VTA may be less functionally salient than division into dorsal and ventral tiers (Joel and Weiner, 2000). As our knowledge of the functional connectivity and projections of subpopulations and regions within the dopamine nuclei remains incomplete, we continue with the widely accepted division between the mesolimbic and nigrostriatal pathways, but acknowledge it is primarily the ventral SNc component of the nigrostriatal pathway that is affected. The pattern of DA cell loss observed here in Pitx3-deficient mice highlights the importance of isolating and assessing the functional characteristics of the dorsal tier of the SNc and the degree to which its function may be similar to that of the VTA or the ventral SNc, a project beyond the scope of the present study.
There is a rich literature on the role of the mesolimbic dopaminergic pathway in mediating hyperlocomotion and behavioral sensitization in response to psychostimulants. The NAc has been clearly demonstrated to be a key neural substrate mediating both the acute and sensitized locomotor response to psychostimulants (Di Chiara, 2002; Everitt and Robbins, 2005; Parkinson et al, 1999; Robinson and Berridge, 2003; Vezina, 2004). Although enhanced release of dopamine in response to repeated exposures to psychostimulants has been demonstrated in both the NAc and DSt (Paulson and Robinson, 1995), enhanced cFOS induction that correlates with locomotor sensitization is only observed in the NAc and not the DSt (Crombag et al, 2002), suggesting that the accumbens is also the key site of locomotor sensitization. The precise efferent pathways through which the NAc increases locomotor activity, both acute and sensitized, however, have not been characterized. Our data suggest that the nigrostriatal pathway serves as a critical effector mechanism for the NAc. In the present study, the CPP and sucrose preference data suggest that the reinforcing effects of cocaine are sensitized in the Pitx3-deficient mice. However, there is a lack of locomotor sensitization in these mice. The most likely interpretation is that in the Pitx3-deficient mice, the NAc sensitizes to repeated cocaine exposures, but the locomotor expression of sensitization, like the acute locomotor response, is dependent on the nigrostriatal pathway. We attribute the inconclusive findings of earlier studies on the role of the nigrostriatal pathway (see Supplemental Material) to the difficulties inherent in achieving sufficiently precise and complete lesions of the nigrostriatal dopamine system. In contrast, in this study, a genetically and developmentally defined subset of dopamine neurons are precisely and completely eliminated (Maxwell et al, 2005).
Understanding the functional relationship between compartments within the basal ganglia has been an enduring challenge. Mogenson et al (1980) hypothesized that the basal ganglia linked limbic information processing with motor output by moving information progressively from the motivationally oriented medial anterior region of the striatum, including the NAc, to final sensorimotor processing in dorsal–caudal regions. On the basis of elegant anatomical studies, Haber (2003) mapped out an architecture of ascending ‘spiral’ loops between the striatum and dopamine nuclei that could mediate the movement of information from ventral, limbic regions of the striatum to dorsal, sensorimotor regions and suggested these loops may serve a ‘teaching’ function between striatal compartments. These studies and others suggest a general principle of striatal organization where incoming information, both descending and ascending, are processed in functionally discrete striatal regions but that the result of that processing is passed on to the next striatal compartment where it is integrated into the functionally discrete processing of that compartment (Calzavara et al, 2007; Haber, 2003; Haber et al, 2006; Joel, 2001; Kelly and Strick, 2004). More specifically, these loops may provide a mechanism whereby sensory and motor cortical processing, as it progresses through the striatum, can be modulated by reward information signaled by complementary mesencephalic-striatal dopamine loops. Dopamine, in turn, can regulate striatal synaptic plasticity (Calabresi et al, 2007; Reynolds and Wickens, 2002; Wolf et al, 2004), modulate reinforcement learning (Schultz, 2002), and alter the expression of motivated behavior (Cagniard et al, 2006; Salamone and Correa, 2002).
In a recent study, Belin and Everitt (2008) directly tested the hypothesis that the dopaminergic connections between the ventral and DSt are critical in mediating drug-seeking behavior. By unilaterally lesioning the NAc core and then applying DA antagonist contralaterally in the DSt, they showed a reduction in second-order responding for cocaine-paired conditioned stimuli while neither the unilateral NAc lesion itself nor unilateral administration of DA antagonist in the DSt alone resulted in decreased responding. These data elegantly demonstrate that established drug-seeking behavior depends on NAc recruitment of dorsally projecting dopamine activity. Our study complements this work by showing that the ability of the NAc to mediate the basic psychostimulant action of cocaine also depends on an intact nigrostriatal system.
An interesting question remains as to the role of the nigrostriatal pathway in the reinforcing effects of cocaine. The mesoaccumbens pathway is clearly associated with drug reinforcement and addiction (Di Chiara, 2002; Everitt and Robbins, 2005; Ito et al, 2004; Kelley, 2004; Robinson and Berridge, 1993, 2003; Sellings et al, 2006; Vezina, 2004). The role of the DSt in addiction, however, has recently received greater recognition and attention (Belin and Everitt, 2008; Everitt and Robbins, 2005; Gerdeman et al, 2003; Letchworth et al, 2001; Porrino et al, 2004; Vanderschuren et al, 2005; Volkow et al, 2006), bringing to the fore questions about the functional relationship between the dorsal and ventral striatal compartments and the mesoaccumbens and nigrostriatal pathways that innervate them. Using Pitx3-deficient mice that have a genetically defined loss of nigrostriatal pathway, we show that intraperitoneal (i.p.)-administered cocaine CPP is intact. In addition, we demonstrate that increased sucrose preference as a result of repeated exposures to cocaine is also intact. This would suggest that the mesoaccumbens pathway mediates the reinforcing properties of cocaine, which are retained in these mice, independent of the nigrostriatal pathway; however, it is unlikely that reinforcement is a unitary phenomenon. Although the ventral striatum has been shown to be important in both CPP (Tzschentke, 1998) and sucrose consumption (Kelley, 2004), other reinforced behaviors have been associated with the DSt, particularly habitual, S-R and action-outcome learning (Yin et al, 2004, 2005). Whether Pitx3-deficient mice acquire cocaine reinforced instrumental responding and exhibit habitual, ‘drug-seeking’ behavior is a critical question for future studies and will provide insight into the role of the nigrostriatal pathway in drug-reinforced learning and the development of addiction.
Some studies have suggested that the reinforcing properties of i.p. but not intravenous (i.v.)-administered cocaine in CPP are dopamine-independent (Mackey and van der Kooy, 1985; Morency and Beninger, 1986; Sellings et al, 2006; Spyraki et al, 1987); however, other studies have demonstrated the opposite (Adams et al, 2001; Cervo et al, 2005; Cervo and Samanin, 1995; Gyertyan and Gal, 2003; Kaddis et al, 1995; Khroyan et al, 1999; Pruitt et al, 1995; Vorel et al, 2002). Reconciling these contradictory findings is difficult due to methodological differences between studies and is beyond the scope of this discussion. However, the effects of DA agonists and antagonists on cocaine CPP are exquisitely sensitive to the relationship between the administered dose of cocaine and the dose of drugs manipulating the DA system (see Tzschentke, 1998, 2007 for comprehensive review). The present study did not address this issue; it remains possible that i.v.-administered cocaine CPP might be disrupted in these mice. However, this seems unlikely as the sucrose preference data demonstrate that repeated administration of cocaine sensitizes the reward system in these mice similar to control mice, further supporting the idea that cocaine reinforcement is intact in these mice.
The genetic approach used here offers a level of precision that is not possible with lesion studies; the disadvantage is the difficulty of identifying and characterizing potential developmental compensations. The loss of a functional pathway by any means, genetic mutation or lesion, can potentially result in compensatory changes in the brain, particularly when the loss occurs very early in development. In the Pitx3-deficient mice, presumably the brain does compensate for the loss of the nigrostriatal pathway, otherwise we would expect these mice to display severe parkinsonian-like symptoms, which they do not (Hwang et al, 2003; Nunes et al, 2003; Smits et al, 2005; van den Munckhof et al, 2003). The nature of these potential compensations has not been characterized, although consistent with 6-OHDA neonatal lesions of the SNc, increased serotonergic innervation of the striatum has been described (Smits et al, 2008). Although we cannot rule out potentially unrecognized developmental compensations, we argue that the most parsimonious explanation of the findings reported here, in the context of the current literature, is to attribute them to the altered dopaminergic innervation of the DSt. First, although we detect mild decreases in dopaminergic innervation of the NAc of the Pitx3-deficient mice as they age, the behavioral deficits we observe are not age dependent, consistent with the developmental failure of the ventral SNc projection in these mice beginning at E12.5 (Maxwell et al, 2005). Second, whatever compensations may exist they do not cause aberrant baseline behavior relevant to the behavioral responses studied here: the mice show similar sucrose preference, similar baseline activity in the open field (notwithstanding the difference evident in the initial 5-min block or the difference in age-related changes in activity levels between the genotypes) and similar stereotypic response to apomorphine. Finally, despite potential developmental compensations, the acute locomotor response to cocaine is not rescued, highlighting that this pathway is essential to the psychostimulant effects of cocaine and that any developmental compensations that may occur are unable to replace this function of the nigrostriatal pathway.
Addressing a long-standing question, this study establishes a role for the nigrostriatal pathway in mediating the psychostimulant effects of cocaine and suggests that it functions as an effector mechanism for the NAc, facilitating the cocaine locomotor response widely attributed to the accumbens. In contrast, other cocaine effects associated with the accumbens, such as CPP, are intact, suggesting that at least some forms of cocaine reinforcement are independent of the nigrostriatal pathway. Future studies detailing differences in these mice in reinforcement learning, motivated behavior, and response to other drugs of abuse will provide insight into the role of the nigrostriatal pathway in these behaviors as well as the functional architecture of the striatum.
Supplementary Material
Acknowledgments
We thank Un Kang for providing us original breeding pairs for the Pitx3-deficient homozygotes and Jessica Loweth for invaluable advice on sensitization protocols. This work was supported in part by NIH F31MH076422 (MAK), F32DA020427 (JAB), and MH66216 (XZ).
Footnotes
Disclosure/Conflict of Interest: The authors declare that except for income received from their primary employer no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interests.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Contributor Information
Mazen A Kheirbek, Email: mazen@uchicago.edu, mk3156@columbia.edu.
Xiaoxi Zhuang, Email: xzhuang@bsd.uchicago.edu.
References
- Adams JU, Careri JM, Efferen TR, Rotrosen J. Differential effects of dopamine antagonists on locomotor activity, conditioned activity and conditioned place preference induced by cocaine in rats. Behav Pharmacol. 2001;12:603–611. doi: 10.1097/00008877-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Prendergast B, Zhuang X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol Behav. 2006;87:870–880. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26:2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Costall B, Marsden CD, Naylor RJ, Pycock CJ. Stereotyped behaviour patterns and hyperactivity induced by amphetamine and apomorphine after discrete 6-hydroxydopamine lesions of extrapyramidal and mesolimbic nuclei. Brain Res. 1977;123:89–111. doi: 10.1016/0006-8993(77)90645-x. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. Amphetamine response in rat after dopamine neurone destruction. Nat New Biol. 1972;238:247–248. doi: 10.1038/newbio238247a0. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Smidt MP, Brussaard AB. Pitx3 deficiency in mice affects cholinergic modulation of GABAergic synapses in the nucleus accumbens. J Neurophysiol. 2006;96:2034–2041. doi: 10.1152/jn.00333.2006. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Abnormal pattern of amphetamine locomotion after 6-OHDA lesion of anteromedial caudate. Pharmacol Biochem Behav. 1979;11:23–30. doi: 10.1016/0091-3057(79)90292-2. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Relationships between selective denervation of dopamine terminal fields in the anterior forebrain and behavioral responses to amphetamine and apomorphine. Brain Res. 1980;201:107–127. doi: 10.1016/0006-8993(80)90779-9. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE. Quantifying the psychomotor activating effects of cocaine in the rat. Behav Pharmacol. 2007;18:297–302. doi: 10.1097/FBP.0b013e3281f522a4. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Gal K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, et al. 3,4-Dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Smith GP. Unilateral mesolimbicocortical dopamine denervation decreases locomotion in the open field and after amphetamine. Pharmacol Biochem Behav. 1980;12:453–457. doi: 10.1016/0091-3057(80)90053-2. [DOI] [PubMed] [Google Scholar]
- Joel D. Open interconnected model of basal ganglia-thalamocortical circuitry and its relevance to the clinical syndrome of Huntington's disease. Mov Disord. 2001;16:407–423. doi: 10.1002/mds.1096. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697:76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- Kas MJ, van der Linden AJ, Oppelaar H, von Oerthel L, Ramakers GM, Smidt MP. Phenotypic segregation of aphakia and Pitx3-null mutants reveals that Pitx3 deficiency increases consolidation of specific movement components. Behav Brain Res. 2008;186:208–214. doi: 10.1016/j.bbr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Fuchs RA, Beck AM, Groff RS, Neisewander JL. Behavioral interactions produced by co-administration of 7-OH-DPAT with cocaine or apomorphine in the rat. Psychopharmacology (Berl) 1999;142:383–392. doi: 10.1007/s002130050903. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Mackey WB, van der Kooy D. Neuroleptics block the positive reinforcing effects of amphetamine but not of morphine as measured by place conditioning. Pharmacol Biochem Behav. 1985;22:101–105. doi: 10.1016/0091-3057(85)90492-7. [DOI] [PubMed] [Google Scholar]
- Maxwell SL, Ho HY, Kuehner E, Zhao S, Li M. Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol. 2005;282:467–479. doi: 10.1016/j.ydbio.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morency MA, Beninger RJ. Dopaminergic substrates of cocaine-induced place conditioning. Brain Res. 1986;399:33–41. doi: 10.1016/0006-8993(86)90598-6. [DOI] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; London: 2001. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van Rossum JM. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia. 1975;41:87–95. doi: 10.1007/BF00421062. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Smith HR, Nader MA. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004;27:813–820. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Pruitt DL, Bolanos CA, McDougall SA. Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur J Pharmacol. 1995;283:125–131. doi: 10.1016/0014-2999(95)00309-9. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rieger DK, Reichenberger E, McLean W, Sidow A, Olsen BR. A double-deletion mutation in the Pitx3 gene causes arrested lens development in aphakia mice. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Robinson S, Sotak BN, During MJ, Palmiter RD. Local dopamine production in the dorsal striatum restores goal-directed behavior in dopamine-deficient mice. Behav Neurosci. 2006;120:196–200. doi: 10.1037/0735-7044.120.1.000. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse. 2006;59:374–377. doi: 10.1002/syn.20247. [DOI] [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006;317:1178–1187. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum Mol Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Ohkuma S. Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav. 2000;66:285–292. doi: 10.1016/s0091-3057(00)00185-4. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Bouwmeester H, Hamers FP, van der Linden AJ, Hellemons AJ, et al. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development. 2004;131:1145–1155. doi: 10.1242/dev.01022. [DOI] [PubMed] [Google Scholar]
- Smits SM, Mathon DS, Burbach JP, Ramakers GM, Smidt MP. Molecular and cellular alterations in the Pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. 2005;30:352–363. doi: 10.1016/j.mcn.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Smits SM, Noorlander CW, Kas MJ, Ramakers GM, Smidt MP. Alterations in serotonin signalling are involved in the hyperactivity of Pitx3-deficient mice. Eur J Neurosci. 2008;27:388–395. doi: 10.1111/j.1460-9568.2008.06032.x. [DOI] [PubMed] [Google Scholar]
- Snyder AM, Zigmond MJ, Lund RD. Sprouting of serotoninergic afferents into striatum after dopamine-depleting lesions in infant rats: a retrograde transport and immunocytochemical study. J Comp Neurol. 1986;245:274–281. doi: 10.1002/cne.902450209. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behav Brain Res. 1987;26:57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Bruno JP, Snyder AM, Stricker EM, Zigmond MJ. Apparent sprouting of striatal serotonergic terminals after dopamine-depleting brain lesions in neonatal rats. Brain Res. 1984;291:164–167. doi: 10.1016/0006-8993(84)90665-6. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EA, Takahashi JS, et al. Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol Behav. 2000;69:269–275. doi: 10.1016/s0031-9384(00)00219-5. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Gilbert F, Chamberland M, Levesque D, Drouin J. Striatal neuroadaptation and rescue of locomotor deficit by l-dopa in aphakia mice, a model of Parkinson's disease. J Neurochem. 2006;96:160–170. doi: 10.1111/j.1471-4159.2005.03522.x. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- van Oosten RV, Verheij MM, Cools AR. Bilateral nigral 6-hydroxydopamine lesions increase the amount of extracellular dopamine in the nucleus accumbens. Exp Neurol. 2005;191:24–32. doi: 10.1016/j.expneurol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum DS, Stevens LC. Aphakia, a new mutation in the mouse. J Hered. 1968;59:147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yue Y, Widmer DA, Halladay AK, Cerretti DP, Wagner GC, Dreyer JL, et al. Specification of distinct dopaminergic neural pathways: roles of the Eph family receptor EphB1 and ligand ephrin-B2. J Neurosci. 1999;19:2090–2101. doi: 10.1523/JNEUROSCI.19-06-02090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.